Abstract
Evolution has favored the utilization of dioxygen (O2) in the development of complex multi-cellular organisms. O2 is actually a toxic mutagenic gas that is highly oxidizing and combustible. It is thought that plants are largely to blame for polluting the earth’s atmosphere with O2 due to the development of photosynthesis by blue green algae over 2 billion years ago. The rise of the plants and atmospheric O2 levels placed evolutionary stress on organisms to adapt or become extinct. This implies that all the surviving creatures on our planet are mutants that have adapted to the “abnormal biology of O2.” Much of the adaptation to the presence of O2 in biological systems comes from well coordinated antioxidant and repair systems that focus on converting O2 to its most reduced form, water (H2O) and the repair and replacement of damaged cellular macromolecules. Biological systems have also harnessed O2’s reactive properties for energy production, xenobiotic metabolism, host defense, and as a signaling messenger and redox modulator of a number of cell signaling pathways. Many of these systems involve electron transport systems and offer many different mechanisms by which antioxidant therapeutics can alternatively produce an antioxidant effect without directly scavenging oxygen-derived reactive species. It is likely that each agent will have a different set of mechanisms that may change depending of the model of oxidative stress, organ system, or disease state. An important point is that all biological processes of aerobes have co-evolved with O2 and this creates a Pandora’s Box for trying to understand the mechanism of action(s) of antioxidants being developed as therapeutic agents.
The Abnormal Biology of Oxygen
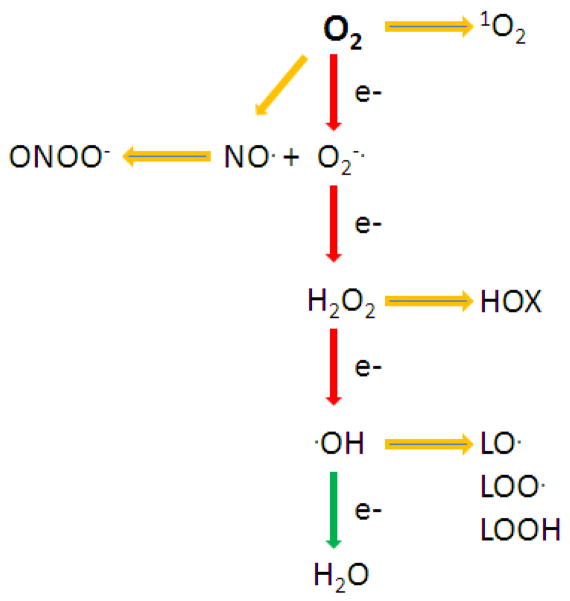
O2 is unique in that it is a relatively stable free radical with two unpaired electrons that have parallel spins. This feature restricts O2 to accept electrons one at a time and is used as an electron acceptor in electron transport chains that are abundant in biological systems. The partial reduction of O2 leads to a cascade of oxygen-derived species that contribute to damaging cellular macromolecules, tissue injury, dysfunction, and disease (Figure 1). All higher eukaryotes require oxygen as the terminal electron acceptor for mitochondrial ATP generation. The mitochondrial electron transport chain accepts electrons from either NADH or FADH2 and passes them on to the terminal cytochrome oxidase which collects electrons on each of its four iron-heme subunits which are added sequentially to O2 to form H2O [1]. The iron-heme subunit is actually a naturally occurring metalloporphyrin and an important cofactor in many different electron transport systems. This is a very efficient process, but a small percentage of the electrons leak as partially reduced O2 species as superoxide (O2−) and hydrogen peroxide (H2O2) [2]. Although both O2− and H2O2 are reactive species, it is thought that most of the oxidative damage to cellular macromolecules occurs through additional reactions of these molecules with transitional metals giving rise to the formation of hydroxyl radicals, a three electron reduction of O2 [3]. Another electron transport system that uses NADPH and produces a prominent oxygen derived free radical, nitric oxide (NO), is nitric oxide synthase (NOS) [4]. Like many of these types of oxidoreductases, NOS can also produce O2− under certain conditions [5]. O2− and NO rapidly react to form peroxynitrite (ONOO−) [6]. ONOO− is a strong oxidizing and nitrating species capable of damaging cellular macromolecules [7]. The haloperoxidases are oxidant generating systems that utilize H2O2 to generate another series of oxygen-derived reactive species known as halous acids (HOX) which are produced for host defense [8–10]. It is ironic that what often separates a pro-oxidant from an anti-oxidant is the efficiency at which the agent or process converts O2 to water. Indeed, biologic systems use very similar process to either generate or scavenge partially reduced oxygen species. There are a number of electron transport systems that generate or leak O2− and H2O2 (Table 1). An example is the use of electrons from NADPH by NADPH oxidases (NOXs) to generate O2− which rapidly dismutates spontaneously or enzymatically to H2O2 [11]. This brings up two important points to consider: 1) that wherever there is O2− there will be H2O2; and 2) superoxide dismutases (SODs) still leave behind H2O2 and thus can’t function alone as an antioxidant and only act as antioxidants in coupled processes that leads to the formation of H2O.
Figure 1.
The partial reduction of dioxygen (O2) and the formation of a number of oxygen-derived reactive species: singlet oxygen (1O2), superoxide (O2−·), nitric oxide (NO·), peroxynitrite (ONOO−), hydrogen peroxide (H2O2), hydroxyl radical (·OH), lipid alkoxyl radical (LO·), lipid peroxyl radical (LOO·), and lipid peroxide (LOOH).
Table 1.
Potential NAD(P)H Dependent Electron Transport Systems for redox reaction with Antioxidants
| Biological Electron Transport Systems | Category |
|---|---|
| NOX/DOUX | Pro-oxidant |
| Xanthine oxidoreductase | Pro-oxidant |
| Cytochrome b5 reductase | Pro-oxidant |
| P450/P450 reductase | Pro-oxidant |
| Nitric oxide synthase | Pro-oxidant |
| Ubiquinone oxidoreductase | Pro-oxidant |
| Heme oxygenase | Antioxidant |
| Biliverdin reductase | Antioxidant |
| Quinone oxidoreductase (NQO1) | Antioxidant |
| Thioredoxin reductase | Antioxidant |
| Glutathione reductase | Antioxidant |
| Monodehydroascorbate reductase | Antioxidant |
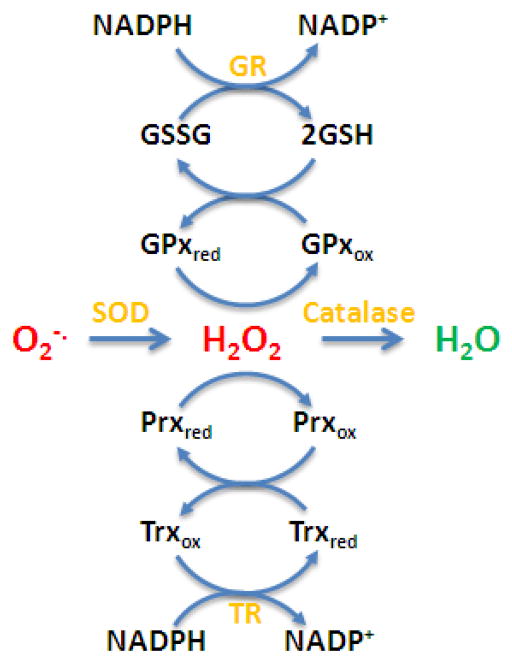
Antioxidant systems that regulate cellular H2O2 levels also rely on NADPH [12] (Table 1). Both the glutathione peroxidase (GPx) and peroxiredoxin (Prx) systems rely on glutathione (GSH) and thioredoxin (Trx), respectively, to scavenge H2O2 and their oxidized products are recycled by glutathione and thioredoxin reductases that utilize NADPH, respectively (Figure 2). Biological systems try to avoid the addition of an electron to H2O2 which can happen with reduced transitional metals or iron-heme complexes. Transitional metals are kept in less reactive bound states by metal-binding proteins and free iron-heme groups are tightly regulated by heme oxygenase, which ironically is another NADPH dependent electron transport system [13]. Although iron-heme groups are often thought to be involved in pro oxidant reactions they are utilized by catalase to perform a two electron dismutation of H2O2 to produce O2 and H2O [14]. Many of the one and two electron transport systems can operate as either oxidant generating or scavenging systems. Any process that augments or inhibits these systems can change the steady-state level of oxygen derived reactive species. As one can see, in biological systems there is not such a clear divide between processes that lead to either a pro-oxidant or antioxidant outcome. This is also true for the development of antioxidants as therapeutic agents that are thought to mimic the biological systems.
Figure 2.
The major endogenous antioxidant pathways that scavenge oxygen-derived reactive species. Superoxide (O2−·) is scavenged by superoxide dismutase (SOD) and the hydrogen peroxide (H2O2) product can be scavenged by catalase or two thiol based systems. The glutathione (GSH) system utilizes glutathione peroxidase (GPx) and GSH. The glutathione disulfide is recycled by glutathione reductase (GR) that utilizes NADPH. The thioredoxin (Trx) system utilizes peroxiredoxins (Prx) that are recycled by thioredoxin (Trx). The oxidized Trx is recycled by thioredxin reductase (TR) that utilizes NADPH.
Role of Oxygen-derived Reactive Species in Oxidative Stress and Cellular Signaling
The term “oxidative stress” is probably one of the most vague and often abused terms in science second only to the term “inflammation.” Often both terms are used together in a chicken and the egg type of scenario. Typically oxidative stress implies that there is an imbalance between oxidant production and antioxidant and repair defenses resulting in the increased steady-state levels of oxidized cellular macromolecules [15]. Many antioxidant agents are often validated by blocking the increased steady-state levels of oxidized cellular macromolecules in animal models involving oxidative stress [16–33]. This popular view does not account for why adaptive responses have failed which is probably more important mechanistically than the simple fact that steady-state levels of oxidized cellular macromolecules are increasing. An important issue is whether the oxidation of biological molecules play a causative role in the pathogenesis of disease or is simply a consequence of the disease process [34, 35]. Although this point is continually raised when antioxidants fail in clinical trials, it is important to consider that successful treatment will require knowing the exact mechanism behind the failure to adapt in order to pick the appropriate antioxidant to intervene. It is this critical gap in our knowledge that has handicapped the successful development of antioxidants as therapeutically useful agents. Aging is an excellent example of a natural process that results in a loss of adaptive responses leading to elevated steady-state levels of oxidized cellular macromolecules [36]. Aging is a common lynchpin of many human diseases and yet we still don’t have a clear picture why adaptive responses slowly fail during the aging process [35]. Many examples of adaptive responses to short oxidant exposures have been described such as brief hyperoxic exposures that substantially raise the burden of oxidants in the lungs but also produce a rapid increase in the antioxidant defenses far above the oxidant burden and are protected against further oxidant exposures [37]. Since most oxidant exposures evoke adaptive responses, many antioxidant compounds may actually have paradoxical effects by blocking adaptive responses which invoke a more coordinated antioxidant protection than provided by the antioxidant agent. The so called “bell shaped dose response curves” observed with many antioxidant agents are likely a consequence of this phenomena. An example of an antioxidant producing paradoxical effects in humans have been documented and occurred with chronic β-carotene supplementation in cigarette smokers which had the paradoxical effect of increasing cancer risk [38–41].
Many of the oxidant-induced adaptive responses are triggered by oxygen derived reactive species directly or indirectly impacting cell signaling pathways. These events have been best studied in bacteria where specific transcriptional factors oxyR and soxRS have been identified [42, 43]. OxyR is activated by H2O2 and induces a number of antioxidant and DNA repair genes products [44, 45]. It is interesting to note that in the soxRS system, the soxR protein that senses the oxidant signal contains an iron-sulfur cluster and only signals soxS when the iron-sulfur cluster is in its oxidized [2Fe-2S]+2 state [46]. A common motif in these types of processes is that a reporter protein needs to be oxidized to affect transcriptional processes and often involves a thiol or metal complex. The best characterized adaptive pathway to oxidants and electrophiles in mammalian systems is the nuclear factor E2-related factor 2 (Nrf2)/antioxidant response element (ARE) signaling pathway [47, 48]. Nrf2 is responsible for both basal and adaptive antioxidant levels in response to oxidative stress. Nrf2 levels are stabilized during oxidative or electrophile stress by altering the binding of Kelch like-ECH-associated protein 1 (Keap1) and Cullin 3 which degrades Nrf2 by the ubiquination pathway [49, 50]. Nrf2 then binds ARE elements in the upstream promoter regions of many genes associated with antioxidant activity and xenobiotic metabolism [47, 51]. Another similar cell signaling pathway that is commonly affected by O2 is the hypoxia-inducible factor (HIF) which under normoxic conditions is rapidly turned over by ubiquination but is stabilized during hypoxia [52, 53]. It is interesting to note that many of the electron transport systems often consume O2 and can cause local hypoxia and may indirectly activate HIF signaling. Conversely, antioxidant dismutation reactions produce O2 that may suppress HIF signaling [54, 55]. A rapidly growing field suggests that oxygen derived reactive species can directly or indirectly affect kinase signaling pathways [56–58]. There have been a number of kinase and phosphatase activities shown to be modulated by oxygen derived reactive species [59, 60]. These kinase pathways control a number of critical cellular functions from cell division and metabolism to cell death. A general theme of these reactions involves critical cysteine residues that are selectively oxidized by peroxide or electrophiles formed during oxidative events such as lipid peroxidation [61, 62]. An example of this is 4-hydroxy-2-nonenal modification of SHP-1 [63]. Another indirect process is the peroxide-mediated formation of an intermediate sulfenic acid that undergoes a glutathionylation reaction as has been shown for PTP1B and PTEN [64, 65]. These types of processes underscore how pharmacologic levels of antioxidants may disrupt these signaling pathways and the difficulty of assigning a specific mechanism of action.
Classic Mechanism of Action for Antioxidant Therapeutics
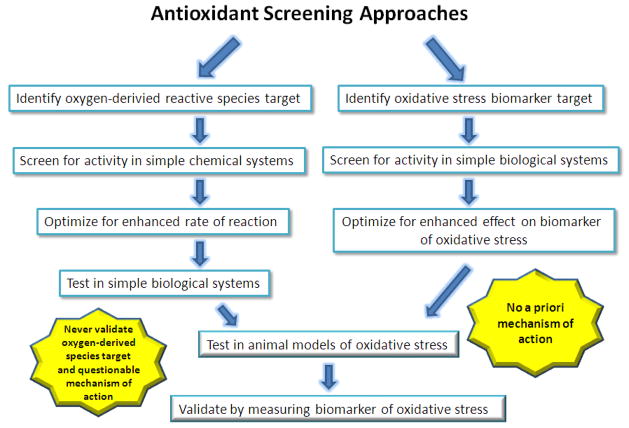
The classical drug development approach is to model the agent based on endogenous antioxidants and to compare the agents ability to scavenge the oxygen derived reactive species (Figure 3). Traditionally, antioxidant therapeutics have been identified from either screens for their ability to scavenge specific oxygen derived species or a more general approach at inhibiting the formation of oxidized cellular macromolecules in a system where oxygen derived species are generated [66–72]. There are advantages and disadvantages to both approaches. The main advantage of the first approach is one can characterize specific oxygen-derived reactive species that can be targeted by the therapeutic and define the rates of reaction in simple buffer systems. Most of the catalytic antioxidants developed to date have been characterized in this manner. Some groups have used this data to denounce other competitor compounds as being non-specific, inferior, or producing their effects by artifacts [73–76], when in reality their approach and compounds suffer the same issues. The major problem with this approach is that it assumes these specific targets are going to be important in the ultimate disease indication and assumes the compound must work only by directly reacting with the specific oxygen derived species at high second order rate constants. This approach also assumes that there will be no other competing reactions or side reactions that will affect the rates of reaction and specificity for the targeted oxygen derived reactive species in more complex biological systems. To my knowledge none of these assumptions have ever been validated in complex biological systems or disease models. The more general approach does not make an assumption on how the compound may be producing its antioxidant effect in the biological system. The advantage of this approach is that a relevant biological antioxidant effect will be achieved and information on the concentration of the agent necessary for the effect. The disadvantage is that one will not have detailed information on the mechanism of action and the effect may be tissue or system specific. In reality, the first approach relies on unproven assumptions and often lacks a proven biological mechanism of action, thus is not much different than the second approach but leads to misconceptions in the field. The next section will start to probe Pandora’s box of not so classy but potentially more biologically relevant antioxidant mechanisms of actions.
Figure 3.
Current concepts and concerns about screening strategies for antioxidant therapeutics.
Pandora’s Box of Potential Antioxidant Mechanism(s) of Action
The most popular and general mechanism of action by which antioxidant therapeutics work is that they protect cellular macromolecules from oxidation that leads to dysfunction [77]. This mechanism is probably most appropriate for injury and disease due to large increases in oxidant exposures that occur during industrial accidents involving oxidizing agents such as chlorine [78] and phosgene gas [79], occupational exposures to metals [80–82], ambient pollution exposures [83–85], cigarette smoke exposures [86–88] and radiation exposure [89, 90]. Under these scenarios, the endogenous antioxidant and repair defenses are overwhelmed causing injury and cell death. However, recent data has suggested that antioxidant agents may be better at affecting the delayed response of endogenous oxygen derived reactive species in response to these types of challenges on the less affected surviving tissue [18, 91–94]. A striking finding in radiation-mediating tumor killing studies was that the antioxidant treatment did not spare the tumor from radiation-induced cell death which implies the antioxidants did not scavenge the initial radiation-induced oxygen derived species. Given the earlier discussion on the numerous ways the body can endogenously produce oxygen-derived reactive species through alterations in electron transport systems one can envision numerous mechanisms that an agent could attenuate these responses without actually directly scavenging oxygen–derived reactive species. For example, the NOX electron transport system often requires proper assemble of its components in order to generate O2− [95]. There are a number of agents that block this process and in doing so produce similar effects as if an agent directly scavenged O2− [96]. Additionally, there are a number of agents that can utilize the electrons transferred from NADPH to flavin subunits and use them to scavenge O2− [72, 97]. There are agents that block the transfer of electrons from NAD(P)H to the flavin subunit [96]. The biological outcomes of these separate mechanisms of action are exactly the same and could be falsely interpreted as a SOD mimic effect. Lastly, there is evidence that some managanese porphyrins can redox cycle with tetrahydrobiopterin which is required by nitric oxide synthase for nitric oxide production thereby suppressing NO production [98]. All these mechanisms could decrease the burden of oxidized cellular macromolecules. The redox potential of metal containing antioxidants is often touted as proof of optimizing a compound’s dismutation activity [74]. However, some of these mechanisms associated with electron transport systems might correlate to a compound’s redox potential but have nothing to do with a dismutation reaction. Now apply this concept to the hundreds of different electron transport systems in organisms and one gets a glimpse of the magnitude of Pandora’s box.
Going back to the concept that organisms have co-evolved with oxygen raises the possibility that some proposed antioxidant agents may actually be weak pro-oxidants and activate biological sensors to evoke a coordinated antioxidant adaptive response. A major sensor for oxidative and electrophilic stress is the nrf2/ARE system [99]. Many natural products are thought to produce their antioxidant effects by activating the nrf2 system. Flavones and chalcone have been shown to induce the nrf2 system by increasing endogenous antioxidant expression [100, 101]. Redox active metal containing compounds are readily reduced in biological systems and can redox cycle with oxygen to paradoxically generate oxygen-derived reactive species [102]. However, they can also scavenge these reactive species so the net effect will be a decrease in their scavenging ability and this will be amplified under condition of higher oxygen tensions. Some groups claim that the so called superoxide specific mimics, such as the macrocyclic agents, are specific because they can only perform one electron transfers [73]. However, it is equally likely that macrocyclic agents can back cycle to produce superoxide under reducing conditions found in biological systems. These types of reactions could lead to paradoxical superoxide production and nrf2 induction. However, it is likely not all compounds will do this equally so this may be more likely for some antioxidant agents than others. For instance, a manganese porphyrin has been shown to not induce nrf2 responsive gene products such as HO-1 and GCL directly in cell culture and actually blocked chalcone-mediated induction of these two gene products [101]. These finding may suggest some antioxidant agents may actual block adaptive responses to oxidative stress and may work best when given after oxidant exposures.
Oxidative stress has been shown to induce HIF signaling and many antioxidants have been shown to attenuate oxidant-induced HIF signaling [103]. HIF signaling components are rapidly degraded under normal oxygen tension due to the requirement of oxygen by prolyl hydroxylases [104]. Prolyl hydroxylases are iron containing dioxygenases and it is interesting to note than iron and manganese porphyrins are often used to model enzymatic hydroxylation reactions [105, 106]. One could speculate that manganese or iron prophyrins could inhibit HIF signaling by directly hydroxylating HIFs. A consequence of dismutation of superoxide and hydrogen peroxide is the generation of oxygen which is known to suppress HIF signaling. SOD and catalase mimics are thought to suppress HIF signaling by scavenging oxygen-derived reactive species but could also be producing biological effects indirectly by suppressing HIF signaling through local increases in oxygen tension. Recent studies with manganese porphyrins have shown suppression of radiation-induced HIF signaling and assumed the mechanism to be through scavenging oxygen-derived reactive species [107–109]. Teasing out the actual mechanism(s) rather than assuming the mechanism based on artificially defined antioxidant activities are more challenging and rarely done.
Redox signaling often involves oxidative modification of cysteine residues that can produce a wide variety of effects that can be beneficial or detrimental during oxidative stress. There are numerous redox active cysteine residues on proteins that upon oxidative modification change the protein’s activity. Some of these effects are direct but many may be indirect such as changing the ratio of reduced to oxidized GSH ratios. A number of higher oxidation state of the cysteine thiol have been found under conditions of oxidative stress including disulfide (RS-SR), sulfenic acid (RSOH), sulfinic acid (RSO2H), and sulfonic acid (RSO3H). Each increasing oxidation state of the thiol is less reversible and less susceptible to antioxidant rescue [110]. A number of studies have shown that some of the antioxidants can attenuate the accumulation of protein thiol oxidation [21, 22, 111]. However, the exact mechanism(s) by which this is achieved is rarely reported.
In summary, there are a large number of potential mechanisms by which antioxidant therapeutic agents can produce protective effects in models of oxidative stress beside the often assumed ability to directly scavenge oxygen-derived reactive species and some groups are starting to acknowledge and explore this reality [112–114]. Many of the mechanisms involve potential interactions with NAD(P)H oxidoreductase electron transport systems such as those listed in table 1. If these NAD(P)H oxidoreductase systems are potentials sources for oxygen-derived reactive species then the interaction will “mimic” an antioxidant effect. Conversely, if these NAD(P)H oxidoreductases are predominantly antioxidant systems then their inhibition may lead to a pro-oxidant effect and evoke endogenous antioxidant responses. Likewise redox cycling with these oxidoreductases with oxygen may also evoke local changes in oxygen tensions that will affect the HIF signaling pathways, potential activation of the nrf2 signaling pathway, and changes in thiol redox balance. All these potential confounding pathways unleashes the full potential of Pandora’s box and often makes it very difficult to fully define the critical mechanism(s) of action by which antioxidant therapeutics produce their beneficial effects in complex biological systems. However the current practice of assuming the mechanism of action of antioxidant therapeutics is due to their chemical reactivity in more simple chemical systems is often deceptive and misleading. It is very likely that each antioxidant agent will have different mechanism(s) of action and that this may change with model and disease state. Welcome to Pandora.
Figure 4.
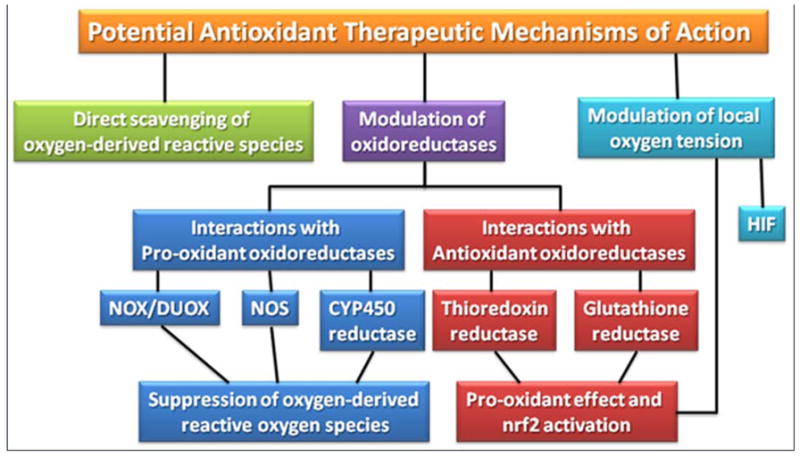
Potential mechanisms of action for antioxidant therapeutics.
Highlights.
During evolution, biological systems have adapted to the abnormal biology of O2.
Cells use O2’s reactive nature in many different electron transport systems.
Disruption of electron transport systems can alter oxidant production.
The numbers of different mechanisms of action for antioxidants are numerous.
Reliance solely on chemical reactivity of an antioxidant can be misleading.
Acknowledgments
I would like to thank Dr. Dean Jones for many enlightening conversations about the role of oxygen in the evolution of biological systems. This work was supported in part by NIH grants HL59602, HL075523, HL084469, ES017582, ES015678 and research grants from Aeolus Pharmaceuticals. Dr. Day is a consultant for and holds equity in Aeolus Pharmaceuticals which is developing metalloporphyrins as therapeutic agents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chance B. Energy-linked cytochrome oxidation in mitochondria. Nature. 1961;189:719–725. doi: 10.1038/189719b0. [DOI] [PubMed] [Google Scholar]
- 2.Turrens JF. Superoxide production by the mitochondrial respiratory chain. Biosci Rep. 1997;17:3–8. doi: 10.1023/a:1027374931887. [DOI] [PubMed] [Google Scholar]
- 3.Halliwell B, Gutteridge JM. Biologically relevant metal ion-dependent hydroxyl radical generation. An update. FEBS Lett. 1992;307:108–112. doi: 10.1016/0014-5793(92)80911-y. [DOI] [PubMed] [Google Scholar]
- 4.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 5.Day BJ, Patel M, Calavetta L, Chang LY, Stamler JS. A mechanism of paraquat toxicity involving nitric oxide synthase. Proc Natl Acad Sci U S A. 1999;96:12760–12765. doi: 10.1073/pnas.96.22.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 7.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrison M, Schonbaum GR. Peroxidase-catalyzed halogenation. Annu Rev Biochem. 1976;45:861–888. doi: 10.1146/annurev.bi.45.070176.004241. [DOI] [PubMed] [Google Scholar]
- 9.Clifford DP, Repine JE. Hydrogen peroxide mediated killing of bacteria. Mol Cell Biochem. 1982;49:143–149. doi: 10.1007/BF00231175. [DOI] [PubMed] [Google Scholar]
- 10.Chandler JD, Day BJ. Thiocyanate: a potentially useful therapeutic agent with host defense and antioxidant properties. Biochem Pharmacol. 2012;84:1381–1387. doi: 10.1016/j.bcp.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambeth JD. Nox/Duox family of nicotinamide adenine dinucleotide (phosphate) oxidases. Curr Opin Hematol. 2002;9:11–17. doi: 10.1097/00062752-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Fourquet S, Huang ME, D’Autreaux B, Toledano MB. The dual functions of thiol-based peroxidases in H2O2 scavenging and signaling. Antioxid Redox Signal. 2008;10:1565–1576. doi: 10.1089/ars.2008.2049. [DOI] [PubMed] [Google Scholar]
- 13.Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- 14.Deisseroth A, Dounce AL. Catalase: Physical and chemical properties, mechanism of catalysis, and physiological role. Physiol Rev. 1970;50:319–375. doi: 10.1152/physrev.1970.50.3.319. [DOI] [PubMed] [Google Scholar]
- 15.Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans. 2007;35:1147–1150. doi: 10.1042/BST0351147. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez PK, Zhuang J, Doctrow SR, Malfroy B, Benson PF, Menconi MJ, Fink MP. EUK-8, a synthetic superoxide dismutase and catalase mimetic, ameliorates acute lung injury in endotoxemic swine. J Pharmacol Exp Ther. 1995;275:798–806. [PubMed] [Google Scholar]
- 17.Mackensen GB, Patel M, Sheng H, Calvi CL, Batinic-Haberle I, Day BJ, Liang LP, Fridovich I, Crapo JD, Pearlstein RD, Warner DS. Neuroprotection from delayed postischemic administration of a metalloporphyrin catalytic antioxidant. J Neurosci. 2001;21:4582–4592. doi: 10.1523/JNEUROSCI.21-13-04582.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabbani ZN, Batinic-Haberle I, Anscher MS, Huang J, Day BJ, Alexander E, Dewhirst MW, Vujaskovic Z. Long-term administration of a small molecular weight catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation-induced injury. Int J Radiat Oncol Biol Phys. 2007;67:573–580. doi: 10.1016/j.ijrobp.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang LP, Huang J, Fulton R, Day BJ, Patel M. An orally active catalytic metalloporphyrin protects against 1-methyl 4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity in vivo. J Neurosci. 2007;27:4326–4333. doi: 10.1523/JNEUROSCI.0019-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Neill HC, White CW, Veress LA, Hendry-Hofer TB, Loader JE, Min E, Huang J, Rancourt RC, Day BJ. Treatment with the catalytic metalloporphyrin AEOL 10150 reduces inflammation and oxidative stress due to inhalation of the sulfur mustard analog 2-chloroethyl ethyl sulfide. Free Radic Biol Med. 2010;48:1188–1196. doi: 10.1016/j.freeradbiomed.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGovern T, Day BJ, White CW, Powell WS, Martin JG. AEOL10150: a novel therapeutic for rescue treatment after toxic gas lung injury. Free Radic Biol Med. 2011;50:602–608. doi: 10.1016/j.freeradbiomed.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang LP, Waldbaum S, Rowley S, Huang TT, Day BJ, Patel M. Mitochondrial oxidative stress and epilepsy in SOD2 deficient mice: attenuation by a lipophilic metalloporphyrin. Neurobiol Dis. 2012;45:1068–1076. doi: 10.1016/j.nbd.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rong Y, Doctrow SR, Tocco G, Baudry M. EUK-134, a synthetic superoxide dismutase and catalase mimetic, prevents oxidative stress and attenuates kainate-induced neuropathology. Proc Natl Acad Sci U S A. 1999;96:9897–9902. doi: 10.1073/pnas.96.17.9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung C, Rong Y, Doctrow S, Baudry M, Malfroy B, Xu Z. Synthetic superoxide dismutase/catalase mimetics reduce oxidative stress and prolong survival in a mouse amyotrophic lateral sclerosis model. Neurosci Lett. 2001;304:157–160. doi: 10.1016/s0304-3940(01)01784-0. [DOI] [PubMed] [Google Scholar]
- 25.Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, Baudry M. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc Natl Acad Sci U S A. 2003;100:8526–8531. doi: 10.1073/pnas.1332809100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brazier MW, Doctrow SR, Masters CL, Collins SJ. A manganese-superoxide dismutase/catalase mimetic extends survival in a mouse model of human prion disease. Free Radic Biol Med. 2008;45:184–192. doi: 10.1016/j.freeradbiomed.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Clausen A, Doctrow S, Baudry M. Prevention of cognitive deficits and brain oxidative stress with superoxide dismutase/catalase mimetics in aged mice. Neurobiol Aging. 2010;31:425–433. doi: 10.1016/j.neurobiolaging.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahmood J, Jelveh S, Calveley V, Zaidi A, Doctrow SR, Hill RP. Mitigation of radiation-induced lung injury by genistein and EUK 207. Int J Radiat Biol. 2011;87:889–901. doi: 10.3109/09553002.2011.583315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doctrow SR, Lopez A, Schock AM, Duncan NE, Jourdan MM, Olasz EB, Moulder JE, Fish BL, Mader M, Lazar J, Lazarova Z. A synthetic superoxide dismutase/catalase mimetic EUK-207 mitigates radiation dermatitis and promotes wound healing in irradiated rat skin. J Invest Dermatol. 2013;133:1088–1096. doi: 10.1038/jid.2012.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahmood J, Jelveh S, Zaidi A, Doctrow SR, Hill RP. Mitigation of radiation-induced lung injury with EUK-207 and genistein: effects in adolescent rats. Radiat Res. 2013;179:125–134. doi: 10.1667/RR2954.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Y, Chaiswing L, Oberley TD, Batinic-Haberle I, St Clair W, Epstein CJ, St Clair D. A mechanism-based antioxidant approach for the reduction of skin carcinogenesis. Cancer Res. 2005;65:1401–1405. doi: 10.1158/0008-5472.CAN-04-3334. [DOI] [PubMed] [Google Scholar]
- 32.Benov L, Batinic-Haberle I. A manganese porphyrin suppresses oxidative stress and extends the life span of streptozotocin-diabetic rats. Free Radic Res. 2005;39:81–88. doi: 10.1080/10715760400022368. [DOI] [PubMed] [Google Scholar]
- 33.Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jiang C, Reboucas JS, Batinic-Haberle I, Vujaskovic Z. Early and late administration of MnTE-2-PyP5+ in mitigation and treatment of radiation-induced lung damage. Free Radic Biol Med. 2010;48:1034–1043. doi: 10.1016/j.freeradbiomed.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Vliet A, Eiserich JP, Marelich GP, Halliwell B, Cross CE. Oxidative stress in cystic fibrosis: does it occur and does it matter? Adv Pharmacol. 1997;38:491–513. doi: 10.1016/s1054-3589(08)60996-5. [DOI] [PubMed] [Google Scholar]
- 35.Floyd RA, Towner RA, He T, Hensley K, Maples KR. Translational research involving oxidative stress and diseases of aging. Free Radic Biol Med. 2011;51:931–941. doi: 10.1016/j.freeradbiomed.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gould NS, Min E, Gauthier S, Chu HW, Martin R, Day BJ. Aging adversely affects the cigarette smoke-induced glutathione adaptive response in the lung. Am J Respir Crit Care Med. 2010;182:1114–1122. doi: 10.1164/rccm.201003-0442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crapo JD, Sjostrom K, Drew RT. Tolerance and cross-tolerance using NO2 and O2. I. Toxicology and biochemistry. J Appl Physiol. 1978;44:364–369. doi: 10.1152/jappl.1978.44.3.364. [DOI] [PubMed] [Google Scholar]
- 38.Meyer F, Bairati I, Fortin A, Gelinas M, Nabid A, Brochet F, Tetu B. Interaction between antioxidant vitamin supplementation and cigarette smoking during radiation therapy in relation to long-term effects on recurrence and mortality: a randomized trial among head and neck cancer patients. Int J Cancer. 2008;122:1679–1683. doi: 10.1002/ijc.23200. [DOI] [PubMed] [Google Scholar]
- 39.Albanes D, Heinonen OP, Huttunen JK, Taylor PR, Virtamo J, Edwards BK, Haapakoski J, Rautalahti M, Hartman AM, Palmgren J, et al. Effects of alpha-tocopherol and beta-carotene supplements on cancer incidence in the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study. Am J Clin Nutr. 1995;62:1427S–1430S. doi: 10.1093/ajcn/62.6.1427S. [DOI] [PubMed] [Google Scholar]
- 40.Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M, Hartman AM, Palmgren J, Freedman LS, Haapakoski J, Barrett MJ, Pietinen P, Malila N, Tala E, Liippo K, Salomaa ER, Tangrea JA, Teppo L, Askin FB, Taskinen E, Erozan Y, Greenwald P, Huttunen JK. Alpha-Tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. J Natl Cancer Inst. 1996;88:1560–1570. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- 41.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Jr, Valanis B, Williams JH, Jr, Barnhart S, Cherniack MG, Brodkin CA, Hammar S. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996;88:1550–1559. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- 42.Demple B, Amabile-Cuevas CF. Redox redux: the control of oxidative stress responses. Cell. 1991;67:837–839. doi: 10.1016/0092-8674(91)90355-3. [DOI] [PubMed] [Google Scholar]
- 43.Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christman MF, Morgan RW, Jacobson FS, Ames BN. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985;41:753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- 45.Morgan RW, Christman MF, Jacobson FS, Storz G, Ames BN. Hydrogen peroxide-inducible proteins in Salmonella typhimurium overlap with heat shock and other stress proteins. Proc Natl Acad Sci U S A. 1986;83:8059–8063. doi: 10.1073/pnas.83.21.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hidalgo E, Demple B. An iron-sulfur center essential for transcriptional activation by the redox-sensing SoxR protein. EMBO J. 1994;13:138–146. doi: 10.1002/j.1460-2075.1994.tb06243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Itoh K, Ishii T, Wakabayashi N, Yamamoto M. Regulatory mechanisms of cellular response to oxidative stress. Free Radic Res. 1999;31:319–324. doi: 10.1080/10715769900300881. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 49.Gao L, Wang J, Sekhar KR, Yin H, Yared NF, Schneider SN, Sasi S, Dalton TP, Anderson ME, Chan JY, Morrow JD, Freeman ML. Novel n-3 fatty acid oxidation products activate Nrf2 by destabilizing the association between Keap1 and Cullin3. J Biol Chem. 2007;282:2529–2537. doi: 10.1074/jbc.M607622200. [DOI] [PubMed] [Google Scholar]
- 50.Villeneuve NF, Lau A, Zhang DD. Regulation of the Nrf2-Keap1 antioxidant response by the ubiquitin proteasome system: an insight into cullin-ring ubiquitin ligases. Antioxid Redox Signal. 2010;13:1699–1712. doi: 10.1089/ars.2010.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayes JD, McMahon M. Molecular basis for the contribution of the antioxidant responsive element to cancer chemoprevention. Cancer Lett. 2001;174:103–113. doi: 10.1016/s0304-3835(01)00695-4. [DOI] [PubMed] [Google Scholar]
- 52.Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- 53.Semenza GL, Agani F, Feldser D, Iyer N, Kotch L, Laughner E, Yu A. Hypoxia, HIF-1, and the pathophysiology of common human diseases. Adv Exp Med Biol. 2000;475:123–130. doi: 10.1007/0-306-46825-5_12. [DOI] [PubMed] [Google Scholar]
- 54.Yang ZZ, Zhang AY, Yi FX, Li PL, Zou AP. Redox regulation of HIF-1alpha levels and HO-1 expression in renal medullary interstitial cells. Am J Physiol Renal Physiol. 2003;284:F1207–1215. doi: 10.1152/ajprenal.00017.2002. [DOI] [PubMed] [Google Scholar]
- 55.BelAiba RS, Djordjevic T, Bonello S, Flugel D, Hess J, Kietzmann T, Gorlach A. Redox-sensitive regulation of the HIF pathway under non-hypoxic conditions in pulmonary artery smooth muscle cells. Biol Chem. 2004;385:249–257. doi: 10.1515/BC.2004.019. [DOI] [PubMed] [Google Scholar]
- 56.Chakraborti S, Chakraborti T. Oxidant-mediated activation of mitogen-activated protein kinases and nuclear transcription factors in the cardiovascular system: a brief overview. Cell Signal. 1998;10:675–683. doi: 10.1016/s0898-6568(98)00014-x. [DOI] [PubMed] [Google Scholar]
- 57.Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 58.Torres M, Forman HJ. Redox signaling and the MAP kinase pathways. Biofactors. 2003;17:287–296. doi: 10.1002/biof.5520170128. [DOI] [PubMed] [Google Scholar]
- 59.Krejsa CM, Schieven GL. Impact of oxidative stress on signal transduction control by phosphotyrosine phosphatases. Environ Health Perspect. 1998;106(Suppl 5):1179–1184. doi: 10.1289/ehp.98106s51179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakashima I, Kato M, Akhand AA, Suzuki H, Takeda K, Hossain K, Kawamoto Y. Redox-linked signal transduction pathways for protein tyrosine kinase activation. Antioxid Redox Signal. 2002;4:517–531. doi: 10.1089/15230860260196326. [DOI] [PubMed] [Google Scholar]
- 61.Claiborne A, Yeh JI, Mallett TC, Luba J, Crane EJ, 3rd, Charrier V, Parsonage D. Protein-sulfenic acids: diverse roles for an unlikely player in enzyme catalysis and redox regulation. Biochemistry. 1999;38:15407–15416. doi: 10.1021/bi992025k. [DOI] [PubMed] [Google Scholar]
- 62.Petersen DR, Doorn JA. Reactions of 4 hydroxynonenal with proteins and cellular targets. Free Radic Biol Med. 2004;37:937–945. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 63.Rinna A, Forman HJ. SHP-1 inhibition by 4-hydroxynonenal activates Jun N-terminal kinase and glutamate cysteine ligase. Am J Respir Cell Mol Biol. 2008;39:97–104. doi: 10.1165/rcmb.2007-0371OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barrett WC, DeGnore JP, Konig S, Fales HM, Keng YF, Zhang ZY, Yim MB, Chock PB. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry. 1999;38:6699–6705. doi: 10.1021/bi990240v. [DOI] [PubMed] [Google Scholar]
- 66.Samuni A, Mitchell JB, DeGraff W, Krishna CM, Samuni U, Russo A. Nitroxide SOD-mimics: modes of action. Free Radic Res Commun. 1991;12–13(Pt 1):187–194. doi: 10.3109/10715769109145785. [DOI] [PubMed] [Google Scholar]
- 67.Weiss RH, Flickinger AG, Rivers WJ, Hardy MM, Aston KW, Ryan US, Riley DP. Evaluation of activity of putative superoxide dismutase mimics. Direct analysis by stopped-flow kinetics. J Biol Chem. 1993;268:23049–23054. [PubMed] [Google Scholar]
- 68.Baudry M, Etienne S, Bruce A, Palucki M, Jacobsen E, Malfroy B. Salen-manganese complexes are superoxide dismutase-mimics. Biochem Biophys Res Commun. 1993;192:964–968. doi: 10.1006/bbrc.1993.1509. [DOI] [PubMed] [Google Scholar]
- 69.Faulkner KM, Liochev SI, Fridovich I. Stable Mn(III) porphyrins mimic superoxide dismutase in vitro and substitute for it in vivo. J Biol Chem. 1994;269:23471–23476. [PubMed] [Google Scholar]
- 70.Baker K, Marcus CB, Huffman K, Kruk H, Malfroy B, Doctrow SR. Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: a key role for reactive oxygen species in ischemic brain injury. J Pharmacol Exp Ther. 1998;284:215–221. [PubMed] [Google Scholar]
- 71.Day BJ, Fridovich I, Crapo JD. Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch Biochem Biophys. 1997;347:256–262. doi: 10.1006/abbi.1997.0341. [DOI] [PubMed] [Google Scholar]
- 72.Kachadourian R, Johnson CA, Min E, Spasojevic I, Day BJ. Flavin-dependent antioxidant properties of a new series of meso-N,N′ dialkyl-imidazolium substituted manganese(III) porphyrins. Biochem Pharmacol. 2004;67:77–85. doi: 10.1016/j.bcp.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 73.Salvemini D, Riley DP, Cuzzocrea S. SOD mimetics are coming of age. Nat Rev Drug Discov. 2002;1:367–374. doi: 10.1038/nrd796. [DOI] [PubMed] [Google Scholar]
- 74.Batinic-Haberle I, Reboucas JS, Spasojevic I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid Redox Signal. 2010;13:877–918. doi: 10.1089/ars.2009.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miriyala S, Spasojevic I, Tovmasyan A, Salvemini D, Vujaskovic Z, St Clair D, Batinic-Haberle I. Manganese superoxide dismutase, MnSOD and its mimics. Biochim Biophys Acta. 2012;1822:794–814. doi: 10.1016/j.bbadis.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reboucas JS, Spasojevic I, Batinic-Haberle I. Pure manganese(III) 5,10,15,20 tetrakis(4-benzoic acid)porphyrin (MnTBAP) is not a superoxide dismutase mimic in aqueous systems: a case of structure-activity relationship as a watchdog mechanism in experimental therapeutics and biology. J Biol Inorg Chem. 2008;13:289–302. doi: 10.1007/s00775-007-0324-9. [DOI] [PubMed] [Google Scholar]
- 77.Halliwell B. Can oxidative DNA damage be used as a biomarker of cancer risk in humans? Problems, resolutions and preliminary results from nutritional supplementation studies. Free Radic Res. 1998;29:469–486. doi: 10.1080/10715769800300531. [DOI] [PubMed] [Google Scholar]
- 78.Martin JG, Campbell HR, Iijima H, Gautrin D, Malo JL, Eidelman DH, Hamid Q, Maghni K. Chlorine-induced injury to the airways in mice. Am J Respir Crit Care Med. 2003;168:568–574. doi: 10.1164/rccm.200201-021OC. [DOI] [PubMed] [Google Scholar]
- 79.Qin XJ, Li YN, Liang X, Wang P, Hai CX. The dysfunction of ATPases due to impaired mitochondrial respiration in phosgene-induced pulmonary edema. Biochem Biophys Res Commun. 2008;367:150–155. doi: 10.1016/j.bbrc.2007.12.111. [DOI] [PubMed] [Google Scholar]
- 80.Snow ET. Metal carcinogenesis: mechanistic implications. Pharmacol Ther. 1992;53:31–65. doi: 10.1016/0163-7258(92)90043-y. [DOI] [PubMed] [Google Scholar]
- 81.Rikans LE, Yamano T. Mechanisms of cadmium-mediated acute hepatotoxicity. J Biochem Mol Toxicol. 2000;14:110–117. doi: 10.1002/(sici)1099-0461(2000)14:2<110::aid-jbt7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 82.Ercal N, Gurer Orhan H, Aykin-Burns N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem. 2001;1:529–539. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- 83.Menzel DB. The toxicity of air pollution in experimental animals and humans: the role of oxidative stress. Toxicol Lett. 1994;72:269–277. doi: 10.1016/0378-4274(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 84.Tao F, Gonzalez-Flecha B, Kobzik L. Reactive oxygen species in pulmonary inflammation by ambient particulates. Free Radic Biol Med. 2003;35:327–340. doi: 10.1016/s0891-5849(03)00280-6. [DOI] [PubMed] [Google Scholar]
- 85.Moller P, Folkmann JK, Forchhammer L, Brauner EV, Danielsen PH, Risom L, Loft S. Air pollution, oxidative damage to DNA, and carcinogenesis. Cancer Lett. 2008;266:84–97. doi: 10.1016/j.canlet.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 86.Chow CK. Cigarette smoking and oxidative damage in the lung. Ann N Y Acad Sci. 1993;686:289–298. doi: 10.1111/j.1749-6632.1993.tb39189.x. [DOI] [PubMed] [Google Scholar]
- 87.MacNee W. Oxidants/antioxidants and COPD. Chest. 2000;117:303S–317S. doi: 10.1378/chest.117.5_suppl_1.303s-a. [DOI] [PubMed] [Google Scholar]
- 88.Cantin AM, Richter MV. Cigarette smoke-induced proteostasis imbalance in obstructive lung diseases. Curr Mol Med. 2012;12:836–849. doi: 10.2174/156652412801318746. [DOI] [PubMed] [Google Scholar]
- 89.Riley PA. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 90.Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Rev. 2004;23:311–322. doi: 10.1023/B:CANC.0000031769.14728.bc. [DOI] [PubMed] [Google Scholar]
- 91.Vujaskovic Z, Batinic-Haberle I, Rabbani ZN, Feng QF, Kang SK, Spasojevic I, Samulski TV, Fridovich I, Dewhirst MW, Anscher MS. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic Biol Med. 2002;33:857–863. doi: 10.1016/s0891-5849(02)00980-2. [DOI] [PubMed] [Google Scholar]
- 92.Decraene D, Smaers K, Gan D, Mammone T, Matsui M, Maes D, Declercq L, Garmyn M. A synthetic superoxide dismutase/catalase mimetic (EUK-134) inhibits membrane-damage-induced activation of mitogen-activated protein kinase pathways and reduces p53 accumulation in ultraviolet B-exposed primary human keratinocytes. J Invest Dermatol. 2004;122:484–491. doi: 10.1046/j.0022-202X.2004.22215.x. [DOI] [PubMed] [Google Scholar]
- 93.Rabbani ZN, Salahuddin FK, Yarmolenko P, Batinic-Haberle I, Thrasher BA, Gauter-Fleckenstein B, Dewhirst MW, Anscher MS, Vujaskovic Z. Low molecular weight catalytic metalloporphyrin antioxidant AEOL 10150 protects lungs from fractionated radiation. Free Radic Res. 2007;41:1273–1282. doi: 10.1080/10715760701689550. [DOI] [PubMed] [Google Scholar]
- 94.Rosenthal RA, Fish B, Hill RP, Huffman KD, Lazarova Z, Mahmood J, Medhora M, Molthen R, Moulder JE, Sonis ST, Tofilon PJ, Doctrow SR. Salen Mn complexes mitigate radiation injury in normal tissues. Anticancer Agents Med Chem. 2011;11:359–372. doi: 10.2174/187152011795677490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Uhlinger DJ, Inge KL, Kreck ML, Tyagi SR, Neckelmann N, Lambeth JD. Reconstitution and characterization of the human neutrophil respiratory burst oxidase using recombinant p47-phox, p67-phox and plasma membrane. Biochem Biophys Res Commun. 1992;186:509–516. doi: 10.1016/s0006-291x(05)80837-x. [DOI] [PubMed] [Google Scholar]
- 96.Jaquet V, Scapozza L, Clark RA, Krause KH, Lambeth JD. Small-molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxid Redox Signal. 2009;11:2535–2552. doi: 10.1089/ars.2009.2585. [DOI] [PubMed] [Google Scholar]
- 97.Ferrer Sueta G, Hannibal L, Batinic-Haberle I, Radi R. Reduction of manganese porphyrins by flavoenzymes and submitochondrial particles: a catalytic cycle for the reduction of peroxynitrite. Free Radic Biol Med. 2006;41:503–512. doi: 10.1016/j.freeradbiomed.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 98.Batinic-Haberle I, Spasojevic I, Fridovich I. Tetrahydrobiopterin rapidly reduces the SOD mimic Mn(III) ortho-tetrakis(N-ethylpyridinium-2-yl)porphyrin. Free Radic Biol Med. 2004;37:367–374. doi: 10.1016/j.freeradbiomed.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 99.Villeneuve NF, Lau A, Zhang DD. Regulation of the Nrf2 Keap1 antioxidant response by the ubiquitin proteasome system: an insight into cullin-ring ubiquitin ligases. Antioxid Redox Signal. 2010;13:1699–1712. doi: 10.1089/ars.2010.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006;72:1439–1452. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 101.Kachadourian R, Pugazhenthi S, Velmurugan K, Backos DS, Franklin CC, McCord JM, Day BJ. 2′,5′ Dihydroxychalcone-induced glutathione is mediated by oxidative stress and kinase signaling pathways. Free Radic Biol Med. 2011;51:1146–1154. doi: 10.1016/j.freeradbiomed.2011.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kachadourian R, Brechbuhl HM, Ruiz-Azuara L, Gracia Mora I, Day BJ. Casiopeina IIgly-induced oxidative stress and mitochondrial dysfunction in human lung cancer A549 and H157 cells. Toxicology. 2010;268:176–183. doi: 10.1016/j.tox.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Haddad JJ. Oxygen homeostasis, thiol equilibrium and redox regulation of signalling transcription factors in the alveolar epithelium. Cell Signal. 2002;14:799–810. doi: 10.1016/s0898-6568(02)00022-0. [DOI] [PubMed] [Google Scholar]
- 104.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJC. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 105.Groves JT, Watanabe Y. Oxygen activation by metalloporphyrins related to peroxidase and cytochrome P-450. Direct observation of the oxygen-oxygen bond cleavage step. J Am Chem Soc. 1986;108:7834–7836. doi: 10.1021/ja00284a058. [DOI] [PubMed] [Google Scholar]
- 106.Jin N, Lahaye DE, Groves JT. A “push-pull” mechanism for heterolytic o-o bond cleavage in hydroperoxo manganese porphyrins. Inorg Chem. 2010;49:11516–11524. doi: 10.1021/ic1015274. [DOI] [PubMed] [Google Scholar]
- 107.Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 108.Jackson IL, Zhang X, Hadley C, Rabbani ZN, Zhang Y, Marks S, Vujaskovic Z. Temporal expression of hypoxia-regulated genes is associated with early changes in redox status in irradiated lung. Free Radic Biol Med. 2012;53:337–346. doi: 10.1016/j.freeradbiomed.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jackson IL, Chen L, Batinic-Haberle I, Vujaskovic Z. Superoxide dismutase mimetic reduces hypoxia-induced O2*, TGF-beta, and VEGF production by macrophages. Free Radic Res. 2007;41:8–14. doi: 10.1080/10715760600913150. [DOI] [PubMed] [Google Scholar]
- 110.Forman HJ, Maiorino M, Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49:835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gould NS, White CW, Day BJ. A role for mitochondrial oxidative stress in sulfur mustard analog 2-chloroethyl ethyl sulfide-induced lung cell injury and antioxidant protection. J Pharmacol Exp Ther. 2009;328:732–739. doi: 10.1124/jpet.108.145037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Day BJ. Catalytic antioxidants: a radical approach to new therapeutics. Drug Discov Today. 2004;9:557–566. doi: 10.1016/S1359-6446(04)03139-3. [DOI] [PubMed] [Google Scholar]
- 113.Rosenthal RA, Fish B, Hill RP, Huffman KD, Lazarova Z, Mahmood J, Medhora M, Molthen R, Moulder JE, Sonis ST, Tofilon PJ, Doctrow SR. Salen Mn complexes mitigate radiation injury in normal tissues. Anticancer Agents Med Chem. 2011;11:359–372. doi: 10.2174/187152011795677490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Day BJ. Catalase and glutathione peroxidase mimics. Biochem Pharmacol. 2009;77:285–296. doi: 10.1016/j.bcp.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]