Abstract
Chronic exposure to manganese (Mn) can cause manganism, a neurodegenerative disorder similar to Parkinson's disease. The toxicity of Mn includes impairment of astrocytic glutamate transporters. 17β-Estradiol (E2) has been shown to be neuroprotective in various neurodegenerative diseases including Parkinson's disease and Alzheimer's disease, and some selective estrogen receptor modulators, including tamoxifen (TX), also possess neuroprotective properties. We have tested our hypothesis that E2 and TX reverse Mn-induced glutamate transporter impairment in astrocytes. The results established that E2 and TX increased glutamate transporter function and reversed Mn-induced glutamate uptake inhibition, primarily via the up-regulation of glutamate/aspartate transporter (GLAST). E2 and TX also increased astrocytic GLAST mRNA levels and attenuated the Mn-induced inhibition of GLAST mRNA expression. In addition, E2 and TX effectively increased the expression of transforming growth factor β1, a potential modulator of the stimulatory effects of E2/TX on glutamate transporter function. This effect was mediated by the activation of MAPK/extracellular signal-regulated kinase (ERK) and phosphoinositide 3-kinase (PI3K)/Akt signaling pathways. These novel findings suggest, for the first time, that E2 and TX enhance astrocytic glutamate transporter expression via increased transforming growth factor β1 expression. Furthermore, the present study is the first to show that both E2 and TX effectively reverse Mn-induced glutamate transport inhibition by restoring its expression and activity, thus offering a potential therapeutic modality in neurodegenerative disorders characterized by altered glutamate homeostasis.
Keywords: 17β-estradiol, astrocytes, astroglial glutamate transporters, glutamate transporters, tamoxifen, transforming growth factor-β1
Elevated extracellular levels of glutamate are associated with excitotoxic neuronal death and neurodegenerative processes (Gillessen et al. 2002). Therefore, removal of glutamate by astrocytic glutamate transporters is critical for maintaining optimal extracellular concentrations of this amino acid (Gegelashvili and Schousboe 1997). Given the critical role of astrocytic glutamate transporters (GLAST and GLT-1) in neuronal cell death, impairment of glutamate transporters has been invoked in neurological disorders including Alzheimer's disease (AD) (Masliah et al. 1996; Ferrarese et al. 1999), Parkinson's disease (PD) (Ferrarese et al. 1999) and amyotrophic lateral sclerosis (ALS) (Rothstein et al. 1992).
Chronic exposure to high levels of occupational or environmental Mn is known to cause neurotoxicity (Cotzias et al. 1968), resulting in manganism (Barbeau 1984; Sloot et al. 1994), a disease resembling PD. Although the underlying mechanisms are not completely understood, glutamate-mediated excitotoxicity plays a key role in its etiology (Brouillet et al. 1993; Plaitakis and Shashidharan 2000; Hazell 2002). Mn decreases astrocytic glutamate uptake (Hazell and Norenberg 1997; Normandin and Hazell 2002; Mutkus et al. 2005) and reduces the expression of the astrocytic glutamate transporter, GLAST (Erikson and Aschner 2002; Erikson et al. 2002), leading to excessive extracellular glutamate levels. Thus, agents that effectively enhance astrocytic glutamate transporter function may serve as potential therapeutics against the Mn-induced neurotoxicity and other neurological disorders characterized by impairment of astrocytic glutamate transporters.
There is abundant evidence in support of 17β-estradiol (E2)'s neuroprotective effects in diverse in vitro and in vivo models as well as clinical neurodegenerative diseases (Shi et al. 1997; Simpkins et al. 1997; Dhandapani and Brann 2002). Because of the undesirable peripheral effects of E2, selective estrogen receptor modulators (SERMs), such as tamoxifen (TX) and raloxifen, have received extensive attention as potential neuroprotectants. Tamoxifen has been shown to exert neuroprotective effects in diverse models of brain injury (Kimelberg et al. 2000; Dhandapani et al. 2005).
Astrocytes play a critical role in E2-induced neuroprotection (Azcoitia et al. 2001; Dhandapani and Brann 2003a, 2007; Sortino et al. 2004). Astrocytes express two types of E2 receptors, ER-α and ER-β, and increase E2-synthesizing aromatase expression upon brain injury (Peterson et al. 2004), suggesting that astrocytic E2 actively participates in response to brain injury. It has been suggested that E2-induced growth factor production is involved in astrocyte-mediated E2's neuroprotection. E2 increases the astrocytic production of transforming growth factor-β1 (TGF-β1), protecting neurons from apoptosis (Dhandapani and Brann 2003b).
In addition to direct neuroprotective effects by E2-induced release of growth factors from astrocytes, these factors may also indirectly protect neurons by modulating astrocytic functions per se, including the expression and function of astrocytic glutamate transporters. E2 increases the expression of GLT-1 and GLAST, enhancing astrocytic glutamate uptake in both the mouse and human AD brain (Liang et al. 2002; Pawlak et al. 2005), thus controlling the levels of glutamate in the extracellular fluid.
The main goal of the present study was to determine whether E2 and/or TX effectively attenuate Mn-induced glutamate transporter impairment in astroglial cells. We hypothesized that: (i) E2 and/or TX restore astrocytic glutamate transporter function via genomic pathways, and (ii) TGF-β1 mediates this process.
Materials and methods
Primary cortical astrocyte cultures
Astrocyte cultures were prepared according to previously established protocols (Aschner et al. 1992). Astrocytes were isolated from cerebral cortices of newborn (1-day-old) Sprague–Dawley rats. Pups were decapitated under halothane-anesthesia, and the cerebral cortices were dissected out. The meninges were removed, and the cortices were digested with bacterial neutral protease (Dispase, Invitrogen, Eugene, OR, USA). Astrocytes were then recovered by the repeated removal of dissociated cells and plated at a density of 1 × 105 cells/mL. Twenty-four hours after the initial plating, the media were changed to preserve the adhering astrocytes and to remove neurons and oligodendrocytes. The cultures were maintained at 37°C in a 95% air/5% CO2 incubator for 3 weeks in minimum essential medium (MEM) with Earle's salts supplemented with 10% fetal bovine serum, 100 U/mL of penicillin and 100 μg/mL of streptomycin. The media were changed twice per week. Our experience dictates that the purity of these cultures is > 95%-positive for the astrocyte-specific marker, glial fibrillary acidic protein. All experiments were performed 3 weeks post-isolation.
Cell viability assay
Cell viability was measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Astrocytes were grown in flasks for 3 weeks and detached from the flasks with 0.25% trypsin. Cells (1 × 105 cells/mL) were seeded in 96-well poly-l-lysine coated plates (100 μL/well) and allowed to attach to the substratum overnight. Subsequently, the cultures were treated with designated compounds or vehicle control in Opti-MEM reduced serum media followed by incubation for the indicated times. Three hours before the end of the incubation period, 10 μL of phosphate-buffered saline (PBS) containing MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide) (5 mg/mL) was added to each well. Next, 100 μL of isopropanol containing 1.0 N HCl was added to each well to dissolve the crystals formed by MTT reagent. Absorbance was measured with a plate reader at 550 nm, using a reference wavelength of 630 nm.
Glutamate uptake assay
Glutamate uptake was measured as previously described (Mutkus et al. 2005), with a minor modification. Astrocytes (24-well plates) were treated with compounds in Opti-MEM reduced serum media for the indicated times. Next, the culture media were washed 2× and replaced with the uptake buffer, pre-warmed HEPES-buffered solution containing 122 mM NaCl, 3.3 mM KCl, 0.4 mM MgSO4, 1.3 mM CaCl2, 1.2 mM KH2PO4, 25 mM HEPES, and 10 mM d-(+)-glucose, pH of 7.4. Five min later, pre-warmed uptake buffer containing 0.25 μCi/ml l-[3H]-glutamate (specific activity: 49.0 Ci/mmol, Amersham Pharmacia Biotech, Piscataway, NJ, USA) and unlabeled glutamate at a final concentration of 100 nM (Liang et al. 2002) was added. Uptake was terminated after 10 min of incubation at 37°C by three washes with ice-cold PBS, immediately followed by cell lysis in 1 mL of 1 N NaOH. An aliquot of 750 μL was neutralized in 75 μL of 10 N HCl, and radioactivity was determined by liquid scintillation counter (LS 6500, Beckman Coulter, Fullerton, CA, USA). Protein content was determined using a 25 μL aliquot neutralized in 1 N HCl, and quantified using the bicinchoninic acid protein assay reagent kit (Pierce, Rockford, IL, USA). Radioactivity counts were corrected for protein levels and calculated as glutamate nmol/mg protein/min. Experiments were performed in quadruplicates in three independent cultures.
Measurement of the extracellular l-glutamate concentrations
A series of experiments was carried out to determine the rate of glutamate disappearance from the media as a surrogate measure of glutamate uptake in astrocytes. Extracellular glutamate levels were measured by a fluorimetric method, using the Amplex Red Glutamic Acid assay kit (Invitrogen). Cells in 96-well plates were treated with Mn, E2 or TX in Opti-MEM media for the indicated times. Immediately thereafter, the uptake buffer containing glutamic acid (20 μM) was replaced with media in each well. After 30 min of incubation, 50 μL of supernatants from each sample was transferred into 96-well plates, followed by the addition of 50 μL of substrate mixture containing 100 μM Amplex Red, 0.25 U/mL horseradish peroxidase, 0.08 U/mL l-glutamate oxidase, 0.5 U/mL l-glutamate-pyruvate transaminase, 200 μM l-alanine and 1× reaction buffer. After 30 min of incubation at 37°C, fluorescence was measured in a fluorescence microplate reader (FlexStation, Molecular Devices, Sunnyvale, CA, USA) with excitation-emission at 530–590 nm.
Western blot analysis
After the indicated treatments, cultures were washed twice with cold Hank's buffered salt solution (HBSS). Subsequently, 50 μL of a RIPA buffer (radio immunoprecipitation assay buffer; 150 mM NaCl, 1.0% IGEPAL® CA-630, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, and 50 mM Tris, pH 8.0) containing a protease inhibitor cocktail (10 μL in 1 mL of lysis buffer; Sigma-Aldrich, St Louis, MO, USA) was added to the each well of 6-well plates, and cells were scraped and transferred to microcentrifuge tubes. After sonication, the suspensions were centrifuged at 3000 g for 10 min at 4°C, and protein solutions from the supernatant were obtained. The protein content of the cell lysates was measured with bicinchoninic acid reagents, samples of 30 μg protein were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (10%) under reducing conditions and proteins were then electrophoretically transferred to a polyvinylidenefluoride membrane (Millipore, Bedford, MA, USA). The membranes were blocked for 1 h at 22–25°C in Tris Buffered Saline (TBS) with 0.1% Tween 20 (TBST) containing 5% non-fat milk. The blots were incubated with primary antibodies (GLAST, 1 : 2000; μ-actin, 1 : 5000; Millipore) for 2 h at 22–25°C and subsequently washed three times in TBST. Then, the membranes were incubated with the secondary antibodies (1 : 3000, anti-guinea pig, anti-mouse IgG peroxidase conjugates, Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 h at 2 h at 22–25°C. After three washes with TBST, the immunoreactive proteins were detected using an enhanced chemiluminescence western blotting detection kit (Pierce).
Immunohistochemistry
After the indicated treatments, astrocytes were washed twice with TBS and fixed at 22–25°C for 10 min with 100% cold methanol. Cells were permeabilized with 0.1% Triton X-100 in PBS for 15 min and then incubated for 1 h in blocking buffer (10% goat serum). After incubation with the primary antibodies, mouse monoclonal anti-glial fibrillary acidic protein (1 : 200, Millipore), anti-estrogen receptors, α and β, (Santa-Cruz Technology) and the anti-GLAST antibodies (1 : 500, Millipore), overnight at 4°C, cells were washed with washing buffer and incubated with secondary antibodies with fluorescein conjugates (1 : 200, Millipore). To visualize cell nuclei, cultures were rinsed and then incubated in 4′,6-diamidino-2-phenylindole dihydrochloride hydrate/antifade diluted 1 : 1000 in TBS for 10 min at 22–25°C. The cover slips were mounted in Immunomount, and pictures were taken with a wide-field fluorescence microscope (Olympus, Pittsburgh, PA, USA).
RT-PCR
Total RNA was extracted from cells using the RNeasy mini RNA isolation kit (Qiagen, Valencia, CA, USA). Two μg total RNA was transcribed to cDNA with poly-dT oligonucleotides and reverse transcriptase. PCR amplifications targeting GLAST and TGF-β1 were performed with polymerase chain reaction kits in a final volume of 50 μL. Sequences of the primers are listed in Table 1. After 30–40 amplification cycles [denaturing at 94°C for 30 s, annealing at 55–64°C for 30 s and extension at 72°C for 1 min (iCycler, Bio-Rad Laboratories, Hercules, CA, USA)], samples were electrophoresed on a 1.5% agarose gel, and nucleic acid bands were visualized by ethidium bromide staining. Glyceraldehyde 3-phosphate dehydrogenase was used as internal standard.
Table 1.
Sequences of primers for RT-PCR reactions and sizes of PCR products
| cDNA | Primers | Size of products (base pairs) |
|---|---|---|
| GLAST | Forward: ACGGTCACTGCTGTCATTG | 121 |
| Reverse: TGTGACGAGACTGGAGATGA | ||
| TGF-β1 | Forward: TGCTTCAGCTCCACAGAGAA | 180 |
| Reverse: TGGTTGTAGAGGGCAAGGAC | ||
| GAPDH | Forward: TCCCTCAAGATTGTCAGCAA | 300 |
| Reverse: AGATCCACAACGGATACATT |
GLAST, glutamate/aspartate transporter; TGF-β1, transforming growth factor-β1; GADPH, glyceraldehyde 3-phosphate dehydrogenase.
Real-time PCR
Real-time PCR was performed for the amplification of GLAST and TGF-β1 with a Cycler real-time PCR detection system (Applied Biosystem, Foster City, CA, USA). The real-time PCR reactions were carried out in a total volume of 5 μL, containing a 2 ng cDNA template of each sample, 0.3 μm of the appropriate primers and the Taqman Mastermix 1× (100 mm KCl, 40 mm Tris-HCl, pH 8.4, 0.4 mm of each dNTP, 50 U/mL iTaq DNA polymerase, 6 mm MgCl2, Taqman probes, 20 nm fluorescein and stabilizers). The PCR protocol was carried out as follows: 15 s denaturation at 95°C and 1 min annealing at 60°C for 40 cycles. Fluorescence was detected at the end of each elongation step. Each sample was normalized with the relative amplification of the glyceraldehyde 3-phosphate dehydrogenase. Relative quantification of mRNA in the samples were performed by the post-run data analysis software (SDS 2.3) provided by the Cycler system from Applied Biosystems.
Biotinylation of cell surface proteins
Biotinylation of cell surface proteins was performed with the Pierce cell surface protein isolation kit. Briefly, astrocytes grown in 10 mm dishes were rinsed twice with ice-cold PBS containing 0.1 mM Ca2+ and 1 mM Mg2+ and incubated for 30 min at 4°C with 7 mL of Sulfo-NHS-SS-biotin (1 mg/mL PBS containing Ca2+ and Mg2+). Unreacted biotin was quenched by a Quenching solution containing 100 mM glycine in PBS Ca2+/Mg2+. Next, cells were scraped and collected by centrifugation at 500 g for 3 min, followed by lysis. Biotinylated proteins were isolated by Immobilized NeutrAvidin Gel loading columns. Once proteins eluted from the columns, protein assay and western blot analysis were performed to detect GLAST as previously described in the western blot section (see above). Beta-actin, which is not expressed in cell surface membranes, was also evaluated to assure the purity of cell surface proteins.
Statistical analysis
The mean and SEM were determined for each set of data. One-way analysis of variance (anova) followed by a Tukey post-hoc test was used for statistical analysis. A probability of 0.05 or less was considered statistically significant.
Results
E2/TX protected astrocytes against Mn-induced cytotoxicity
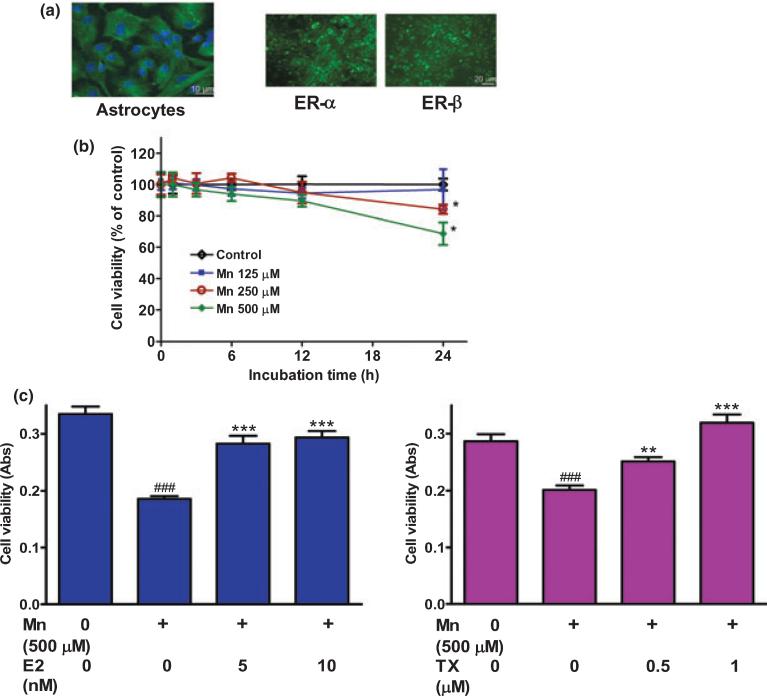
Figure 1(a) shows that the purity of the astroglial cultures that were used in the present study, was estimated at > 95%. These cultures expressed both ER-α and ER-β, indicating that E2 could exert its physiological action via classical estrogen receptor activation, as it has been reported previously (Dhandapani et al. 2005). The effect of Mn on cell viability is shown in Fig. 1(b). Mn caused a time- and concentration-dependent cell death. Treatment of astrocytes for 24 h with Mn at 250 and 500 μM induced significant cell death compared with controls (p < 0.05). Based on these findings, Mn at 500 μM was chosen for additional studies aimed at determining the efficacy of E2/TX for protecting astrocytes from cell death. As shown in Fig. 1(c), pre-treatment of astrocytes for 24 h with E2 or TX effectively rescued astrocytes from Mn-induced cell death in a concentration-dependent manner. E2 was used at 5–20 nM, a concentration shown to be physiologically relevant; TX was used at 1–2 μM, a concentration shown to be pharmacologically relevant (Dhandapani et al. 2005).
Fig. 1.
E2/TX protects astrocytes from Mn toxicity in astroglial cultures. (a) Both E2 receptor subtypes, ER-α and ER-β, are expressed in astrocytic cultures. (b) Mn induced astrocytic cytotoxicity occurs in a time- and concentration-dependent manner. Cell viability was assessed by MTT assay as described in the Methods section. (c) E2/TX protects astrocytes from Mn toxicity. Astrocytes were pre-treated for 24 h with E2 (5 and 10 nM) or with TX (0.5 and 1 μM) prior to the addition of Mn (500 μM), followed by the MTT assay. (###p < 0.001 vs. control; **p < 0.01, ***p < 0.001 vs. the Mn treatment; Tukey's test following anova). Data are expressed as the mean ± SEM (n = 5–8). Experiments were performed in three independent sets of cultures.
E2/TX attenuated Mn-induced glutamate uptake inhibition
Next, we assessed the following: (i) the ability of Mn to inhibit glutamate uptake; (ii) the relative contributions of GLAST and GLT-1 to glutamate uptake; and (iii) the ability of E2/TX to reverse Mn-induced inhibition of glutamate transporters.
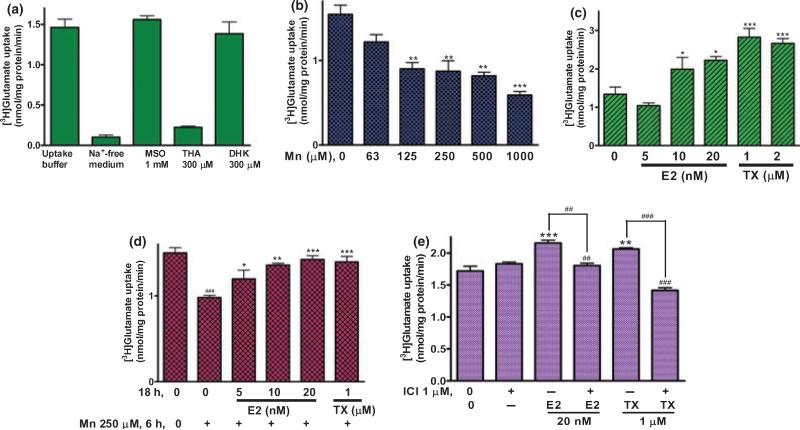
As shown in Fig. 2(a), [3H]glutamate uptake in Na+-free media was completely blocked (Fig. 2a), establishing that glutamate uptake in these cultures is Na+-dependent. As astrocytic glutamate is metabolized to glutamine by glutamine synthetase, methionine sulfoximine, a specific inhibitor of this enzyme, was used. The addition of 1 mM methionine sulfoximine to the buffer did not alter glutamate uptake (Fig. 2a), indicating that glutamate to glutamine metabolism within the experimental period is negligible.
Fig. 2.
E2/TX reverses Mn-induced glutamate uptake inhibition in astrocytes. After treatment with Mn or E2/TX, cells were incubated with 100 nM of unlabeled glutamate containing 0.25 μCi of [3H]glutamate (specific activity: 49.0 Ci/mmol) for 10 min. The uptake was terminated by washing the cells with ice-cold PBS buffer as described in the Methods section. (a) Glutamate uptake was THA-sensitive, Na+-dependent and GLAST subtype-sensitive. [3H]glutamate uptake was measured in uptake buffer, Na+-free medium, in the presence of THA (a non-selective inhibitor of glutamate transporters), MSO (an inhibitor of glutamine synthase) or DHK (a selective inhibitor of GLT-1). (MSO, methionine sulfoximine; THA, dl-threo-μ-hydroxyaspartic acid; DHK, dihydrokainic acid). (b) Mn (6 h) inhibited glutamate uptake in a concentration-dependent manner. (c) E2/TX (24 h) increased glutamate uptake. (d) E2/TX (18 h pre-treatment) reversed Mn (6 h)-induced glutamate uptake inhibition. (e) E2/TX-induced enhancement of glutamate uptake was ER-dependent. ###p < 0.001 vs. control (d); *p < 0.05, **p < 0.01, ***p < 0.001, ##p < 0.01 vs. control (b,c,e) or Mn treatment (d); Tukey's test following anova. Data are expressed as the mean ± SEM (n = 5–8).
To assure that the glutamate uptake assay was mediated by glutamate transporters, the non-specific glutamate transporter inhibitor, dl-threo-O-hydroxy-aspartic acid (THA, 300 μM), was used. dl-threo-O-hydroxy-aspartic acid almost completely blocked [3H]glutamate uptake (Fig. 2a), indicating that uptake is specific to glutamate transporters. To ascribe the contribution of glutamate uptake to each of the specific transporters, dihydrokainic acid (DHK, 300 μM), a GLT-1 specific inhibitor (Suzuki et al. 2001) was used. Dihydrokainic acid did not inhibit glutamate uptake (Fig. 2a), indicating that glutamate uptake in the cultured astrocyte occurred predominantly through the GLAST transporter, which corroborates the previous report (Kondo et al. 1995).
The next experiment addressed the effect of Mn exposure for 6 h on glutamate uptake for 10 min. This time period was selected based on the results shown in previous reports (Mutkus et al. 2005). We aimed to separate the direct effect of Mn on glutamate uptake that is associated with transporter expression and function from uptake inhibition that is secondary to cell death. Treatment of astrocytes for 6 h with Mn inhibited glutamate uptake in a concentration-dependent manner (Fig. 2b), indicating that glutamate uptake inhibition precedes cell death, which is observed only after 24 h of exposure to Mn (Fig. 1b).
When astrocytes were treated for 24 h with E2 or TX, a time period sufficient to effectively increase glutamate uptake, E2 (10 and 20 nM) and TX (1 and 2 μM) significantly increased glutamate uptake (Fig. 2c). Pre-treatment of astrocytes for 18 h with E2 or TX prior to Mn exposure for 6 h reversed Mn-induced glutamate uptake inhibition (Fig. 2d). The E2/TX-induced enhancement of glutamate uptake was estrogen receptor (ER)-dependent (Fig. 2e), given that the ER antagonist, ICI182,780, fully abolished the E2/TX-induced enhancement of astrocytic glutamate uptake.
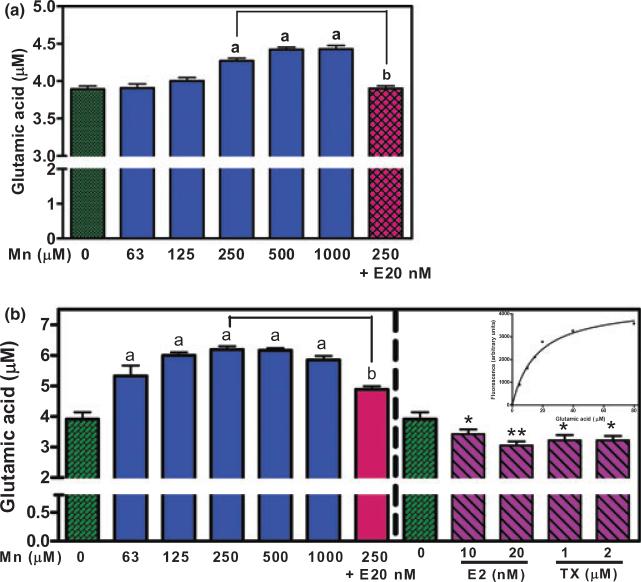
Additional studies addressed glutamate disappearance from the culture media, a surrogate measure of Mn-induced impairment in glutamate uptake. The standard glutamate assay validated that the addition of glutamate up to 20 μM was linear (Fig. 3b, inserted graph). Treatment of astrocytes for 6 h with Mn (250 μM up to 1000 μM) led to a concentration-dependent glutamate accumulation (by interfering uptake), and pre-treatment with E2 (20 nM) for 24 h blocked this effect (Fig. 3a). To determine whether a longer Mn treatment increases glutamate accumulation in the medium, astrocytes were treated for 24 h with Mn. As shown in Fig. 3(b), Mn (63, 125 and 250 μM) increased glutamate accumulation in the medium in a concentration-dependent manner (34.5 ± 8.7%, 53.4 ± 3.1% and 58.3 ± 2.9%, respectively). Pre-treatment of astrocytes for 24 h with E2 (20 nM) prior to the addition of Mn (250 μM) for 24 h attenuated Mn-induced glutamate accumulation from 58.3 ± 2.9% to 24.9 ± 2.8%. To better understand the E2/TX-attenuation mechanism, astrocytes were treated for 24 h with E2 or TX alone. The results showed that both E2 (10 and 20 nM) and TX (1 and 2 μM) significantly reduced glutamate accumulation and that this effect was most pronounced with E2 (20 nM), which decreased glutamate accumulation by 23 ± 3.6% (Fig. 3b).
Fig. 3.
Mn leads to glutamate accumulation in the astrocytic media, whereas E2/TX reverses this effect. Cells were treated for 6 or 24 h with Mn or E2/TX, after which 20 μM glutamate were added to the cell cultures, followed by 30 min of incubation. Glutamate levels in the media were measured by a fluorimetric method. (a) Astrocytes were treated for 6 h with Mn. (b) Astrocytes were treated for 24 h with Mn or E2/TX. For one set of each experiment, cells were pre-treated for 24 h with E2 20 nM prior to Mn (250 μM for 6 or 24 h) treatment. ap < 0.05 vs. control, bp < 0.05 vs. Mn 250 μM, *p < 0.05, **p < 0.01 vs. control; Tukey's test following anova. Data are expressed as the mean ± SEM (n = 5–8).
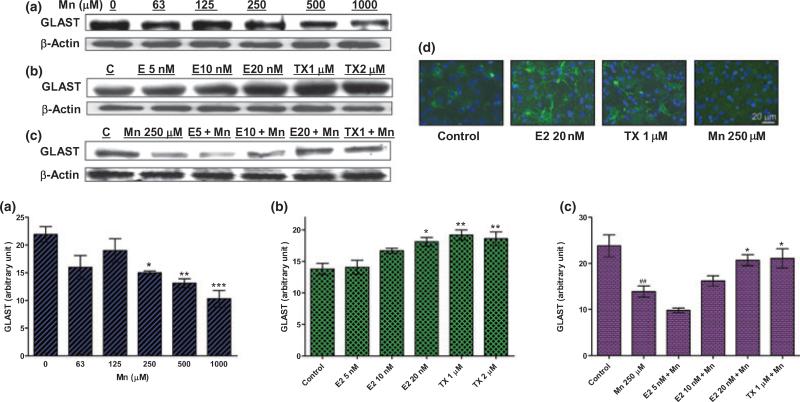
E2/TX attenuated the Mn-induced inhibition of GLAST protein expression in whole cell lysates
As GLAST was implicated as the main contributor to astrocytic glutamate uptake of our experimental condition (Fig. 2a), the next experiment was designed to determine whether E2/TX altered its protein expression. Treatment of astrocytes for 6 h with Mn decreased (Fig. 4a), whereas treatment for 24 h with E2 and TX increased GLAST expression (Fig. 4b). Pre-treatment for 18 h with E2 or TX prior to Mn treatment for an additional 6 h (in the presence of E2 or TX) attenuated Mn-induced GLAST inhibition (Fig. 4c). E2/TX treatment led to an increase, whereas Mn treatment led to a decrease in GLAST protein expression (as shown by immunocytochemical staining, Fig. 4d).
Fig. 4.
Mn-induced inhibition of GLAST protein expression in whole cell lysates is reversed by E2/TX. (a) Treatment of astrocytes for 6 h with Mn decreased GLAST expression in a concentration-dependent manner. (b) Treatment of astrocytes for 24 h with E2 (20 nM) and TX (1 and 2 μM) increased GLAST protein expression. (c) Pre-treatment of E2 (10 and 20 nM) and TX (1 μM) for 18 h prior to Mn treatment for 6 h attenuated the Mn-induced inhibitory effects of GLAST expression. Western blot data were also quantified. ##p < 0.01, *p < 0.05, **p < 0.01, ***p < 0.001 vs. control; Tukey's test following anova. Data are expressed as the mean ± SEM (n = 3). (d) Immunocytochemistry study showed that E2 and TX increased astrocytic GLAST expression after 24 h, whereas Mn inhibited GLAST protein expression after 6 h.
Mn decreased, but E2/TX increased GLAST expression and reversed the Mn-induced inhibitory effects on GLAST expression in astroglial plasma membranes
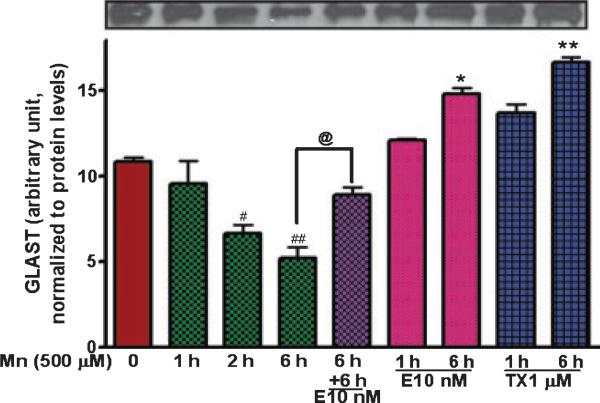
Glutamate transporter function, like other plasma membrane proteins, is regulated by trafficking to or from the plasma membrane. Accordingly, we sought to determine if Mn or E2/TX alters the membrane trafficking of GLAST. Astrocytes were treated with E2/TX (1 or 6 h) or Mn (1, 2 or 6 h). These time periods are shorter than those described in the previous studies because GLAST trafficking to or from the membrane occurs faster than its regulation at translational and transcriptional levels (Sheldon and Robinson 2007). As shown in Fig. 5, Mn decreased plasma membrane GLAST protein expression within 2 h of exposure, whereas pre-treatment for 6 h with E2 (10 nM) prior to Mn exposure (for additional 6 h in the presence of E2 or TX) attenuated the Mn-induced inhibitory effects on plasma membrane GLAST expression. Treatment of astrocytes for 6 h with E2 (10 nM) or TX (1 μM) alone increased astrocytic membrane GLAST expression by 36.4 ± 3.7% and 53.9 ± 2.8%, respectively.
Fig. 5.
E2/TX enhances GLAST expression on the astroglial plasma membranes and reverses Mn-induced reduction by promoting GLAST trafficking. Astroglial plasma membranes were isolated by biotinylation of surface membrane proteins using a biotinylation kit (Pierce) followed by western blot analysis. All samples were adjusted to contain the same amount of proteins prior to loading on the gel. Mn (500 μM) reduced, while E2/TX increased GLAST expression on astroglial cell surface membranes. E2 reversed the Mn-induced inhibition of GLAST expression (#p < 0.05, ##p < 0.01 vs. control; *p < 0.05, **p < 0.01 vs. control; @p < 0.05; Tukey's test following anova). Data are expressed as the mean ± SEM (n = 3).
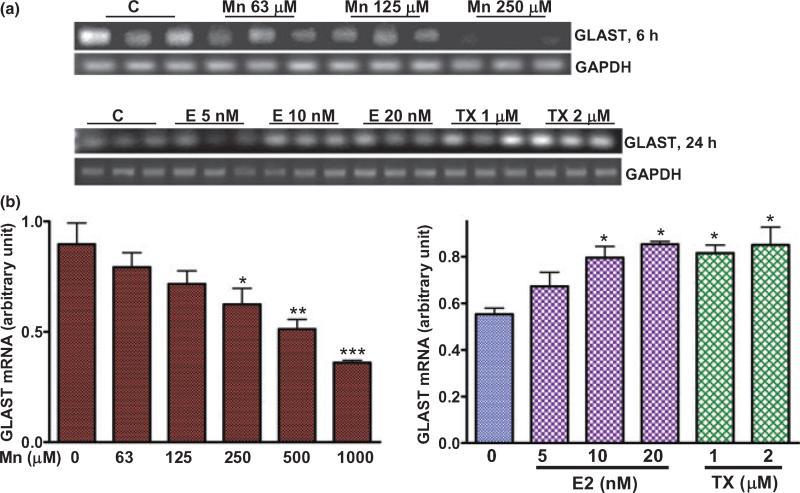
Mn inhibited the expression of GLAST mRNA, whereas E2/TX increased GLAST mRNA
As Mn decreased, but E2/TX increased GLAST protein expression in astrocytes (Fig. 4), we next sought to determine whether transcriptional levels of GLAST expression were altered by Mn or E2/TX treatments. Semi-quantitative RT-PCR and quantitative real time PCR analyses were carried out. Mn (6 h) decreased GLAST mRNA expression in a concentration-dependent manner (RT-PCR, Fig. 6a; real time PCR assay, Fig. 6b). Treatment of astrocytes for 24 h with E2/TX led to increased GLAST mRNA expression, as shown with both conventional RT-PCR and real time PCR (Fig. 6a and b, respectively). The real time PCR data established that E2 (10 and 20 nM) and TX (1 and 2 μM) increased GLAST mRNA levels by 43.2 ± 6.4%, 53.6 ± 2.5%, 47.6 ± 3.1% and 53.1 ± 7.6%, respectively (Fig. 6b).
Fig. 6.
Mn inhibits, whereas E2/TX increases expression of GLAST mRNA. (a) Incubation of astrocytes for 6 h with Mn decreased the expression of GLAST mRNA, while treatment of astrocytes for 24 h with E2/TX increased GLAST expression by RT-PCR, semi-quantitative analysis of GLAST mRNA. (b) Treatment of astrocytes for 6 h with Mn decreases GLAST expression, whereas treatment of astrocytes for 24 h with E2 and TX increased GLAST mRNA expression by the real-time PCR analysis. *p < 0.05, **p < 0.01, ***p < 0.001 vs. control; Tukey's test following anova. Data are expressed as the mean ± SEM (n = 4).
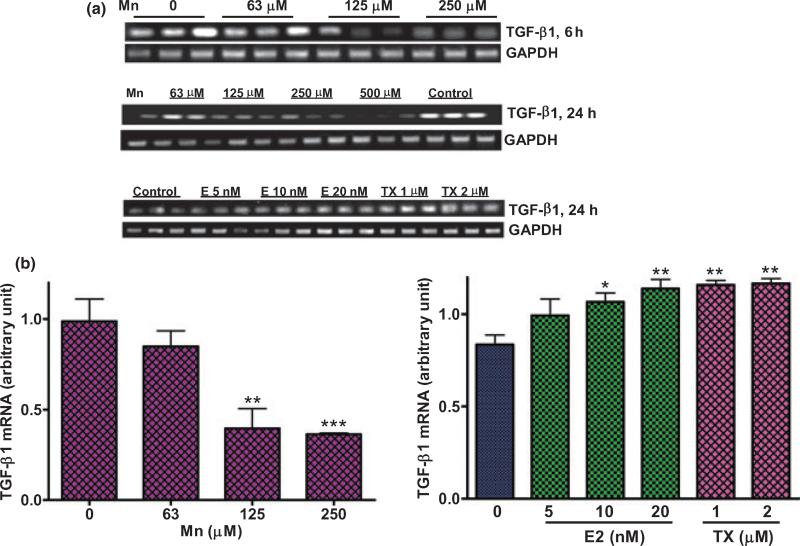
Mn and E2/TX exerted opposite effects on the expression of TGF-β1 mRNA
Glutamate transporters are regulated by growth factors (Suzuki et al. 2001; Figiel et al. 2003), such as epidermal growth factor and basic fibroblast growth factor (Figiel et al. 2003). As Mn and E2/TX exerted opposite effects on glutamate transporter functions (Figs 2 and 3), we next sought to determine whether TGF-β1 mediates the effects of Mn or E2/TX on GLAST mRNA expression. As shown in Fig. 7(a), astrocytic TGF-β1 mRNA levels were decreased by Mn (24 h), whereas E2/TX increased TGF-β1 mRNA expression (24 h; Fig. 7a). The quantitative real time PCR data showed that E2 (10 and 20 nM) and TX (1 and 2 μM) increased TGF-β1 mRNA levels by 27.5 ± 3.1%, 36.2 ± 2.7%, 38.5 ± 2.4% and 39.5 ± 5%, respectively (Fig. 7b).
Fig. 7.
Mn and E2/TX exert opposite effects on the expression of astrocytic transforming growth factor β1 (TGF-β1) mRNA. (a) Treatment of astrocytes for 6 h or 24 h with Mn decreased TGF-β1 expression, whereas E2/TX treatment for 24 h increased TGF-β1 expression by RT-PCR analysis. (b) Real time-PCR analysis showed that treatment of astrocytes for 24 h with Mn decreased TGF-β1 mRNA expression, but E2/TX increased TGF-β1 mRNA expression. *p < 0.05, **p < 0.01 ***p < 0.001 vs. control; Tukey's test following anova. Data are expressed as the mean ± SEM (n = 4). Experiments were performed in three independent sets of cultures for verification of results.
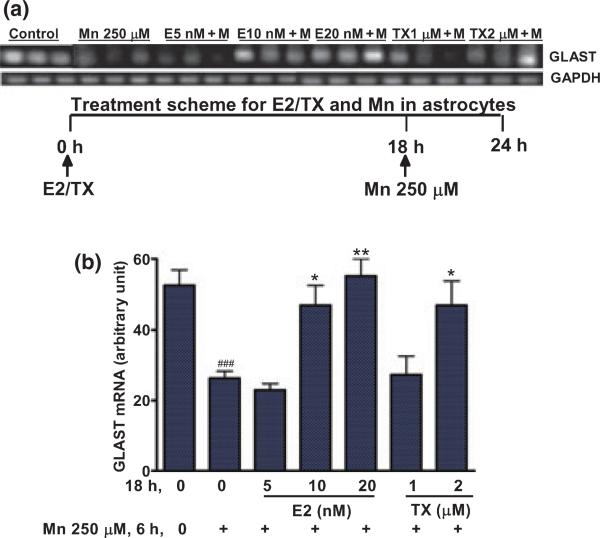
E2/TX attenuated the Mn-induced inhibition of GLAST mRNA
Next, we sought to determine if E2/TX could block or attenuate the Mn-induced inhibitory effects on the expression of GLAST. As shown in Fig. 8, treatment of astrocytes for 18 h with E2/TX prior to Mn treatment for 6 h in the presence of E2/TX led to attenuation in Mn-induced GLAST inhibition.
Fig. 8.
E2/TX attenuates the Mn-induced inhibition of GLAST expression in astrocytes. Pre-treatment of astrocytes for 18 h with E2/TX prior to Mn exposure for 6 h protected against the Mn-induced inhibition of GLAST mRNA expression by RT-PCR (a) and real time-PCR analyses (b) (###p < 0.001 vs. control; *p < 0.05, **p < 0.01 vs. the Mn treatment; Tukey's test following anova). Data are expressed as the mean ± SEM (n = 4). Experiments were performed in three independent sets of cultures for verification of results.
TGF-β1 mediated the E2/TX-induced enhancement of glutamate transporter functions
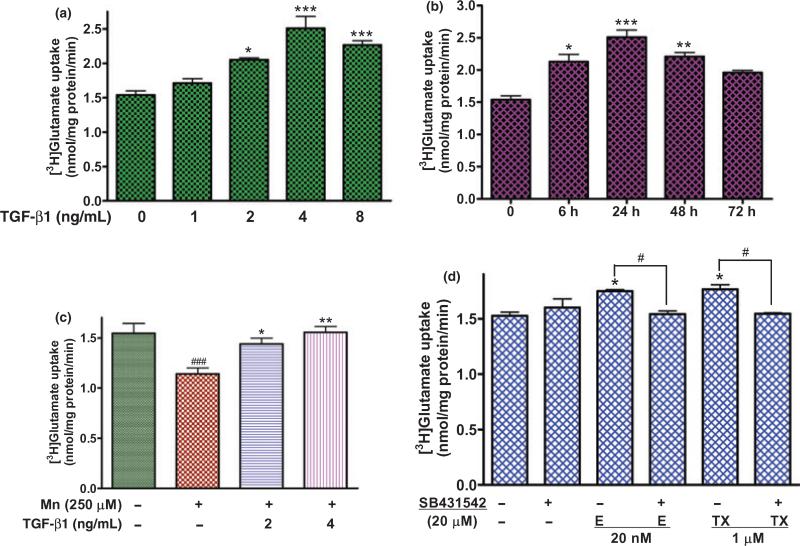
As we have postulated that E2/TX-induced TGF-β1 expression might mediate the E2/TX-induced enhancement of glutamate transporters, we next sought to determine whether TGF-β1 could increase glutamate transporter function. As shown in Fig. 9(a), TGF-β1 increased astrocytic glutamate uptake in a concentration-dependent manner. In another set of experiments, TGF-β1 (4 ng/mL) was also shown to lead to a time-dependent increase in glutamate uptake (Fig. 9b). Pre-treatment of astrocytes for 18 h with TGF-β1 (2, 4 ng/mL) prior to Mn treatment for 6 h attenuated the Mn-induced inhibition of glutamate uptake (Fig. 9c). The specific inhibitor of the TGF-β1 receptor, SB431542, abolished E2- or TX-enhancement of glutamate uptake (Fig. 9d).
Fig. 9.
TGF-β1 increases glutamate uptake in a concentration- and time-dependent manner in astrocytes. (a) Treatment of astrocytes for 24 h with TGF-β1 (2, 4, 8 ng/mL) increased glutamate uptake. (b) TGF-β1 at 4 ng/mL increased glutamate uptake most effectively after 24 h treatment in astrocytes. (c) Pre-treatment with TGF-β1 (18 h) prior to Mn treatment (6 h) reversed the Mn-induced glutamate uptake inhibition in astrocytes. (d) SB431542 (a TGFR inhibitor, 20 μM) abolished the E2/TX-induced enhancement of glutamate uptake (###p < 0.01 vs. control; *p < 0.05, **p < 0.01, ***p < 0.001 vs. control (a,b,d), or the Mn treatment (c), #p < 0.05; Tukey's test following anova). Data are expressed as the mean ± SEM (n = 4).
MAPK/ERK and PI3K/Akt signaling pathways might be involved in the E2/TX and TGF-β1-induced enhancement of glutamate transporter functions
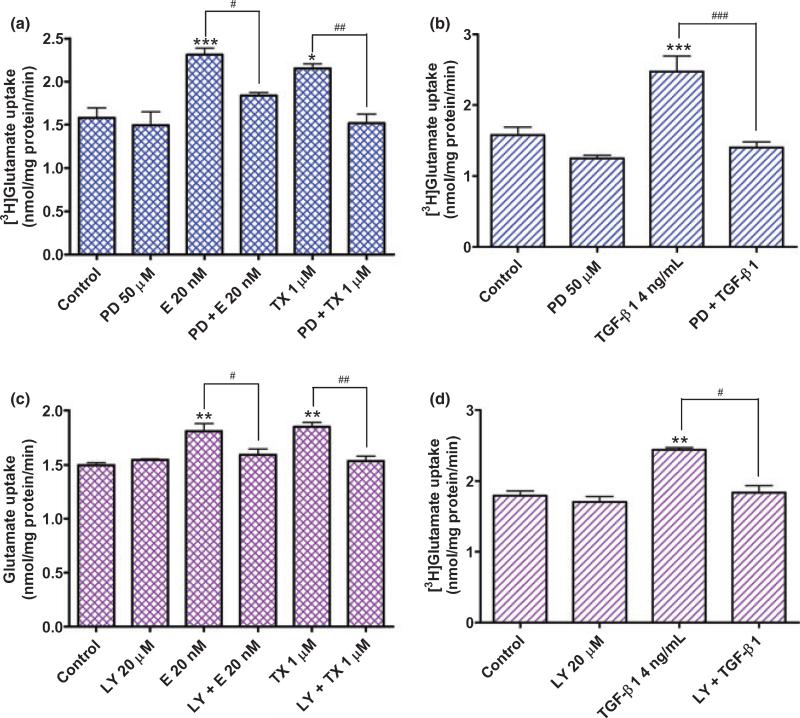
Astroglial glutamate uptake is regulated, at least in part, by the MAPK/extracellular signal-regulated kinase (ERK) (Abe and Saito 2001) and phosphoinositide 3-kinase (PI3K)/Akt (Koeberle and Bahr 2008) signaling pathways. Therefore, we tested whether these signaling pathways are involved in E2/TX- and TGF-β1-induced enhancement of glutamate transporter function. As shown in Fig. 10(a) and (b), PD98059, an inhibitor of MAPK, abolished the stimulatory effects of E2/TX and TGF-β1 on glutamate uptake. Likewise, an inhibitor of PI3K/Akt, LY294002, also blocked the enhancing effects of E2/TX and TGF-β1 (Fig. 10c and d) on glutamate uptake. These results indicate that the activation of MAPK and PI3K/Akt signaling pathways is involved in the TGF-β1-mediated enhancement of astrocytic glutamate transporter function by E2/TX.
Fig. 10.
(a,b) Activation of the MAPK/ERK signaling pathway is required for both E2/TX(a)- and TGF-β1(b)-induced enhancement of glutamate uptake in astrocytes. The addition of PD98059 50 μM, an inhibitor of MAPK/ERK, to the culture media 30 min prior to E2/TX or TGF-β1 treatment for 24 h abolished the E2/TX- or TGF-β1-induced enhancement of glutamate uptake (PD98059 was dissolved in DMSO as a concentration of 6.5 mg/mL and diluted with Opti-MEM experimental media). (c,d) Activation of the PI3K/Akt signaling pathway is required for both E2/TX(c)- and TGF-β1(d)-induced enhancement of glutamate uptake in astrocytes. The addition of LY294002 20 μM, an inhibitor of PI3K/Akt, to the culture media 30 min prior to E2/TX or TGF-β1 treatment for 24 h abolished the E2/TX- or TGF-β1-incuded enhancement of glutamate uptake (LY294002 was dissolved in DMSO at a concentration of 25 mg/mL and diluted with Opti-MEM media). The same concentration of DMSO was used as a control. (#p < 0.05, ##p < 0.01, ###p < 0.001; *p < 0.05, **p < 0.01, ***p < 0.001 vs. control; Tukey's test following anova). Data are expressed as the mean ± SEM (n = 4).
Discussion
Manganese has been widely reported to induce the impairment of glutamate uptake function as well as GLAST mRNA expression (Hazell and Norenberg 1997; Erikson et al. 2002; Mutkus et al. 2005). Given the important role of astrocytic glutamate transporters in maintaining extracellular concentrations of this excitotoxic amino acid (Rothstein et al. 2005), we explored the ability of E2 and TX to reverse the neurotoxic effects of Mn. We do not maintain that astrocytes are the only cell type affected by Mn. Nevertheless, there is abundant evidence in support of their pivotal role in mediating neurotoxicity, establishing astrocytes as a unique and relevant experimental model for the assessment of mechanisms underlying Mn-induced cytotoxicity and potential avenues for reversing its effects.
There is compelling evidence for the roles of glutamate-mediated excitotoxicity in Mn neurotoxicity and possible involvement of astrocytic glutamate transporters. Both Mn uptake and glutamate uptake predominantly occur in astrocytes (Aschner et al. 1999; Danbolt 2001). Mn injection into the rat striatum induces NMDA excitotoxic lesions in intact brain; prior treatment with the non-competitive NMDA antagonist MK801 blocks these lesions (Brouillet et al. 1993). These findings indicate that Mn neurotoxicity involves a NMDA receptor-mediated process (Spadoni et al. 2000). Mn decreases astrocytic glutamate uptake (Hazell and Norenberg 1997; Normandin and Hazell 2002) and the expression of the astrocytic glutamate transporter, GLAST, suggesting a potential mechanism by which extracellular glutamate levels could be increased (Erikson and Aschner 2002). These results indicate that the glutamatergic system of the basal ganglia is disrupted in Mn neurotoxicity, establishing a solid rationale to study glutamatergic dysfunction in this model of neurodegeneration.
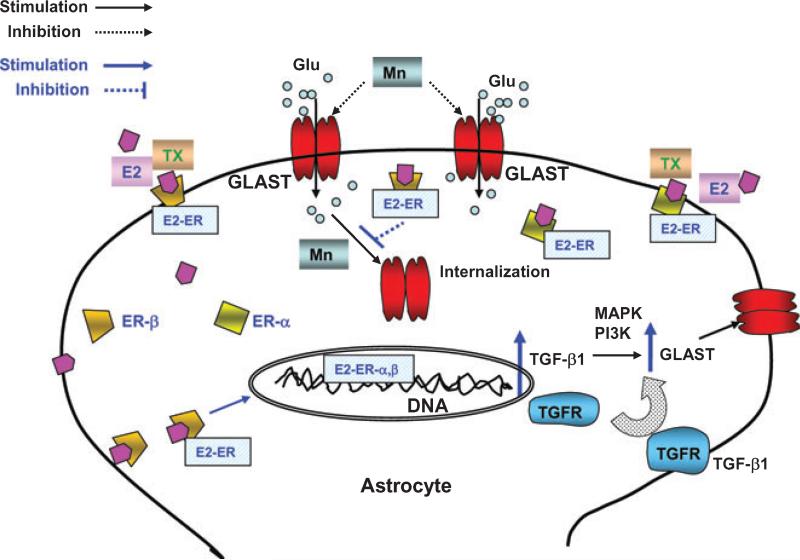
For the first time, we have demonstrated that: (i) E2 and TX attenuate Mn-induced astrocytic glutamate transporter impairment by restoring glutamate function via estrogen receptor-mediated genomic action, and (ii) TGF-β1 plays an important role in the E2 and TX-mediated expression of GLAST (Fig. 11). Our results establish that the inhibitory effects of Mn on glutamate uptake and GLAST expression are fully reversed by E2 or TX, suggest the possibility that E2 and TX may also be potentially efficacious in restoring glutamate homeostasis in neurodegenerative disorders generally characterized by glutamate uptake inhibition (Rothstein et al. 1996; Tao et al. 2001). The impairment of astrocytic glutamate transporters has been invoked in the etiology of AD (Masliah et al. 1996), PD (Ferrarese et al. 1999) and ALS (Rothstein et al. 1992). Consistent with these observations, enhancement of astrocytic transporter function by the β-lactam antibiotic, ceftriaxone, was shown to be neuroprotective in a model of ALS (Rothstein et al. 2005).
Fig. 11.
Proposed mechanism of E2/TX-induced restoration on Mn-induced astroglial glutamate transporter impairment. E2/TX may act via genomic actions to increase the expression of TGF-β1, which in turn increases GLAST expression in astrocytes. This action is ER-dependent. Modulation of GLAST trafficking by Mn or E2/TX may also play a role in the ability of E2/TX to attenuate Mn-induced glutamate transporter inhibition. Activation of MAPK/ERK and PI3K/Akt signaling is required for E2/TX and TGF-β1 in restoring glutamate transporter function (expression). Glu, glutamate; E2, 17β-estradiol; TX, tamoxifen; ER, estrogen receptor; GLAST, glutamate aspartate transporter; TGFR, transforming growth factor receptor.
Our results establish that E2 and TX restore Mn-induced glutamate transporter impairment by increasing the expression of both GLAST protein and mRNA expression. Both E2 and TX were most effective in attenuating the Mn-induced inhibition of glutamate transporters after 18 h of pre-treatment, suggesting a genomic action on GLAST expression. Specificity of ER-subtype involved, such as ER-α and ER-β, was not pursued in the present study. E2 10–20 nM were shown effective in induction of genomic GLAST protein expression; TX appeared more efficacious than E2, suggesting that SERMs offer a greater potential for neuroprotectant therapeutics. However, it is premature to exclude the possibility that E2 and TX restore GLAST transporter function via ER-independent mechanisms. For example, both E2 and TX possess antioxidant properties, effectively attenuating reactive oxygen species (ROS) production in astroglial cultures by Mn (unpublished data), glutamate and hydrogen peroxide (Moosmann and Behl 1999; Wang et al. 2006). Toxic Mn concentrations present in relevant mammalian cells can be estimated from the literature. Concentrations of Mn used in the present study were up to 500 μM in most experiments. Notably, concentrations of Mn (6 h exposure) that effectively induce glutamate transporter impairment are not associated with cell death (Fig. 1). Moreover, in in vivo exposure conditions, chronic low-dose of Mn exposure can lead to accumulation of Mn at concentrations as high as 200 μM in the striatal and globus pallidus (Erikson and Aschner 2002). In addition, weekly injections of Mn over a 3-month period in striatum and Globus Pallidus (GP) of monkeys (0, 2.25, 4.5, and 9 g), produce dose-related clinical signs, which are more severe in the higher dose range (Suzuki et al. 1975). At the highest dose, the Mn concentration is increased 12-fold in the striatum and nine-fold in the GP. The ‘physiological range’ (no symptoms) is ~75–100 μM, and clinical signs increase in frequency and severity above this level, suggesting this concentration is near the threshold of toxicity. Accordingly, the concentrations used herein represent Mn concentrations that are relevant to chronic Mn in vivo exposures in an in vitro set up.
Functional glutamate transporters are located on cell surface membranes. The activities of these transporters are regulated by a redistribution of these proteins to or from the plasma membrane (Robinson 2002), governed largely by signaling pathways. As shown in Fig. 5, Mn decreased, whereas E2/TX increased GLAST expression on the cell surface membranes by modulating GLAST trafficking. Although these modulatory effects on cell surface expression by Mn or E2/TX might not be significant enough to alter glutamate uptake, as Mn or E2/TX did not change glutamate uptake within 2 h or 6 h upon exposure, respectively (Fig. 2), long-term effects of Mn or E2/TX by modulating signaling pathways cannot be ruled out. The mechanism underlying the effect(s) of Mn or E2/TX on GLAST trafficking remains to be established, but Protein Kinase C (PKC) signaling pathways might be involved. Activation of PKC is known to induce internalization of various transporters such as serotonin, dopamine, norepinephrine and GLT-1 (Robinson 2002; Sheldon and Robinson 2007). Furthermore, Mn activates PKC-α in astrocytes (Liao et al. 2007).
Interestingly, TX showed similar effects to that of E2 on glutamate transporters, indicating TX might have agonistic functions associated with glutamate transporter function enhancement, in agreement with other studies in various injury models (O'Neill et al. 2004a,b; Dhandapani et al. 2005). This is noteworthy because the pharmacological effects of TX could provide information for the development of NeuroSERMs (Zhao et al. 2005) that possess neuroprotective effects without E2-like undesired effects, such as breast and uterine tissue growth.
Transforming growth factor-β1 is a multifunctional pleiotropic cytokine involved in the differentiation, growth and survival of neuronal cells (Zhu et al. 2002; Wyss-Coray 2004; Vivien and Ali 2006). This, to our knowledge, is the fist report to implicate TGF-β1 in the regulation of GLAST expression and function in astrocytes. TGF-β1 is up-regulated following ischemia-induced brain damage, thereby exerting neuroprotective effects. Notably, astrocytes have been shown to represent the major target for TGF-β1-mediated responses, including the up-regulation of type-1 plasminogen activator inhibitor expression (Buisson et al. 2003). Moreover, the astroglial production of TGF-β1 plays a critical role in E2-induced neuroprotection as noted in injury models associated with b-amyloid (Sortino et al. 2004) and apoptotic cell death (Dhandapani et al. 2005). TGF-β1 effectively rescues neurons from an excitotoxicity model, while ita neuroprotective effects require the presence of astrocytes (Buisson et al. 1998). Our studies further establish a critical role for TGF-β1 in the regulation of glutamate transporter function, demonstrating that Mn decreased TGF-β1 mRNA expression, while E2 and TX had opposite effects. These changes were also mirrored by the glutamate uptake profiles associated with these respective treatments (Fig. 2). Combined with previous reports, our findings indicate that this cytokine modulates effects on glutamate transporters that are directly related to excitotoxic-mediated neuronal death. This suggests that TGF-β1 is a major mediator in the ability of E2/TX to reverse the Mn-induced impairment of glutamate transporters. These findings provide an important basis for the future development of therapeutic strategies for glutamate-associated neurological disorders (Rothstein et al. 2005; Dunlop 2006; Dunlop and Butera 2006).
Additional findings implicate the involvement of both MAPK/ERK and PI3K/Akt signaling pathways in the E2/TX-induced TGF-β1 regulation of glutamate transporter function. Several studies have reported that signaling pathways, such as those of MAPK/ERK and PI3K/Akt, are involved in regulating glutamate transporters (Abe and Saito 2001; Koeberle and Bahr 2008). The regulation of glutamate transporter expression by growth factors, such as insulin-like growth factor-1 and basic fibroblast growth factor, is also associated with the activation of signaling pathways, including MAPK and PI3K/Akt pathways (Leof 2000). In line with these observations, the present study establishes that the enhancing effects of E2/TX on glutamate transporter functions via enhanced TGF-β1 expression are dependent on MAPK/ERK and PI3K/Akt activation, given the ability of a specific blocker (SB431542) of the TGF-β1 receptor to attenuate the TGF-β1-induced enhancement of glutamate uptake.
Taken together, the results from the present study indicate that E2 or SERMs, such as TX, possess promising therapeutic potential for treating neurological disorders associated with glutamate transporter impairment via increased TGF-β1 expression. By utilizing analogous models of Mn-induced injury, future in vivo studies or co-culture studies could be profitably directed at better defining the cellular/molecular mechanisms by which E2/TX enhances glutamate transporter expression and function.
Acknowledgements
The authors gratefully acknowledge partial support by grants from NIEHS 10563 and DoD W81XWH-05-1-0239 (MA).
Abbreviations used
- AD
Alzheimer's disease
- ALS
amyotrophic lateral sclerosis
- E2
17β-estradiol
- ER
E2 receptor
- GLAST
glutamate/aspartate transporter
- MEM
minimum essential medium
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PBS
phosphate-buffered saline
- PD
Parkinson's disease
- SERMs
selective estrogen receptor modulators
- TBS
Tris Buffered Saline
- TGF-β1
transforming growth factor-β1
- TX
Tamoxifen
References
- Abe K, Saito H. Possible linkage between glutamate transporter and mitogen-activated protein kinase cascade in cultured rat cortical astrocytes. J. Neurochem. 2001;76:217–223. doi: 10.1046/j.1471-4159.2001.00062.x. [DOI] [PubMed] [Google Scholar]
- Aschner M, Gannon M, Kimelberg HK. Manganese uptake and efflux in cultured rat astrocytes. J. Neurochem. 1992;58:730–735. doi: 10.1111/j.1471-4159.1992.tb09778.x. [DOI] [PubMed] [Google Scholar]
- Aschner M, Vrana KE, Zheng W. Manganese uptake and distribution in the central nervous system (CNS). Neurotoxicology. 1999;20:173–180. [PubMed] [Google Scholar]
- Azcoitia I, Garcia-Ovejero D, Chowen JA, Garcia-Segura LM. Astroglia play a key role in the neuroprotective actions of estrogen. Prog. Brain Res. 2001;132:469–478. doi: 10.1016/S0079-6123(01)32096-4. [DOI] [PubMed] [Google Scholar]
- Barbeau A. Manganese and extrapyramidal disorders (a critical review and tribute to Dr. George C. Cotzias). Neurotoxicology. 1984;5:13–35. [PubMed] [Google Scholar]
- Brouillet EP, Shinobu L, McGarvey U, Hochberg F, Beal MF. Manganese injection into the rat striatum produces excitotoxic lesions by impairing energy metabolism. Exp. Neurol. 1993;120:89–94. doi: 10.1006/exnr.1993.1042. [DOI] [PubMed] [Google Scholar]
- Buisson A, Nicole O, Docagne F, Sartelet H, Mackenzie ET, Vivien D. Up-regulation of a serine protease inhibitor in astrocytes mediates the neuroprotective activity of transforming growth factor beta1. FASEB J. 1998;12:1683–1691. [PubMed] [Google Scholar]
- Buisson A, Lesne S, Docagne F, Ali C, Nicole O, MacKenzie ET, Vivien D. Transforming growth factor-beta and ischemic brain injury. Cell. Mol. Neurobiol. 2003;23:539–550. doi: 10.1023/A:1025072013107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotzias GC, Horiuchi K, Fuenzalida S, Mena I. Chronic manganese poisoning. Clearance of tissue manganese concentrations with persistance of the neurological picture. Neurology. 1968;18:376–382. doi: 10.1212/wnl.18.4.376. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog. Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Dhandapani KM, Brann DW. Protective effects of estrogen and selective estrogen receptor modulators in the brain. Biol. Reprod. 2002;67:1379–1385. doi: 10.1095/biolreprod.102.003848. [DOI] [PubMed] [Google Scholar]
- Dhandapani K, Brann D. Neuroprotective effects of estrogen and tamoxifen in vitro: a facilitative role for glia? Endocr. 2003a;21:59–66. doi: 10.1385/endo:21:1:59. [DOI] [PubMed] [Google Scholar]
- Dhandapani KM, Brann DW. Transforming growth factor-beta: a neuroprotective factor in cerebral ischemia. Cell Biochem. Biophys. 2003b;39:13–22. doi: 10.1385/CBB:39:1:13. [DOI] [PubMed] [Google Scholar]
- Dhandapani KM, Brann DW. Role of astrocytes in estrogen-mediated neuroprotection. Exp. Gerontol. 2007;42:70–75. doi: 10.1016/j.exger.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Dhandapani KM, Wade FM, Mahesh VB, Brann DW. Astrocyte-derived transforming growth factor-{beta} mediates the neuroprotective effects of 17{beta}-estradiol: involvement of nonclassical genomic signaling pathways. Endocrinology. 2005;146:2749–2759. doi: 10.1210/en.2005-0014. [DOI] [PubMed] [Google Scholar]
- Dunlop J. Glutamate-based therapeutic approaches: targeting the glutamate transport system. Curr. Opin. Pharmacol. 2006;6:103–107. doi: 10.1016/j.coph.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Dunlop J, Butera JA. Ligands targeting the excitatory amino acid transporters (EAATs). Curr. Top. Med. Chem. 2006;6:1897–1906. doi: 10.2174/156802606778249829. [DOI] [PubMed] [Google Scholar]
- Erikson K, Aschner M. Manganese causes differential regulation of glutamate transporter (GLAST) taurine transporter and metallothionein in cultured rat astrocytes. Neurotoxicology. 2002;23:595–602. doi: 10.1016/s0161-813x(02)00012-8. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Suber RL, Aschner M. Glutamate/aspartate transporter (GLAST), taurine transporter and metallothionein mRNA levels are differentially altered in astrocytes exposed to manganese chloride, manganese phosphate or manganese sulfate. Neurotoxicology. 2002;23:281–288. doi: 10.1016/s0161-813x(02)00041-4. [DOI] [PubMed] [Google Scholar]
- Ferrarese C, Zoia C, Pecora N, et al. Reduced platelet glutamate uptake in Parkinson's disease. J. Neural. Transm. 1999;106:685–692. doi: 10.1007/s007020050189. [DOI] [PubMed] [Google Scholar]
- Figiel M, Maucher T, Rozyczka J, Bayatti N, Engele J. Regulation of glial glutamate transporter expression by growth factors. Exp. Neurol. 2003;183:124–135. doi: 10.1016/s0014-4886(03)00134-1. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Schousboe A. High affinity glutamate transporters: regulation of expression and activity. Mol. Pharmacol. 1997;52:6–15. doi: 10.1124/mol.52.1.6. [DOI] [PubMed] [Google Scholar]
- Gillessen T, Budd SL, Lipton SA. Excitatory amino acid neurotoxicity. Adv. Exp. Med. Biol. 2002;513:3–40. doi: 10.1007/978-1-4615-0123-7_1. [DOI] [PubMed] [Google Scholar]
- Hazell AS. Astrocytes and manganese neurotoxicity. Neurochem. Int. 2002;41:271–277. doi: 10.1016/s0197-0186(02)00013-x. [DOI] [PubMed] [Google Scholar]
- Hazell AS, Norenberg MD. Manganese decreases glutamate uptake in cultured astrocytes. Neurochem. Res. 1997;22:1443–1447. doi: 10.1023/a:1021994126329. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Feustel PJ, Jin Y, Paquette J, Boulos A, Keller RW, Jr, Tranmer BI. Acute treatment with tamoxifen reduces ischemic damage following middle cerebral artery occlusion. Neuroreport. 2000;11:2675–2679. doi: 10.1097/00001756-200008210-00014. [DOI] [PubMed] [Google Scholar]
- Koeberle PD, Bahr M. The upregulation of GLAST-1 is an indirect antiapoptotic mechanism of GDNF and neurturin in the adult CNS. Cell Death Differ. 2008;15:471–483. doi: 10.1038/sj.cdd.4402281. [DOI] [PubMed] [Google Scholar]
- Kondo K, Hashimoto H, Kitanaka J, Sawada M, Suzumura A, Marunouchi T, Baba A. Expression of glutamate transporters in cultured glial cells. Neurosci. Lett. 1995;188:140–142. doi: 10.1016/0304-3940(95)11408-o. [DOI] [PubMed] [Google Scholar]
- Leof EB. Growth factor receptor signalling: location, location, location. Trends Cell Biol. 2000;10:343–348. doi: 10.1016/s0962-8924(00)01795-5. [DOI] [PubMed] [Google Scholar]
- Liang Z, Valla J, Sefidvash-Hockley S, Rogers J, Li R. Effects of estrogen treatment on glutamate uptake in cultured human astrocytes derived from cortex of Alzheimer's disease patients. J. Neurochem. 2002;80:807–814. doi: 10.1046/j.0022-3042.2002.00779.x. [DOI] [PubMed] [Google Scholar]
- Liao SL, Ou YC, Chen SY, Chiang AN, Chen CJ. Induction of cyclooxygenase-2 expression by manganese in cultured astrocytes. Neurochem. Int. 2007;50:905–915. doi: 10.1016/j.neuint.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Masliah E, Alford M, DeTeresa R, Mallory M, Hansen L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer's disease. Ann. Neurol. 1996;40:759–766. doi: 10.1002/ana.410400512. [DOI] [PubMed] [Google Scholar]
- Moosmann B, Behl C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc. Natl Acad. Sci. USA. 1999;96:8867–8872. doi: 10.1073/pnas.96.16.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutkus L, Aschner JL, Syversen T, Shanker G, Sonnewald U, Aschner M. In vitro uptake of glutamate in GLAST- and GLT-1-transfected mutant CHO-K1 cells is inhibited by the ethylmercury-containing preservative thimerosal. Biol. Trace Elem. Res. 2005;105:71–86. doi: 10.1385/BTER:105:1-3:071. [DOI] [PubMed] [Google Scholar]
- Normandin L, Hazell AS. Manganese neurotoxicity: an update of pathophysiologic mechanisms. Metab. Brain Dis. 2002;17:375–387. doi: 10.1023/a:1021970120965. [DOI] [PubMed] [Google Scholar]
- O'Neill K, Chen S, Brinton RD. Impact of the selective estrogen receptor modulator, raloxifene, on neuronal survival and outgrowth following toxic insults associated with aging and Alzheimer's disease. Exp. Neurol. 2004a;185:63–80. doi: 10.1016/j.expneurol.2003.09.005. [DOI] [PubMed] [Google Scholar]
- O'Neill K, Chen S, Diaz Brinton R. Impact of the selective estrogen receptor modulator, tamoxifen, on neuronal outgrowth and survival following toxic insults associated with aging and Alzheimer's disease. Exp. Neurol. 2004b;188:268–278. doi: 10.1016/j.expneurol.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Pawlak J, Brito V, Kuppers E, Beyer C. Regulation of glutamate transporter GLAST and GLT-1 expression in astrocytes by estrogen. Brain Res. Mol. Brain Res. 2005;138:1–7. doi: 10.1016/j.molbrainres.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Lee DW, Fernando G, Schlinger BA. Radial glia express aromatase in the injured zebra finch brain. J. Comp. Neurol. 2004;475:261–269. doi: 10.1002/cne.20157. [DOI] [PubMed] [Google Scholar]
- Plaitakis A, Shashidharan P. Glutamate transport and metabolism in dopaminergic neurons of substantia nigra: implications for the pathogenesis of Parkinson's disease. J. Neurol. 2000;247(Suppl 2):II25–II35. doi: 10.1007/pl00007757. [DOI] [PubMed] [Google Scholar]
- Robinson MB. Regulated trafficking of neurotransmitter transporters: common notes but different melodies. J. Neurochem. 2002;80:1–11. doi: 10.1046/j.0022-3042.2001.00698.x. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin LJ, Kuncl RW. Decreased gluta-mate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N. Engl. J. Med. 1992;326:1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Sheldon AL, Robinson MB. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem. Int. 2007;51:333–355. doi: 10.1016/j.neuint.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Zhang YQ, Simpkins JW. Effects of 17beta-estradiol on glucose transporter 1 expression and endothelial cell survival following focal ischemia in the rats. Exp. Brain Res. 1997;117:200–206. doi: 10.1007/s002210050216. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Green PS, Gridley KE, Singh M, de Fiebre NC, Rajakumar G. Role of estrogen replacement therapy in memory enhancement and the prevention of neuronal loss associated with Alzheimer's disease. Am. J. Med. 1997;103:19S–25S. doi: 10.1016/s0002-9343(97)00260-x. [DOI] [PubMed] [Google Scholar]
- Sloot WN, van der Sluijs-Gelling AJ, Gramsbergen JB. Selective lesions by manganese and extensive damage by iron after injection into rat striatum or hippocampus. J. Neurochem. 1994;62:205–216. doi: 10.1046/j.1471-4159.1994.62010205.x. [DOI] [PubMed] [Google Scholar]
- Sortino MA, Chisari M, Merlo S, Vancheri C, Caruso M, Nicoletti F, Canonico PL, Copani A. Glia mediates the neuroprotective action of estradiol on beta-amyloid-induced neuronal death. Endocrinology. 2004;145:5080–5086. doi: 10.1210/en.2004-0973. [DOI] [PubMed] [Google Scholar]
- Spadoni F, Stefani A, Morello M, Lavaroni F, Giacomini P, Sancesario G. Selective vulnerability of pallidal neurons in the early phases of manganese intoxication. Exp. Brain Res. 2000;135:544–551. doi: 10.1007/s002210000554. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Mouri T, Nishiyama K, Fujii N. Study of sub-acute toxicity of manganese dioxide in monkeys. Tokushima J. Exp. Med. 1975;22:5–10. [PubMed] [Google Scholar]
- Suzuki K, Ikegaya Y, Matsuura S, Kanai Y, Endou H, Matsuki N. Transient upregulation of the glial glutamate transporter GLAST in response to fibroblast growth factor, insulin-like growth factor and epidermal growth factor in cultured astrocytes. J. Cell Sci. 2001;114:3717–3725. doi: 10.1242/jcs.114.20.3717. [DOI] [PubMed] [Google Scholar]
- Tao F, Lu SD, Zhang LM, Huang YL, Sun FY. Role of excitatory amino acid transporter 1 in neonatal rat neuronal damage induced by hypoxia-ischemia. Neuroscience. 2001;102:503–513. doi: 10.1016/s0306-4522(00)00485-1. [DOI] [PubMed] [Google Scholar]
- Vivien D, Ali C. Transforming growth factor-beta signalling in brain disorders. Cytokine Growth Factor Rev. 2006;17:121–128. doi: 10.1016/j.cytogfr.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Wang X, Dykens JA, Perez E, Liu R, Yang S, Covey DF, Simpkins JW. Neuroprotective effects of 17beta-estradiol and nonfeminizing estrogens against H2O2 toxicity in human neuroblastoma SK-N-SH cells. Mol. Pharmacol. 2006;70:395–404. doi: 10.1124/mol.106.022384. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T. Transforming growth factor-beta signaling pathway as a therapeutic target in neurodegeneration. J. Mol. Neurosci. 2004;24:149–153. doi: 10.1385/JMN:24:1:149. [DOI] [PubMed] [Google Scholar]
- Zhao L, Chen S, Ming Wang J, Brinton RD. 17beta-estradiol induces Ca2+ influx, dendritic and nuclear Ca2+ rise and subsequent cyclic AMP response element-binding protein activation in hippocampal neurons: a potential initiation mechanism for estrogen neurotrophism. Neuroscience. 2005;132:299–311. doi: 10.1016/j.neuroscience.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Yang GY, Ahlemeyer B, Pang L, Che XM, Culmsee C, Klumpp S, Krieglstein J. Transforming growth factor-beta 1 increases bad phosphorylation and protects neurons against damage. J. Neurosci. 2002;22:3898–3909. doi: 10.1523/JNEUROSCI.22-10-03898.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]