Abstract
This unit describes general procedures for the lab cultivation and storage of the Gram-positive facultative intracellular bacterium Listeria monocytogenes. The basic protocols are relevant for a wide scope of applications including microbial genetics, and both in vitro and in vivo infection studies. Commonly used L. monocytogenes strains, serotypes, and growth parameters are also discussed.
Keywords: Listeria monocytogenes, Gram-positive, intracellular pathogen, listeriosis
INTRODUCTION
Listeria monocytogenes are intracellular bacteria that are capable of thriving in divergent environments and thus, are ubiquitous in nature (Freitag et al., 2009; Ramaswamy et al., 2007). The direct contamination of foods or food processing equipment with L. monocytogenes can result in outbreaks of food borne disease called listeriosis. Ingestion of L. monocytogenes can cause varying degrees of gastroenteritis and systemic spread of the bacteria can be lethal in susceptible hosts (Allenberger et al., 2010). Although Listeria is capable of adapting to many different growth conditions in the environment, the procedures described here focus on growth conditions that are commonly used for animal and cell culture models of infection.
In Basic Protocol 1, growth of L. monocytogenes in liquid medium is described. Basic Protocol 2 describes how to produce enumerated aliquots for use in short-term experiments. Basic Protocol 3 is used to prepare bacterial stocks for long-term storage.
CAUTION: Listeria monocytogenes is a Biosafety Level 2 (BSL-2) pathogen. All procedures must be performed following the appropriate guidelines for handling pathogenic microbes. See UNIT 1A.1 and other pertinent resources (APPENDIX 1B) for more information.
BASIC PROTOCOL 1
GROWTH OF L. MONOCYTOGENES IN LIQUID MEDIUM
L. monocytogenes are auxotrophic for seven amino acids including leucine, isoleucine, valine, methionine, arginine, cysteine, and glutamine (Premaratne et al., 1991). The bacteria also require four additional vitamins including riboflavin, thiamine, biotin, and thioctic acid (Premaratne et al., 1991). Therefore, L. monocytogenes need to be grown in a rich culture medium that provides all of these growth factors. Brain Heart Infusion (BHI) is the most commonly used non-selective media for cultivation of Listeria species. Chemically defined minimal media that supports the growth of L. monocytogenes has also been developed (Ralovich et al., 1977; Premaratne et al., 1991). Some strains, such as L. monocytogenes 10403s, can also be cultivated in Tryptic Soy Broth (TSB). In the laboratory, L. monocytogenes are grown in liquid media at temperatures that reflect either environmental conditions (4 to 30 °C) or mammalian body temperature (~37 °C). Broth cultures of Listeria are required for an array of applications, ranging from the isolation of genetic material to the production of enumerated aliquots for infection studies (see Basic Protocol 2).
Materials
L. monocytogenes (from frozen stock, agar stab, or freeze-dried pellet)
Sterile BHI agar (Difco) plates
Sterile BHI broth
Sterile inoculating loop
Sterile culture flasks or test tubes
Incubator (with orbital shaker if desired)
- Prepare isolated colonies of L. monocytogenes (see Appendix 4A or Basic Protocol 2 of Elbing and Brent, 2002).
- From frozen stock:
- Use a sterile inoculating loop to scrape a small quantity of frozen material and streak for isolated colonies on BHI agar.
- From an agar stab:
- Push a sterile inoculating loop into the stabbed area to draw out some bacteria and streak for isolated colonies on BHI agar.
- From freeze-dried pellet:
- Follow instructions on product sheet to rehydrate bacteria. Use a sterilized metal or disposable inoculating loop to collect a loop-full of bacterial solution and streak for isolated colonies on BHI agar.
Incubate agar plate overnight (18–24 h) at desired temperature (see Commentary).
- Using a heat-sterilized metal or sterile disposable plastic inoculating loop, pick up a single, freshly isolated colony of L. monocytogenes.
- Isolated colonies of L. monocytogenes on BHI agar are small (~1 mm), creamy white in color, and dome-shaped.
- Introduce the inoculum into sterile BHI broth in a culture flask or tube by gently swirling the loop within the broth.
- The volume of medium in a culture flask should be no more than 20% of the total culture flask volume. Maintaining a consistent media volume to total flask volume ratio will standardize aeration levels and the resulting growth rates.
- Incubate the Listeria culture at the desired temperature with or without orbital shaking at 200–250 rpm.
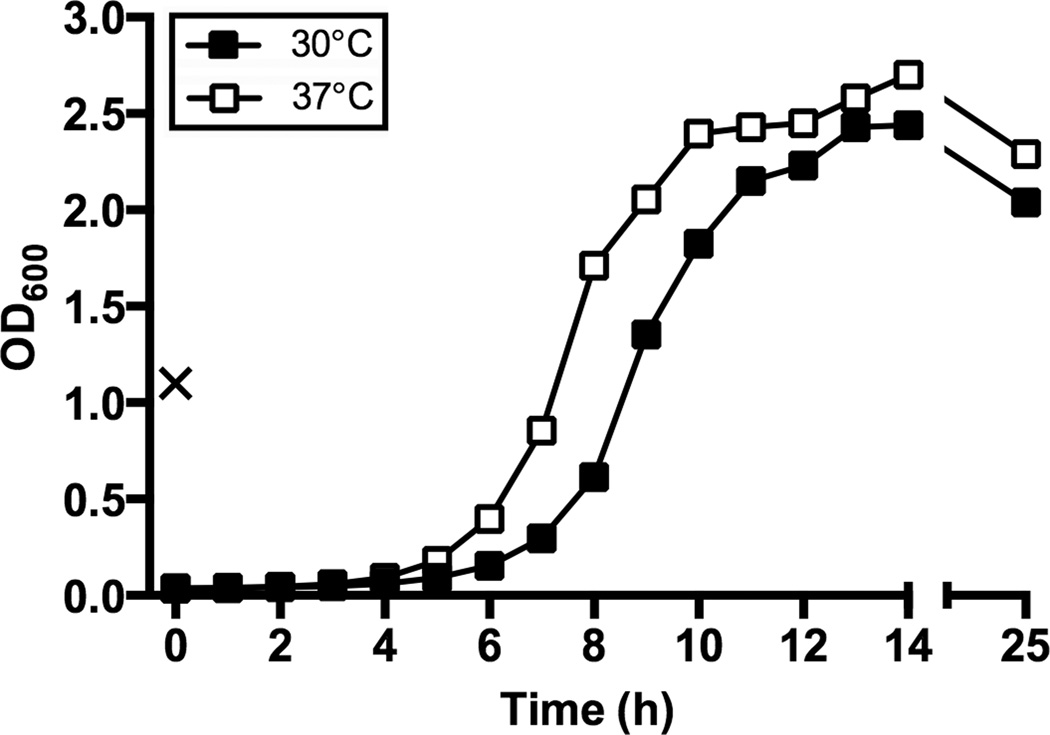
- Overnight cultures (16–24 h) inoculated with a single colony of L. monocytogenes can reach an OD600 of 1.0–2.0 depending on the incubation temperature (see Fig. 1).
- To prepare bacteria in the exponential phase of growth, dilute overnight cultures to an OD600 of 0.05–0.1 and incubate for 6–10 hours.
- The growth rate of the bacteria will be dependent on the incubation temperature (see Fig. 1).
Figure 1.
Growth curves of L. monocytogenes 10403s. A freshly isolated colony of L. monocytogenes 10403s was used to inoculate 4 ml BHI broth which was incubated statically at 30 °C overnight (~16 h). The OD600 of the culture was measured the following day and is denoted by “X”. The overnight growth was back-diluted into fresh BHI to give an OD600 of 0.05 in a total volume of 25 ml in a 125 ml flask. Cultures were incubated with orbital shaking at 200 rpm at either 30 °C (black symbols) or 37 °C (white symbols), and OD600 measurements were taken hourly.
BASIC PROTOCOL 2
SHORT-TERM STORAGE OF LISTERIA ALIQUOTS
This protocol describes a procedure that can be used to infect cells or animals with L. monocytogenes of a known titer, and requires only 2 hours of advance preparation time. Listeria broth cultures are incubated until the growth phase suitable for future studies has been reached, and then aliquots are prepared. The cryostability of L. monocytogenes offers investigators the convenience of freezing aliquots at –80 °C in liquid medium without the addition of cryopreservative (Azizoglu et al., 2009). These frozen aliquots of L. monocytogenes are ideal for infection studies requiring consistent dosages or multiplicity of infection ratios.
Materials
L. monocytogenes grown on BHI agar
Sterile BHI broth
Sterile BHI agar (Difco) plates
Sterile inoculating loop
Sterile culture flasks
Sterile 50 ml polypropylene centrifuge tube
Sterile pipets
Sterile 1.5 ml microcentrifuge tubes
–80 °C freezer
- Inoculate BHI broth in a sterile flask with a freshly isolated colony of L. monocytogenes. Incubate culture at desired temperature with or without orbital shaking until the desired growth phase is reached.
- Culture volume is dependent on usage; however, flask size should be at least 5 times the volume of broth. Incubation temperature and growth conditions should be determined by each investigator (see Commentary). Cultures can be incubated overnight to yield stationary phase bacteria or back-diluted and grown 6–10 hours for exponential phase growth bacteria (see Fig. 1).
- Transfer the entire culture into a sterile 50 ml centrifuge tube.
- Transferring the culture into a centrifuge tube allows for efficient vortexing during the following step.
- Prepare aliquots by transferring 0.5 ml of the broth culture into individual microcentrifuge tubes.
- It is crucial that the bacterial culture is frequently vortexed to ensure preparation of homogenous aliquots that will yield consistent titers.
- Transfer aliquots to a –80 °C freezer.
- Listeria aliquots can be expected to remain viable for at least one to two years (see Commentary).
- To determine the titer of the aliquots, thaw one tube on ice and then transfer a known volume (typically 100–400 µl) to a sterile 125 ml flask containing 9.6–9.9 ml of BHI broth for a final volume of 10 ml.
- Before determining aliquot titer, keep the tube in the –80 °C freezer for at least several hours to account for loss in viability due to the initial effects of freezing.
- Allow bacteria to recover from the thaw by incubating the flask for 1.5 hours.
- Recovery temperature is dependent on the growth temperature used to prepare the bacteria and the intended application (e.g., in vivo infection studies would be best suited to room temperature or lower to mimic environmental conditions, while in vitro infection of mammalian cells is better suited to a recovery temperature of 37 °C.
- Perform multiple serial dilutions and spread relevant dilutions on BHI agar plates. By counting the resulting number of colonies, the titer of each Listeria aliquot (CFU/ml) can be determined (Appendix 4A or Elbing and Brent, 2002).
- Assuming the primary culture has been homogenously prepared into aliquots, the resulting titers should vary no more than 2–4 fold among aliquot tubes.
BASIC PROTOCOL 3
LONG-TERM STORAGE OF L. MONOCYTOGENES
Laboratory stocks of L. monocytogenes can be stored indefinitely in 20% glycerol at –80 °C. The following protocol describes the preparation of long-term glycerol stocks of L. monocytogenes starting with either growth on agar plates or broth cultures.
Materials
L. monocytogenes grown on BHI agar or in BHI broth culture
20% glycerol (v/v) BHI broth or 40% glycerol (v/v) BHI broth, sterile (see recipe)
Sterile cotton tip applicator
Sterile pipets
1 ml cryogenic storage vial, sterile
–80 °C freezer
Cultivate L. monocytogenes overnight to yield moderately heavy growth.
- Transfer bacteria to a cryovial containing glycerol.
- From broth culture:
- Transfer 0.5 ml of Listeria broth culture into a sterile cryogenic vial containing 0.5 ml of 40% glycerol/BHI broth.
- Final concentration of glycerol will be 20% (v/v).
- From agar plate:
- Transfer 1 ml of 20% glycerol/BHI broth to a sterile cryogenic vial. Using a sterile cotton tip applicator, swab and collect the entire surface growth of Listeria. Swirl the tip of the applicator in the 20% glycerol/BHI broth to inoculate the solution.
Close the vial tightly and briefly vortex to thoroughly mix the bacteria in the glycerol solution.
Briefly centrifuge the vial (0.5 s) or tap on bench-top to remove any solution trapped in cap.
- Store vial at –80 °C.
- Flash freezing may prolong storage life but is not necessary.
REAGENTS AND SOLUTIONS
20% glycerol (v/v) BHI broth
80 ml BHI broth
20 ml glycerol
Autoclave for 15 min. at 121 °C
Store at room temperature
40% glycerol (v/v) BHI Broth
60 ml BHI broth
40 ml glycerol
Autoclave for 15 min. at 121 °C
Store at room temperature
NOTE: Deionized and distilled water shouldbe used in all recipes.
COMMENTARY
Background Information
L. monocytogenes are Gram-positive, facultative intracellular bacteria capable of causing a human foodborne disease known as listeriosis. Listeria species are readily isolated from animals, soil, and stagnant water supplies, and these reservoirs can lead to the contamination of dairy products and produce (Farber and Peterkin, 1991). In addition, ready-to-eat meat products have also been contaminated with L. monocytogenes, potentially due to the growth of biofilms on food processing equipment (Blackman and Frank, 1996; Borucki et al., 2003). Recent data suggests that L. monocytogenes is responsible for 19% of all mortality caused by foodborne illnesses in the United States, with the majority of deaths occurring in immune compromised individuals, elderly adults, pregnant women, and neonates (Scallan et al., 2011; Ramaswamy et al., 2007).
In addition to being a human pathogen, Listeria is also commonly used as a model organism. Cell biologists have been able to study the dynamics of actin polymerization and cell signaling pathways by following the intracellular life cycle of L. monocytogenes in vitro (Hamon et al., 2006). The mouse model of infection is widely used by immunologists to study the strong T-cell-mediated immune response that results in adaptive immunity (Stavru et al., 2011). Listeria also offers molecular biologists a platform for studying the shifts in gene expression that are needed to survive during exposure to a variety of external stresses including extremes of temperature, pH and salt concentration, as well as entry into mammalian cells (Freitag et al., 2009; Camejo et al., 2009; Gray et al., 2006; van der Veen et al., 2008).
Critical Parameters and Troubleshooting
If poor growth of L. monocytogenes occurs in liquid culture medium, it may be due to a loss in viability of the culture used for inoculation. Optimal growth occurs when using an isolated colony from a freshly streaked plate. Bacteria can be recovered from an agar plate or stab that has been stored either refrigerated or at room temperature for up to 3 weeks, however, the OD600 will not reach the values shown in Fig. 1.
Strain lineages and serotypes
L. monocytogenes strains can be divided into serotypes based on somatic and flagellar antigens. At least 13 serotypes of L. monocytogenes have been identified; however, serotypes 1/2a, 1/2b, and 4b cause the vast majority of human disease (Schuchat et al., 1991; Nadon et al., 2001). More recently, virulence gene polymorphisms and ribotyping have been used to designate at least four evolutionary lineages of L. monocytogenes (Rasmussen et al., 1995; Wiedmann et al., 1997; Orsi et al., 2011). Molecular subtyping has implicated that L. monocytogenes lineage I strains (containing serotypes 1/2b and 4b) are responsible for most cases (up to 65%) of listeriosis in humans with lineage II strains (containing serotype 1/2a) causing the remaining 35% of human cases (Jeffers et al., 2001). Lineage III and IV strains of L. monocytogenes are rare and have been mainly isolated from animal infections (Orsi et al., 2011).
L. monocytogenes EGDe (serotype 1/2a) was the first strain to be isolated from the infected laboratory animals of E.G.D. Murray (Murray et al., 1926) and this strain is still commonly used in laboratories today. Another widely used reference strain is L. monocytogenes 10403s (serotype 1/2a), a streptomycin-resistant derivative of a clinical isolate (Edman et al., 1968; Bishop and Hinrichs, 1987). The vast majority of published research on Listeria has used either 10403s or EGDe. However, some studies suggest that a large number of the major food borne outbreaks of listeriosis over the past 30 years were caused by serotype 4b strains, and these bacteria may be better adapted for growth in mammalian hosts (Vázquez-Boland et al., 2001). In order to maintain the virulence of laboratory passaged strains such as EGDe and 10403s, investigators should periodically passage the bacteria through animals such as mice or guinea pigs (Bou Ghanem et al., 2013; Disson, et al., 2009).
Incubation temperature
L. monocytogenes is a psychrotrophic bacterium (capable of surviving and growing at cold temperatures), a property that allows the bacteria to multiply in contaminated food products even when they are properly refrigerated. Thus, growth of L. monocytogenes can occur at temperatures ranging from ~0°C to 45°C. In the laboratory, L. monocytogenes is commonly cultivated at either 30°C or 37°C. Previous work has shown that the thermo-regulated virulence genes of L. monocytogenes are optimally expressed at 37°C and repressed below 30°C (Leimeister-Wächter et al., 1992). Thus, bacteria grown at 37°C are well suited for in vitro infection of cultured cells, or assays designed to mimic the growth state of the bacteria in vivo. In contrast, L. monocytogenes grown at 30°C have features more similar to those found in the environment, and more accurately represent the transmission phase of an infection.
Anticipated Results
Growth in liquid media
Liquid cultures of L. monocytogenes begin to become visibly turbid as the bacteria reach exponential phase of growth and cultures increase in turbidity through the stationary phase of growth. Listeria are facultative anaerobes and microaerophiles, so broth cultures can be incubated with or without shaking and result in minimally altered growth rates. Growth rate is more heavily dependent on incubation temperature and can be inferred from the data shown in Figure 1.
Frozen storage
L. monocytogenes possess a surprisingly high tolerance for repeated freeze-thaw cycles even in the absence of cryopreservatives (Azizoglu et al., 2009). Basic Protocol 2 takes advantage of this feature and provides investigators with a means of preparing frozen aliquots of L. monocytogenes that have a known titer. Unpublished observations from our lab suggest that aliquots prepared following Basic Protocol 2 can be used for more than four years with no loss in viability compared to the original titer.
Time Considerations
The doubling time of L. monocytogenes grown at 37°C in BHI broth is approximately 45–60 minutes. Thus, bacteria inoculated into liquid media at an initial OD600 of 0.05–0.1 will reach exponential phase within 6–10 hours, and will reach stationary phase after overnight growth. Likewise, visible colony growth can be observed on BHI agar plates after 18–24 hours of incubation at 37°C.
Literature Cited
- Allerberger F, Wagner M. Listeriosis: a resurgent foodborne infection. Clin. Microbiol. Infect. 2010;16:16–23. doi: 10.1111/j.1469-0691.2009.03109.x. [DOI] [PubMed] [Google Scholar]
- Azizoglu RO, Osborne J, Wilson S, Kathariou S. Role of growth temperature in freeze-thaw tolerance of Listeria spp. Appl. Environ. Microbiol. 2009;75:5315–5320. doi: 10.1128/AEM.00458-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DK, Hinrichs DJ. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 1987;139:2005–2009. [PubMed] [Google Scholar]
- Blackman IC, Frank JF. Growth of Listeria monocytogenes as a biofilm on various food-processing surfaces. J. Food Prot. 1996;59:827–831. doi: 10.4315/0362-028X-59.8.827. [DOI] [PubMed] [Google Scholar]
- Borucki MK, Peppin JD, White D, Loge F, Call DR. Variation in biofilm formation among strains of Listeria monocytogenes. App. and Environ. Micro. 2003;69:7336–7342. doi: 10.1128/AEM.69.12.7336-7342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bou Ghanem EN, Myers-Morales T, Jones GS, D’Orazio SE. Oral transmission of Listeria monocytogenes in mice via ingestion of contaminated food. J. Vis. Exp. 2013;(75) doi: 10.3791/50381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camejo A, Buchrieser C, Couve´ E, Carvalho F, Reis O, Ferreira P, Sousa S, Cossart P, Cabanes D. In Vivo Transcriptional Profiling of Listeria monocytogenes and Mutagenesis Identify New Virulence Factors Involved in Infection. PLoS Pathog. 2009;5:e1000449. doi: 10.1371/journal.ppat.1000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disson O, Nikitas G, Grayo S, Dusserget O, Cossart P, Lecuit M. Modeling human listeriosis in natural and genetically engineered animals. Nature Protoc. 2009;4:799–810. doi: 10.1038/nprot.2009.66. [DOI] [PubMed] [Google Scholar]
- Edman DC, Pollock MB, Hall ER. Listeria monocytogenes L forms. I. Induction maintenance, and biological characteristics. J. Bacteriol. 1968;96:352–357. doi: 10.1128/jb.96.2.352-357.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbing K, Brent R. Growth on solid media. Curr. Protoc. Mol. Biol. 2002;59:1.3.1–1.3.6. doi: 10.1002/0471142727.mb0103s59. [DOI] [PubMed] [Google Scholar]
- Farber JM, Peterkin PI. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag NE, Port GC, Miner MD. Listeria monocytogenes — from saprophyte to intracellular pathogen. Nature Rev. Micro. 2009;7:623–628. doi: 10.1038/nrmicro2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MJ, Freitag NE, Boor KJ. How the Bacterial Pathogen Listeria monocytogenes Mediates the Switch from Environmental Dr. Jekyll to Pathogenic Mr. Hyde. Infect. Immun. 2006;74:2505–2512. doi: 10.1128/IAI.74.5.2505-2512.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon M, Bierne H, Cossart P. Listeria monocytogenes: a multifaceted model. Nat. Rev. Microbiol. 2006;4:423–434. doi: 10.1038/nrmicro1413. [DOI] [PubMed] [Google Scholar]
- Jeffers GT, Bruce JL, McDonough PL, Scarlett J, Boor KJ, Wiedmann M. Comparative genetic characterization of Listeria monocytogenes isolates from human and animal listeriosis cases. Microbiology. 2001;147:1095–1104. doi: 10.1099/00221287-147-5-1095. [DOI] [PubMed] [Google Scholar]
- Leimeister-Wächter M, Domann E, Chakraborty T. The expression of virulence genes in Listeria monocytogenes is thermoregulated. J. Bacteriol. 1992;174:947–952. doi: 10.1128/jb.174.3.947-952.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EGD, Webb RA, Swann HBR. A disease of rabbits characterized by a large mononuclear leucocytosis caused by a hitherto undescribed bacillus Bacterium monocytogenes (n.sp.) J. Pathol. Bacteriol. 1926;29:407–439. [Google Scholar]
- Nadon CA, Woodward DL, Young C, Rodgers FG, Wiedmann M. Correlations between molecular subtyping and serotyping of Listeria monocytogenes . J Clin Microbiol. 2001;39:2704–2707. doi: 10.1128/JCM.39.7.2704-2707.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsi RH, den Bakker HC, Wiedmann M. Listeria monocytogenes lineages: Genomics, evolution, ecology, and phenotypic characteristics. Int. J. Med. Microbiol. 2011;301:79–96. doi: 10.1016/j.ijmm.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Premaratne RJ, Lin WJ, Johnson EA. Development of an Improved Chemically Defined Minimal Medium for Listeria monocytogenes . Appl. Environ. Microbiol. 1991;57:3046–3048. doi: 10.1128/aem.57.10.3046-3048.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralovich BS, Shahamat M, Woodbine M. Further data on the characters of Listeria strains. Med. Microbiol. Immunol. 1977;163:125–139. doi: 10.1007/BF02121827. [DOI] [PubMed] [Google Scholar]
- Ramaswamy V, Cresence VM, Rejitha JS, Lekshmi MU, Dharsana KS, Prasad SP, Vijila HM. Listeria—review of epidemiology and pathogenesis. J. Microbiol. Immunol. Infect. 2007;40:4–13. [PubMed] [Google Scholar]
- Rasmussen OF, Skouboe P, Dons L, Rossen L, Olsen JE. Listeria monocytogenes exists in at least three evolutionary lines: evidence from flagellin, invasive associated protein and listeriolysin O genes. Microbiology. 1995;141:2053–2061. doi: 10.1099/13500872-141-9-2053. [DOI] [PubMed] [Google Scholar]
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States-major pathogens. Emerg. Infect. Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchat A, Swaminathan B, Broome CV. Epidemiology of Human Listeriosis. Clin. Microbiol. Rev. 1991;4:169–183. doi: 10.1128/cmr.4.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavru F, Archambaud C, Cossart P. Cell biology and immunology of Listeria monocytogenes infections: novel insights. Immunol. Rev. 2011;240:160–184. doi: 10.1111/j.1600-065X.2010.00993.x. [DOI] [PubMed] [Google Scholar]
- van der Veen S, Moezelaar R, Abee T, Wells-Bennik MH. The growth limits of a large number of Listeria monocytogenes strains at combinations of stresses show serotype--and niche-specific traits. J. Appl. Microbiol. 2008;105:1246–1258. doi: 10.1111/j.1365-2672.2008.03873.x. [DOI] [PubMed] [Google Scholar]
- Vázquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Domínguez-Bernal G, Goebel W, González-Zorn B, Wehland J, Kreft J. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 2001;14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmann M, Bruce JL, Keating C, Johnson AE, McDonough PL, Batt CA. Ribotypes and virulence gene polymorphisms suggest three distinct Listeria monocytogenes lineages with differences in pathogenic potential. Infect. Immun. 1997;65:2707–2716. doi: 10.1128/iai.65.7.2707-2716.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]