Abstract
One of the most abundant antioxidants in the lung is glutathione (GSH), a low molecular weight thiol, which functions to attenuate both oxidative stress and inflammation. GSH is concentrated in the epithelial lining fluid (ELF) of the lung and can be elevated in response to the increased oxidant burden from cigarette smoke (CS). However, the transporter(s) responsible for the increase in ELF GSH with cigarette smoke are not known. Three candidate apical GSH transporters in the lung are CFTR, BCRP and MRP2, but their potential role in ELF GSH transport in response to CS has not been investigated. In vitro, the inhibition of CFTR, BCRP, or MRP2 resulted in decreased GSH efflux in response to cigarette smoke extract. In vivo, mice deficient in CFTR, BCRP, or MRP2 were exposed to either air or acute CS. CFTR deficient mice had reduced basal and CS induced GSH in the ELF, while BCRP or MRP2 deficiency had no effect on ELF GSH basal or CS exposed levels. Furthermore, BCRP and MRP2 deficiencies had little effect on lung tissue GSH. These data indicate that CFTR is predominantly involved in maintaining basal ELF GSH and increasing ELF GSH in response to CS.
Introduction
Glutathione (GSH), a tripeptide comprised of glutamate, cysteine, and glycine is an important antioxidant found ubiquitously across many different cell types. Intracellular GSH is maintained at very high concentrations, in the millimolar range, while its extracellular levels can vary greatly. In the plasma, for example, the concentration is in low micromolar range, while in the epithelial lining fluid (ELF) of the lung it can range from 100 to 1000 μM or more in some instances [1, 2].
The reason GSH is maintained at higher concentrations in the ELF compared to the plasma may simply be because there is more chance for damage caused by inhaled gases, particles or pathogens. The high levels of GSH, and the ability to raise those levels, acts as an adaptive mechanism in response to environmental stressors such as bacterial infection or cigarette smoke [2]. One of the pivotal functions that is necessary to establish this adaptive response is to transport GSH into the apical space, yet little is known about the transporters involved.
Of those proteins shown to transport GSH or its’ conjugates several are classified in the ATP-binding cassette sub-family C (ABCC) proteins, commonly known as the Multi-drug associated resistance proteins (MRP) of which the cystic fibrosis transmembrane regulator (CFTR) protein is also a member [3-5]. Even fewer have been shown to be apically expressed in the lung. Currently only CFTR is known to be involved in transport of GSH into the ELF. However, knocking out CFTR only decreases ELF GSH levels to roughly half of normal and CFTR KO mice can still raise ELF GSH levels, albeit to a much lower level than normal [6, 7]. These findings suggest there must be other transporters involved in the efflux of GSH into the ELF in addition to CFTR [5, 8, 9]. Recently the breast cancer related protein (BCRP, ABCG2) a member of the G sub-family of ABC proteins, which is apically expressed, has been shown to transport GSH [10]. Additionally, evidence of MRP2, another apical transporter shown to transport GSH, has been shown in the lung [11].
Cigarette smoke contains a large amount of reactive species and the GSH adaptive response that occurs after acute exposure has been shown to be quite robust [12]. Furthermore, the ability to maintain high levels of ELF GSH has been shown to be important in preventing excess oxidation and inflammation with cigarette smoke [12]. Therefore in the current study the role of CFTR, BCRP, or MRP2 in transport of GSH in response to cigarette smoke was examined.
Methods
Chemicals
All chemicals and reagents including amiloride, Hoechst 3342 and MK571 were obtained from Sigma.
Cigarette smoke extract (CSE) preparation
The smoke of one 3R4F Kentucky reference cigarette was bubbled through 7 mL of room temperature PBS. The extract was measured spectrophotometrically at a wavelength of 210 nm, a range of 1.3-2.0 was considered acceptable. The resulting extract was deemed 100% CSE, and was diluted to a final concentration of 20% CSE in normal cell media.
Cell Culture and treatments
The transformed human bronchial epithelial cell line, 16HBE, were grown in DMEM supplemented with glutamine, 10% FBS and antibiotics. Some commonly used inhibitors of both CFTR and BCRP could not be used due to toxicity at concentrations that block GSH efflux [13]. To alleviate these issues, amiloride an inhibitor of CFTR function and Hoechst 3342, a substrate of BCRP and competitive inhibitor of GSH efflux through BCRP were used. Additionally, the MRP inhibitor MK571 has been shown to be an effective inhibitor of MRP2 function [14]. The 16HBE cells were exposed to 20% CSE and treated with or without amiloride, Hoechst 3342, or MK571 for a total of 48 hours.
Animals
Male C57B/6 mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Male CFTR KO mice that posses the S489X truncation mutation with gut corrected recombinant human CFTR were obtained from our in house colony as previously reported [6]. CFTR KO mice are congenic on a C57B/6 background. Male FVB, BCRP KO, and MRP2 KO mice were obtained from Taconic, both knockout strains are a FVB background and as such, the FVB strain was used as the control strain for these mice. All mice were housed in microisolator cages and given access to water and standard mouse chow ad libitum. All animal experiments were approved by the National Jewish Health IACUC office.
Cigarette smoke exposure
The mice were exposed to CS from Kentucky reference cigarette 3R4F (University of Kentucky) for 5 h as previously described [12]. The average particulate matter was 120 mg/m3 and carbon monoxide was below 350 ppm. Mice were sacrificed 16 h after the CS exposure via cardiac exanguastion and bronchoalveolar lavage (BAL) was performed using two 750 μL rinses of cold isotonic potassium phosphate solution. BAL cells were removed by centrifugation and counted using a Coulter counter and analyzed for glutathione. The dilution of the ELF was calculated by measuring urea in both the BAL fluid (BALF) and plasma as previously reported [12].
Measurement of glutathione
Both total (GSH) and glutathione disulfide (GSSG) were measured spectrophotometrically as previously described [12]. GSH was measured by adding the standard or sample to 100 μL of a 1:1 mixture of 3 units/mL glutathione reductase with 0.67 mg/mL 5,5’-Dithiobis(2-nitrobenzoic acid) (DTNB). The reaction was initiated by the addition of 20 μL of 0.67 mg/mL NADPH and the increase in absorbance at 412 nm was monitored. For GSSG measurements the samples were incubated with 4-vinyl pyridine for 1h to conjugate any reduced GSH prior to analysis, GSSG samples were analyzed the same way as GSH samples. Values measured in BALF are normalized to urea, values in tissues and in vitro are normalized to protein content. The limits of detection were 0.2 μM for both GSH and GSSG.
Statistical analysis
Data is expressed as mean ± standard error. Data was graphed using Prism 5 software (Graphpad). Two-way analysis of variance was performed with Bonferroni post test to compare between Air and CS or WT and KO strain.
Results
Cigarette smoke extract induced mRNA expression
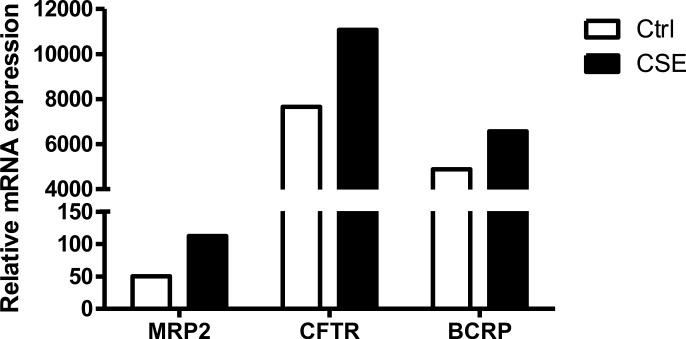
mRNA expression of all transporters in the ABC family was profiled in 16HBE cells under either basal conditions or treatment with cigarette smoke extract (CSE). Due to the xenobiotic response elements that are upstream of a number of genes, most showed increases in mRNA expression (data not shown), therefore to narrow the candidate genes down, only those that have been shown to transport GSH and expressed apically in the lung are shown. This resulted in only three candidate proteins, CFTR, MRP2, and BCRP (Fig 1), for the purpose of investigating the role of apical transport in the lung, all others were not pursued.
Figure 1.
Cigarette smoke extract induces mRNA expression of GSH transporters. Relative mRNA expression in 16HBE cells of the three transporters of GSH known to be expressed apically in the lung. Both basal mRNA levels (open bars) and CSE induced mRNA levels (closed bars) of various transporters were examined. Values were normalized to glyceraldehydes-3-phosphate dehydrogenase standard curve and are representative of the pooled samples of four individual replicates.
Inhibition of CFTR, BCRP, and MRP2 in vitro
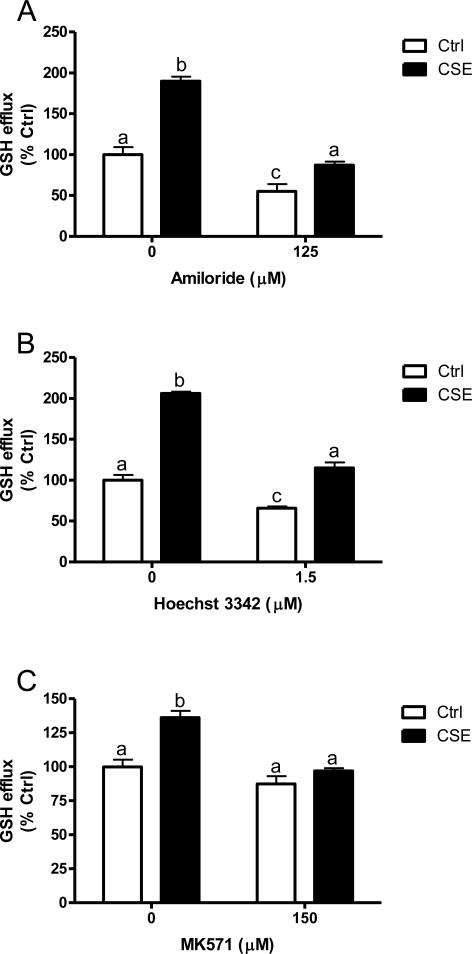
Based on the increase in mRNA expression, which led to three potential targets, the effect of CFTR, BCRP, or MRP2 function on the transport of GSH in vitro was examined. Cells were treated with either 125 μM amiloride to inhibit CFTR (Fig 2A), 1.5 μM Hoechst 33342 to inhibit BCRP (Fig 2B) or 150 μM MK571 to inhibit MRP2 (Fig 2C) and the GSH efflux under basal and CSE stimulated conditions was examined. Under basal conditions the inhibition of CFTR resulted in 55% GSH efflux, BCRP inhibition resulted in 65% efflux and MRP2 inhibition resulted in 87% GSH efflux. Under CSE stimulation alone GSH efflux ranged from 150-200% of control levels while CFTR inhibition resulted in 45%, BCRP inhibition resulted in 65% and MRP2 inhibition resulted in 70% of the CSE induced GSH efflux. This data suggests that all three may be involved in GSH export in response to CSE with some such as CFTR and BCRP being involved in maintaining basal levels while MRP2 may only be involved as a stress response.
Figure 2.
GSH efflux with the inhibition of CFTR, BCRP, or MRP2. 16HBE cells were treated with 125 μM Amiloride to inhibit CFTR (A), 1.5 μM Hoechst 33342 to inhibit BCRP (B) or 150 μM MK571 to inhibit MRP2 (C) in the presence or absence of 20% CSE (closed bars). Extracellular GSH was analyzed after 48 h treatment. Data represented as mean ± SEM, different letters denote significant differences (p < 0.05) between groups.
Effect of CFTR, BCRP, or MRP2 deficiency on ELF GSH
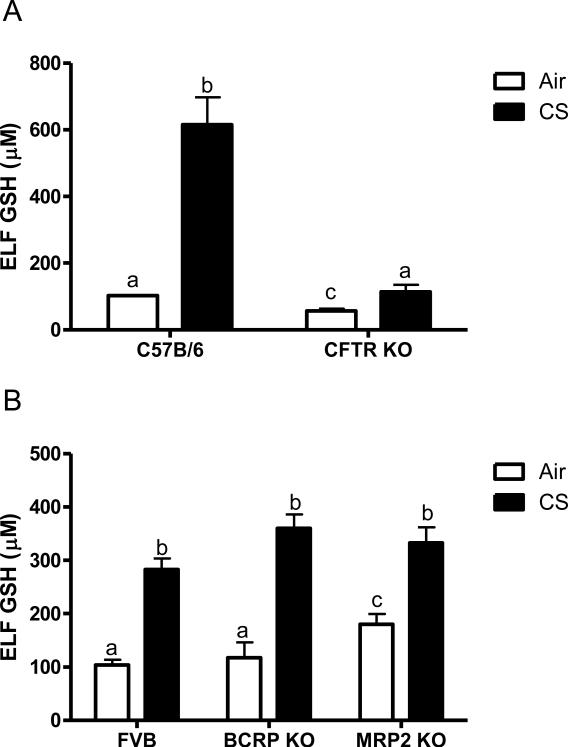
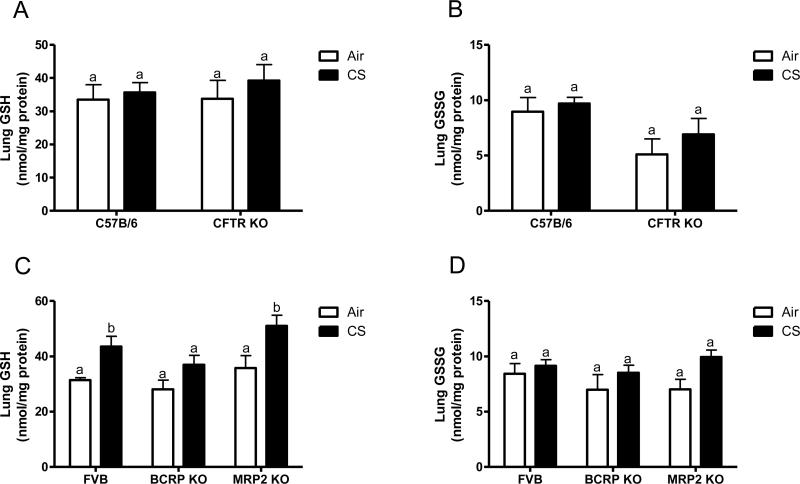
In addition to contributing to altered chloride conductance across membranes, CFTR is known to have a negative impact on GSH levels in the epithelial lining fluid (ELF) [8]. CFTR KO mice have been backcrossed onto a C57B6 background in contrast to the BCRP, and MRP2 KO mice which are an FVB background strain. In comparison to the wild type C57B6 mice the CFTR KO mice have half the levels of GSH in the ELF under basal conditions (Fig 3A). In contrast neither the BCRP KO or MRP2 KO mice have altered basal levels of GSH in the ELF (Fig 3B).
Figure 3.
Epithelial lining fluid (ELF) GSH is only affected by CFTR. The ELF GSH was analyzed in vivo when exposed to air (open bars) or acute cigarette smoke (closed bars). CFTR KO mice were compared to the C57B/6 background strain (A) while the BCRP and MRP2 KO mice were compared to the FVB background strain (B). Data represented as mean ± SEM, different letters denote significant differences (p < 0.05) between groups.
Arguably one of the most important functions of GSH is to protect the lung from oxidant mediated damage, and to increase and maintain high levels of GSH in the ELF requires transport. Therefore the ability of the CFTR, BCRP and MRP2 KO mice to mount an adaptive response to cigarette smoke was examined (closed bars). Interestingly the CFTR KO mice can increase the levels of ELF GSH when exposed to CS, but only up to 100 μM compared with the C57B6 which have increases up to 600 μM. In contrast both the BCRP and MRP2 KO mice were able to increase the ELF GSH to similar levels as the FVB CS exposed mice. Due to the dilution of the ELF the GSSG levels were below detection.
CS exposure increases BAL cell GSH independent of transport expression
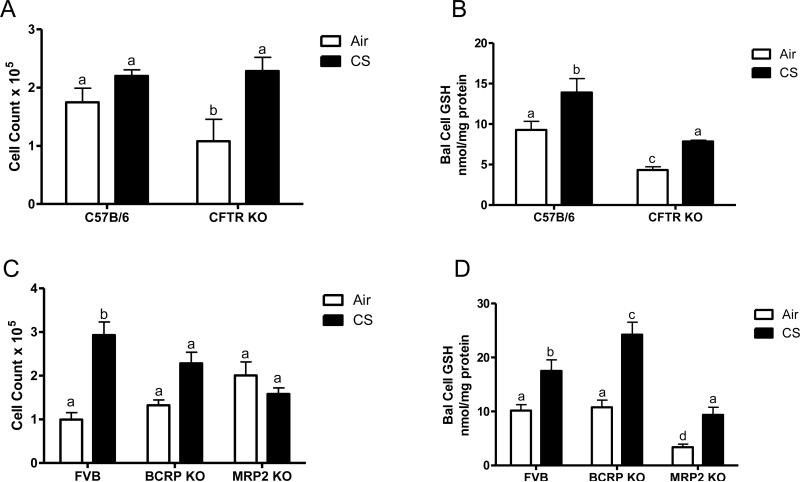
The number of BAL cells found in the airways can be an indicator of a higher degree of stress in the lungs. The number of cells was initially lower in the CFTR KO mice compared to the C57B6, but with the CS exposure there was no increase in cell number in the C57B6 mice while there was a significant increase in the CFTR KO mice (Fig 4A). In contrast, the BCRP KO mice had similar cell numbers as the FVB control while the MRP2 KO mice had slightly elevated, but comparable number of cells as the air controls (Fig 4C). In contrast, both the FVB and BCRP KO mice had higher number of cells with the CS exposure, while there was not any change in the cell count of the MRP2 KO mice.
Figure 4.
Analysis of BAL cell number and GSH content in the CFTR, BCRP, and MRP2 KO mice. The cell count was analyzed in the CFTR (A), BCRP and MRP2 (C) and the respective controls from the cells recovered during bronchalveolar lavage. Furthermore, the BAL cell GSH was also analyzed in the same BAL cells (B and D). Data represented as mean ± SEM, different letters denote significant differences (p < 0.05) between groups.
The vast majority of the BAL cells recovered were macrophages (data not shown), and intracellular GSH can be a major determinant of the sensitivity of the macrophages to becoming activated and releasing proinflammatory cytokines [15], therefore the BAL cell GSH was also examined. Similar to the ELF GSH levels, the CFTR KO mice had roughly half of the amount of intracellular BAL cell GSH as the C57B6 mice and while the CS exposure did cause an increase, the CFTR KO mice still had lower levels than the C57B6 mice (Fig 4B). On the other hand the FVB and BCRP KO mice had similar basal levels of BAL cell GSH and a similar increase with the CS exposure while the MRP2 KO mice had lower levels than either the FVB or BCRP KO mice (Fig 4D).
Lung tissue GSH is not affected by transporter deficiency
By affecting the ability to transport GSH into the apical space it is conceivable that the intracellular levels of GSH may in fact be higher than normal. When the lung tissue GSH (Fig 5A) and GSSG (Fig 5B) was examined in the C57B6 and CFTR KO mice there was no difference between the two strains. Additionally neither the BCRP or MRP2 KO mice had different basal levels of lung GSH (Fig 5C) or GSSG (Fig 5D). Interestingly the FVB and MRP2 KO mice did have a small increase in lung GSH when exposed to CS, while there was no change with the BCRP KO mice.
Figure 5.
Transporter deficiencies do not affect lung tissue GSH or GSSG. Lung tissue GSH (A and C) and GSSG (B and D) was analyzed in the CFTR, BCRP, and MRP2 KO mice and their respective controls exposed to air (open bars) or CS (closed bars). Data represented as mean ± SEM, different letters denote significant differences (p < 0.05) between groups.
Discussion
Glutathione and its functions in biological systems has been investigated for years, and yet there is little known about its regulation in the extracellular space, especially in the epithelial lining fluid of the lung. GSH in the plasma, another extracellular compartment, is thought to be secondary to cysteine/cystine levels, which is viewed as the predominant thiol, due to the low levels and high oxidization of GSH [16]. However, the thiol balance is reversed in the ELF; GSH is the predominant thiol and cysteine by contrast is not as concentrated. Therefore it stands to reason that GSH is playing more of a functional role in the ELF versus the plasma. While the increase in ELF GSH in response to stimuli, such as inhaled oxidants like CS or even pathogens, is well established the transporter(s) involved in maintaining or increasing ELF GSH are not well known.
CFTR is the only known apical transporter to have a role in the transport of GSH in the lung and yet others have found that CFTR expression and function decline with CSE [17, 18]. If CFTR is decreasing, and remaining low, then its inhibition or deletion should not affect the ability to transport GSH. The current study shows the exact opposite, either the inhibition or deletion of CFTR had drastic effects on the ability to export GSH, resulting in at least 50% or greater reduction in extracellular GSH. One way to rationalize why some groups have seen decreases in CFTR may be the time between treatment and examination of CFTR expression. Under conditions that have shown decreased CFTR expression and function with CSE, these were measured shortly after the exposure [17, 19], whereas the current study examines GSH transport after a longer time point. In contrast to others, the current study shows that CFTR gene expression is increased with CSE after a 48 h CSE exposure. It is conceivable that acutely CFTR might be decreased, but as the cell upregulates antioxidant adaptive responses CFTR expression is induced as well.
Secondly, several studies have shown that oxidants and cigarette smoke alike can inactivate CFTR [17, 20]. These studies are based on decreased nasal transepithelial potential differences or cAMP dependent 125I efflux [17]. For one, the function of CFTR in the nasal epithelium would not necessarily be a predictor of the function of CFTR in the lung. The environments that nasal epithelium and lung epithelium are exposed to, even in smokers, can be vastly different and it is conceivable the nasal epithelium could be exposed to much higher concentrations of CS than that of the lung epithelium. One of the most likely causes of the decreased function would be the increase in heavy metals found in CS, particularly cadmium, which has been shown to inactivate CFTR [21]. Furthermore, it may be possible that other anions may block the efflux of 125I, and once properly cleared, CFTR function would regain [22, 23].
One interesting aspect of CFTR function that has been proposed is the formation of a complex with MRP2 and other scaffolding proteins [24]. It has been suggested that CFTR by forming a complex with MRP2 could actually increase MRP2 function. This certainly could have implications in transporter function if CFTR truly is affecting MRP2 function, especially with the deletion of CFTR. In vitro, MRP2 seemed to function as a stress response protein, in which it contributed to the increase in GSH in response to CSE, however in vivo the same trend did not occur. In vivo, MRP2 seemed to have no effect on GSH transport into the ELF. This could be due to several reasons, yet the most plausible is that MRP2 is not expressed widely enough throughout the lung to have an effect on the overall pool of GSH [25], which is highlighted by the low mRNA expression levels in our human bronchial epithelial cell line.
Another transporter protein that was investigated as a potential contributor to the ELF GSH pool was BCRP. Unlike both CFTR and MRP2, BCRP is a member of the G-subfamily of transporters and until recently was not known to transport GSH [10]. However, there is evidence to suggest that it is apically located in the lung and coupled with the recent discovery that it transports GSH provided another target to investigate [10, 26]. Based on in vitro data, BCRP could be involved in both maintaining basal levels of GSH as well as in response to oxidants. Similar to MRP2 though, in vivo, BCRP had little effect on either basal levels or CS induced levels of ELF GSH. A similar explanation of why BCRP may not affect ELF GSH levels is that its expression level, is fairly low, and relegated mainly to a side population of stem cells in the lung [25, 27, 28]. The low expression of both BCRP and MRP2 is highlighted by the inability to detect protein expression of either in normal lung tissue by us and others [29]. Additionally there are limitations to the inhibitors used and the lack of specificity of both the BCRP and MRP2 inhibitors as well as altered expression in cell lines in vitro may be other explanations in the differential effects in vitro versus in vivo. While BCRP does transport GSH, the amount of cells in the lung that express it may not be high enough to change the overall concentration of ELF GSH. Since BCRP and MRP2 do not influence the ELF GSH levels and the ability to adapt to an oxidant insult, CFTR is still the major contributor to both the basal levels of ELF GSH as well as the GSH adaptive response to CS.
There are numerous different animal models used to study the involvement of CFTR, many of which focus primarily on the progression of Cystic Fibrosis [30]. While the study of this disease is important, it is also important to note that there is spontaneous inflammation, oxidative stress, and neutrophil infiltration that occur in many of these animal models [31]. This spontaneous occurrence of inflammation and oxidative stress can actually cause changes in GSH levels and potentially induce an adaptive response. The CFTR KO mouse model used in the present study does not develop spontaneous inflammation, like other CFTR KO models, which makes it an ideal system to study the effects of CFTR transport without the confounding effects of neutrophil infiltration and excess inflammation in the airways. While CFTR is certainly not the only transporter involved in maintaining or increasing ELF GSH, as evidence by the increase in ELF GSH with CS in CFTR KO mice, of the three apically expressed transporters in the lung it is clearly the dominant one. Other transporters may come to light in their ability to transport GSH, others may include members of the organic anion transport proteins (OATP) or even other ABC transporters that have not been characterized for the ability to transport GSH as of yet [4, 32]. In any instance it is clear that CFTR is responsible for maintaining the majority of the basal ELF GSH as well as at least a part of the CS induced adaptive response.
Highlights.
Cigarette smoke (CS) exposure induces an increase in the lung ELF GSH levels.
CFTR mediates 50% of the ELF GSH under both basal and CS exposure.
Loss of GSH transporting proteins BCRP and MRP2 had no affects on ELF GSH levels.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jones DP, Carlson JL, Mody VC, Cai J, Lynn MJ, Sternberg P. Redox state of glutathione in human plasma. Free Radic Biol Med. 2000;28:625–635. doi: 10.1016/s0891-5849(99)00275-0. [DOI] [PubMed] [Google Scholar]
- 2.Gould NS, Day BJ. Targeting maladaptive glutathione responses in lung disease. Biochem Pharmacol. 2010 doi: 10.1016/j.bcp.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awasthi YC, Chaudhary P, Vatsyayan R, Sharma A, Awasthi S, Sharma R. Physiological and pharmacological significance of glutathione-conjugate transport. J Toxicol Environ Health B Crit Rev. 2009;12:540–551. doi: 10.1080/10937400903358975. [DOI] [PubMed] [Google Scholar]
- 4.Ballatori N, Krance SM, Marchan R, Hammond CL. Plasma membrane glutathione transporters and their roles in cell physiology and pathophysiology. Mol Aspects Med. 2009;30:13–28. doi: 10.1016/j.mam.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day BJ, van Heeckeren AM, Min E, Velsor LW. Role for cystic fibrosis transmembrane conductance regulator protein in a glutathione response to bronchopulmonary pseudomonas infection. Infect Immun. 2004;72:2045–2051. doi: 10.1128/IAI.72.4.2045-2051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gould NS, Gauthier S, Kariya CT, Min E, Huang J, Day BJ. Hypertonic saline increases lung epithelial lining fluid glutathione and thiocyanate: two protective CFTR-dependent thiols against oxidative injury. Respir Res. 2010;11:119. doi: 10.1186/1465-9921-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kariya C, Chu HW, Huang J, Leitner H, Martin RJ, Day BJ. Mycoplasma pneumoniae infection and environmental tobacco smoke inhibit lung glutathione adaptive responses and increase oxidative stress. Infect Immun. 2008;76:4455–4462. doi: 10.1128/IAI.00136-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velsor LW, van Heeckeren A, Day BJ. Antioxidant imbalance in the lungs of cystic fibrosis transmembrane conductance regulator protein mutant mice. Am J Physiol Lung Cell Mol Physiol. 2001;281:L31–38. doi: 10.1152/ajplung.2001.281.1.L31. [DOI] [PubMed] [Google Scholar]
- 9.Kariya C, Leitner H, Min E, van Heeckeren C, van Heeckeren A, Day BJ. A role for CFTR in the elevation of glutathione levels in the lung by oral glutathione administration. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1590–1597. doi: 10.1152/ajplung.00365.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brechbuhl HM, Gould N, Kachadourian R, Riekhof WR, Voelker DR, Day BJ. Glutathione transport is a unique function of the ATP-binding cassette protein ABCG2. J Biol Chem. 2010;285:16582–16587. doi: 10.1074/jbc.M109.090506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cherrington NJ, Hartley DP, Li N, Johnson DR, Klaassen CD. Organ distribution of multidrug resistance proteins 1, 2, and 3 (Mrp1, 2, and 3) mRNA and hepatic induction of Mrp3 by constitutive androstane receptor activators in rats. J Pharmacol Exp Ther. 2002;300:97–104. doi: 10.1124/jpet.300.1.97. [DOI] [PubMed] [Google Scholar]
- 12.Gould NS, Min E, Gauthier S, Chu HW, Martin R, Day BJ. Aging adversely affects the cigarette smoke-induced glutathione adaptive response in the lung. Am J Respir Crit Care Med. 2010;182:1114–1122. doi: 10.1164/rccm.201003-0442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pick A, Klinkhammer W, Wiese M. Specific inhibitors of the breast cancer resistance protein (BCRP). ChemMedChem. 2010;5:1498–1505. doi: 10.1002/cmdc.201000216. [DOI] [PubMed] [Google Scholar]
- 14.Wortelboer HM, Usta M, van Zanden JJ, van Bladeren PJ, Rietjens IM, Cnubben NH. Inhibition of multidrug resistance proteins MRP1 and MRP2 by a series of alpha,beta-unsaturated carbonyl compounds. Biochem Pharmacol. 2005;69:1879–1890. doi: 10.1016/j.bcp.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Gould NS, Min E, Day BJ. Macropinocytosis of Extracellular Glutathione Ameliorates Tumor Necrosis Factor alpha Release in Activated Macrophages. PLoS One. 2011;6:e25704. doi: 10.1371/journal.pone.0025704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Go YM, Jones DP. Cysteine/cystine redox signaling in cardiovascular disease. Free Radic Biol Med. 2011;50:495–509. doi: 10.1016/j.freeradbiomed.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, Zielenski J, Durie P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med. 2006;173:1139–1144. doi: 10.1164/rccm.200508-1330OC. [DOI] [PubMed] [Google Scholar]
- 18.Kogan I, Ramjeesingh M, Li C, Kidd JF, Wang Y, Leslie EM, Cole SP, Bear CE. CFTR directly mediates nucleotide-regulated glutathione flux. EMBO J. 2003;22:1981–1989. doi: 10.1093/emboj/cdg194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Virgin FW, Azbell C, Schuster D, Sunde J, Zhang S, Sorscher EJ, Woodworth BA. Exposure to cigarette smoke condensate reduces calcium activated chloride channel transport in primary sinonasal epithelial cultures. Laryngoscope. 2010;120:1465–1469. doi: 10.1002/lary.20930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cantin AM, Bilodeau G, Ouellet C, Liao J, Hanrahan JW. Oxidant stress suppresses CFTR expression. Am J Physiol Cell Physiol. 2006;290:C262–270. doi: 10.1152/ajpcell.00070.2005. [DOI] [PubMed] [Google Scholar]
- 21.Rennolds J, Butler S, Maloney K, Boyaka PN, Davis IC, Knoell DL, Parinandi NL, Cormet-Boyaka E. Cadmium regulates the expression of the CFTR chloride channel in human airway epithelial cells. Toxicol Sci. 2010;116:349–358. doi: 10.1093/toxsci/kfq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linsdell P, Hanrahan JW. Flickery block of single CFTR chloride channels by intracellular anions and osmolytes. Am J Physiol. 1996;271:C628–634. doi: 10.1152/ajpcell.1996.271.2.C628. [DOI] [PubMed] [Google Scholar]
- 23.Linsdell P, Tabcharani JA, Hanrahan JW. Multi-Ion mechanism for ion permeation and block in the cystic fibrosis transmembrane conductance regulator chloride channel. J Gen Physiol. 1997;110:365–377. doi: 10.1085/jgp.110.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li C, Schuetz JD, Naren AP. Tobacco carcinogen NNK transporter MRP2 regulates CFTR function in lung epithelia: implications for lung cancer. Cancer Lett. 2010;292:246–253. doi: 10.1016/j.canlet.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Deen M, de Vries EG, Timens W, Scheper RJ, Timmer-Bosscha H, Postma DS. ATP-binding cassette (ABC) transporters in normal and pathological lung. Respir Res. 2005;6:59. doi: 10.1186/1465-9921-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leitner HM, Kachadourian R, Day BJ. Harnessing drug resistance: using ABC transporter proteins to target cancer cells. Biochem Pharmacol. 2007;74:1677–1685. doi: 10.1016/j.bcp.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salcido CD, Larochelle A, Taylor BJ, Dunbar CE, Varticovski L. Molecular characterisation of side population cells with cancer stem cell-like characteristics in small-cell lung cancer. Br J Cancer. 2010;102:1636–1644. doi: 10.1038/sj.bjc.6605668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith PJ, Furon E, Wiltshire M, Campbell L, Feeney GP, Snyder RD, Errington RJ. ABCG2-associated resistance to Hoechst 33342 and topotecan in a murine cell model with constitutive expression of side population characteristics. Cytometry A. 2009;75:924–933. doi: 10.1002/cyto.a.20800. [DOI] [PubMed] [Google Scholar]
- 29.Scheffer GL, Pijnenborg AC, Smit EF, Muller M, Postma DS, Timens W, van der Valk P, de Vries EG, Scheper RJ. Multidrug resistance related molecules in human and murine lung. J Clin Pathol. 2002;55:332–339. doi: 10.1136/jcp.55.5.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kukavica-Ibrulj I, Levesque RC. Animal models of chronic lung infection with Pseudomonas aeruginosa: useful tools for cystic fibrosis studies. Lab Anim. 2008;42:389–412. doi: 10.1258/la.2007.06014e. [DOI] [PubMed] [Google Scholar]
- 31.Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, Hanfland RA, Wohlford-Lenane C, Dohrn CL, Bartlett JA, Nelson GA, Chang EH, Taft PJ, Ludwig PS, Estin M, Hornick EE, Launspach JL, Samuel M, Rokhlina T, Karp PH, Ostedgaard LS, Uc A, Starner TD, Horswill AR, Brogden KA, Prather RS, Richter SS, Shilyansky J, McCray PB, Jr., Zabner J, Welsh MJ. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med. 2010;2:29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ballatori N, Hammond CL, Cunningham JB, Krance SM, Marchan R. Molecular mechanisms of reduced glutathione transport: role of the MRP/CFTR/ABCC and OATP/SLC21A families of membrane proteins. Toxicol Appl Pharmacol. 2005;204:238–255. doi: 10.1016/j.taap.2004.09.008. [DOI] [PubMed] [Google Scholar]