Abstract
MarA activates two membrane dependent mechanisms of resistance to different antibiotics, such as ciprofloxacin and tetracycline, including promotion of outflux and inhibition of influx of antibiotics. Thus, MarA causes multiple antibiotic resistance phenotype. The activation of these mechanisms needs overexpression of marA. This could happen through mutation in marR. Thus, the aim of this study was to measure marA expression in ciprofloxacin resistant E. coli gyrA mutants and clones with or without marR mutation. For this purpose, real time PCR was used to measure relative expression of marA in above mutants and clones. Results showed that two clones, C14 and C17 overexpressed marA. It is concluded that the level of marA expression is important for activation of above mechanisms.
Key Words: acrAB operon, gyrA mutants, marA gene, marR mutation
Introduction
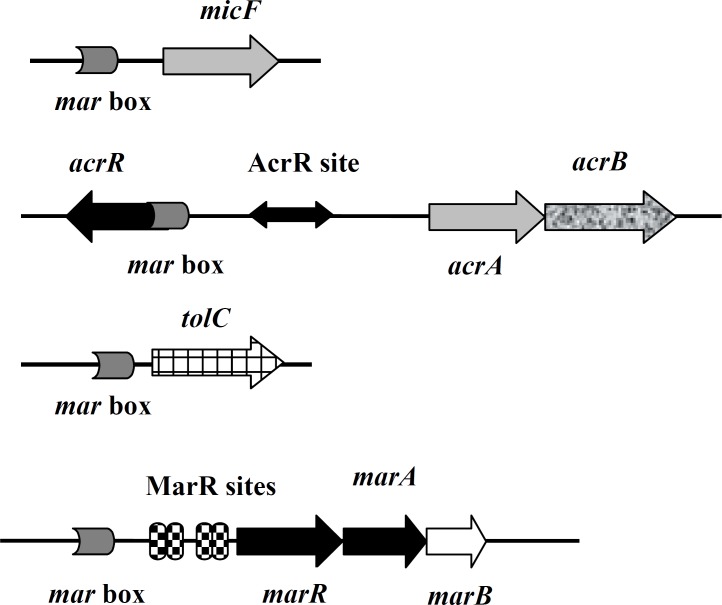
MarA is a member of the AraC family of transcriptional activators found in gram negative bacteria, including E. coli (1, 2). It activates its own transcription and that of mar regulon genes by binding as a monomer to 20 base pair asymmetric sequences known as marboxes via its N-terminal helix-turn-helix (HTH) motif involved in interaction with DNA (3, 4). These marboxes are located upstream of -35 site of the promoters of target genes (5). One of these target genes is micF, which produces an antisense RNA (Figure 1) that downregulates the expression of ompF gene by base pairing to its mRNA (6). ompF encodes an outer membrane protein called OmpF (7). This is a drug entry site for fluoroquinolones (8). Members of this family of antibiotics, such as ciprofolaxin are the antibiotic of choice for treatment of E. coli infections (8, 9).
Figure 1.
Operons and genes under the positive control of MarA and negative control of AcrR and MarR. Modified and adapted from Dzwokai et al (12).
The other examples of MarA target genes are acrAB operon and tolC which altogether encode a membrane three component efflux transporter called AcrAB-TolC (10). This extrudes drugs such as ciprofloxacin and tetracycline out of cells (10). AcrR is the repressor of acrAB operon (11). Its encoding gene is located upstream of this operon in the opposite direction (Figure 1).
Taken together, downregulation of OmpF and upregulation of AcrAB-TolC by overexpression of MarA make the cells resistant to multiple irrelevant antibiotics, including ciprofloxacin, tetracycline and chloramphenicol (3, 13, 14).
Normally, marA expression is suppressed by MarR which is encoded by marR. This along with marA, is a member of marRAB operon. MarR binds as a dimer to two sites (site I and site II) in operator region of this operon (marO) which is downstream of marbox (Figure 1). Site I is located between -35 and -10 region while site II is near translation start site. Site I is important for repression as deletion of it leads to MDR phenotype (15).
Attachment of different ligands, such as antibiotics to MarR repressor dissociates it from the operator site of marRAB operon (16). Then, binding of MarA to mar box activates the expression of marRAB operon (4, 5). Overexpression of marA could happen via mutations in marR (3, 15). In the previous study we described gyrA mutants with or without a mutation in marR (17). These mutants were ciprofloxacin and slightly tetracycline resistant. Their derivative clones posses intermediate levels of resistance to tetracycline (submitted for publication). To understand whether or not marA overexpresses, the level of marA expression was measured by real time PCR in these mutants and clones.
Experimental
Antimicrobial agent and chemicals
Tetracycline hydrochloride (Tc) was obtained from Sigma to induce MDR phenotype. Stock solution was 4 mg/mL.
Bacterial strain and mutants
MG1655 was parent strain. gyrA mutants with or without a mutation in marR gene isolated in previous work (17) are listed in Table 1.
Table 1.
Bacterial strain and mutants
| Strain/Mutant/Clone | Relevant properties |
MIC
|
Source/Reference | |
|---|---|---|---|---|
| Cip (µg/ml) | Tc (µg/ml) | |||
| MG1655 | Wild type | 0.035 | 3 | A gift from Prof. R. G. Lloyd |
| W25 | Wild type; gyrA (Ser83→Leu) and marR (Met74→Thr) | 0.075 | 4 | (17, 18) |
| W26 | Wild type; gyrA (Ser83→Leu) | 0.075 | 4 | (17, 18) |
| W49 | Wild type; gyrA and marOR (20 bp duplication in operator) | 0.625 | 4 | (17, 18) |
| C6 | W25; selected on tetracycline (5 µg/ml) | 1 | 45 | Submitted for publication |
| C14 | W26; selected on tetracycline (5 µg/ml) | 1 | 30 | Submitted for publication |
| C17 | W49; selected on tetracycline (5 µg/ml) | 1 | 30 | Submitted for publication |
Cip and Tc are abbreviations for ciprofloxacin and tertracycline, respectively
As mentioned previously mutants W25, W26 and W49 were isolated from cultivation of wild type strain on LBA containing ciprofloxacin (18). Clones C6, C14 and C17 were derived from cultivation of above mutants on LBA agar in the presence of Tc (submitted for publication). It was explained that resistance to ciprofloxacin can be divided to three levels, including low levels of resistance (MIC: 0.063 to 1 μg/mL), intermediate levels of resistance (MIC: 1 to 32 μg/mL) and high levels of resistance (MIC: >32 μg/mL) (19). Additionally, It was described that resistance to tetracycline can also be divided to three levels, including low levels of resistance (MIC: 1 to 10 μg/mL), intermediate levels of resistance (MIC: 10 to 50 μg/mL) and high levels of resistance (MIC: >50 μg/mL) (20). Based on above definitions mutants have low to intermediate levels of resistance to ciprofloxacin and tetracycline.
Media
LB broth (Merck) and LBA containing 1.5% agar (Merck) were used for cultivation of strain and mutants.
Expression analysis of marA
Real time PCR was used to quantify gene expression of marA and gapA as housekeeping gene. Overnight cultures on LB broth were grown on LB broth plus 3 μg/mL Tc (except for wild type) at 37ºC with shaking at 150 rpm to mid-logarithmic phase (OD600 of 0.6), as described previously (13, 21). Before extraction of total RNA, each culture was stabilized by RNA protect bacterial reagent (Qiagen, Germany) and then pelleted by centrifugation (Sigma, Germany). RNA was extracted immediately after lysozyme-proteinase K digestion of bacteria using an RNeasy Mini Kit (Qiagen, Germany). Contaminating genomic DNA was digested by RNase-free DNase I (Fermentas, Life science research) according to the manufacturer’s instruction (Fermentas, Life science research). RNA purity and concentration was estimated at OD260 by spectrophotometer (Ultrospec 1100, Amersham Pharmacia Biothech). Reverse transcription was conducted using the RevertAid Reverse Transcriptase kit (Fermentas, Life science research), random hexamer and Purified total RNA (2 μg). The negative controls without reverse transcriptase were used to confirm the lack of contaminating DNA in the RNA samples. The cDNAs obtained from reverse transcription and negative controls were amplified by PCR reaction to first verify that negative controls do not produce PCR products and second to find the best annealing temperature for real time PCR. Then, diluted cDNA (2 μL of a 1:10), obtained from reverse transcriptase, were used to quantify the level of marA and gapA with specific primers as mentioned in Table 2 by real time PCR in a Rotor Gene 6000 thermocycler (Corbett Research, Australia) using a SYBR Green kit (Takara, Japan). Serial dilutions of marA and gapA cDNAs were used as standards in real time PCR reactions. Thermal cycling conditions were described previously (21). Relative gene expression was calculated using the efficiency method pfaffl (ratio of marA expression to gapA expression) (22). All data on marA expression are the average of triplicate analyses. The data were recorded as mean ± SD.
Table 2.
List of primers
| Reference | Length of amplicon (bp) | Primer sequence (5′-3′) | Gene |
|---|---|---|---|
| (21) | 187 | CATAGCATTTTGGACTGGAT | marA |
| TACTTTCCTTCAGCTTTTGC | |||
| (21) | 170 | ACTTACGAGCAGATCAAAGC | gapA |
| AGTTTCACGAAGTTGTCGTT |
Statistical analysis
Statistically significant differences in gene expression were determined by Student’s t test (two paired samples, with two tailed distribution), using SPSS version 16 software.
Results
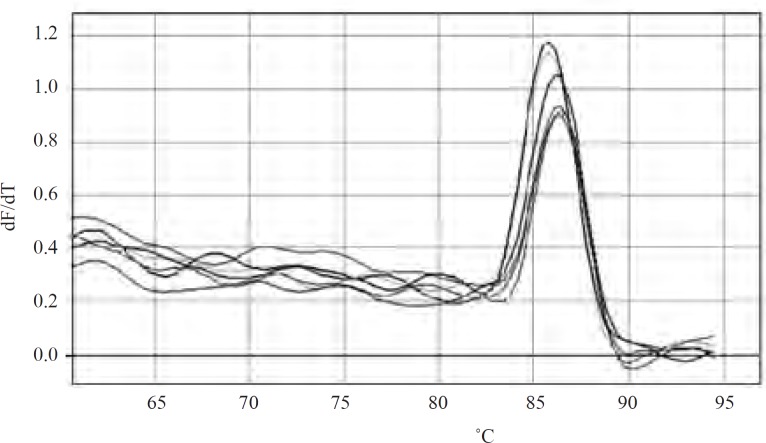
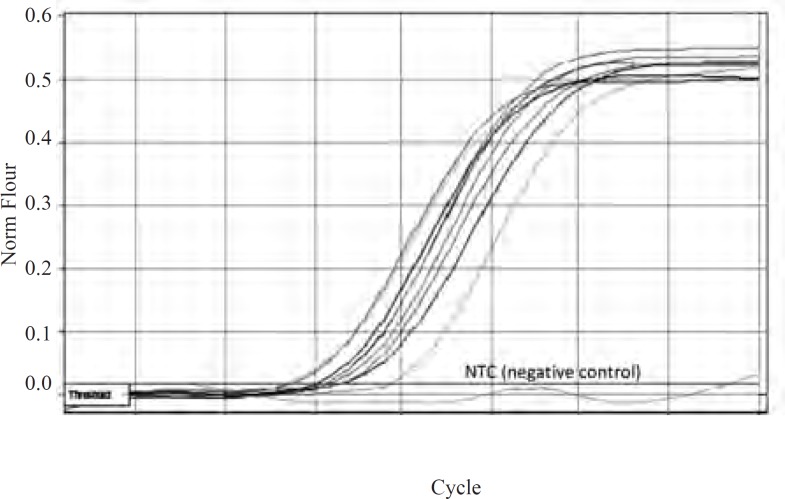
As mutations used in this study harbor either or not a mutation in marR, it was possible that they increase marA expression. All negative controls without reverse transcriptase did not show bands on gel after PCR amplification and gel electrophoresis. Thus, all RNA samples were purified and without DNA contamination. They were used for real time analysis. The suitable annealing temperature was 52˚C as the result of gel electrophoresis following PCR reaction showed (Figure 2). Data derived from standard curves of marA and gapA showed that the efficiency of marA and gapA were 1.92 and 2.1, respectively and coefficient of determination (r2) of marA and gapA were 0.996 and 0.999, respectively. The melting curve of two genes showed just one major peak which indicates the purity of samples. Figure 3 shows the melting curve of marA. The melting point of marA and gapA was 86 degree centigrade. Figure 4 shows the amplification curve of marA. Cts (treshhold cycles) of control strain and mutants obtained from this figure and amplification curve of gapA used to calculate relative marA expression. Table 3 shows the marA relative expression in these mutants. The T-test analysis showed no significant difference between wild type and original mutants for expression of marA (p < 0.05). The reason for this result may due to low level of resistance to Tc. However, for two clones (C14 and C17) this comparison showed significant difference (p < 0.05).
Figure 2.
Gel analysis of PCR product. Lane M and A contain 1 kb DNA ladder and marA PCR product, respectively
ّFigure 3.
Melting curves of marA in wild type and mutants. The yellow color curve belongs to wild type and other colored curves belong to mutants
Figure 4.
Amplification curves of marA in wild type and mutants. The pale blue curve belongs to wild type and other colored curves belong to mutants
Table 3.
Relative expression of marA in wild type (MG1655) and mutants as determined by real time PCR
| Strain/mutant/clone | Relative expression |
|---|---|
| Wild type (MG1655) | 1±0 |
| W25 | 0.9±0.04 |
| W26 | 0.92±0.02 |
| W49 | 0.956±0.02 |
| C6 | 0.97±0.015 |
| C14 | 2.83±0.05 |
| C17 | 3.21±0.04 |
Expression relative to MG1655, mean values from three independent experiments. Figures are the ratio of gene expression between the target gene (marA) and the reference gene (gapA). An effect on gene expression was considered significant when the corresponding ratios were >2 or <0.6 with a P value of less than 0.05.
Discussion
MarA regulates two membrane dependent mechanisms of resistance to fluoroquinolones and also other structurally irrelevant antibiotics (14). These mechanisms cause multiple antibiotic resistance phenotype and mainly associated to decreased entrance of antibiotics because of low synthesis of OmpF and extrusion of drugs via over activation of AcrAB-TolC pump (14).
Normally, the expression of MarA encoded by marA, is inhibited by MarR repressor (4). However, mutation in its encoding gene, marR, alleviates this suppression (15). In the previous study, it was revealed that some gyrA mutants with different MIC for ciprofloxacin had either or not a mutation in marR (17). These mutants and their derivative clones were more or less resistant to tetracycline as well. To study the contribution of MarA regulator to ciprofloxacin and tetracycline resistance, the level of marA expression was measured by real time PCR in these gyrA mutants and clones.
It was shown that the binding of ligands, such as Tc to MarR leads to dissociation of MarR from the operator site of this operon (16). The finding that none of original mutants especially those with mutations in marOR overexpresses marA may imply that occurrence of mutation in marR is not enough by itself to eliminate MarR repression and complete derepression of marRAB operon still needs long exposure to Tc.
On the other hand, it was found that clones C14 and C17 with lower MIC for Tc than clone C6 (Table 1) had marA overexpression. This may imply that besides the exposure to Tc, the genetic background is also important for induction of marRAB operon. However, it was found that C14 and C17 could not overexpress acrA and micF (submitted for publication).
The finding that clone C17 with mutation in operator site of marRAB operon could overexpress marA is consistent with previous finding obtained for this type of mutation (15). Also it was shown that the replacement of Met with another hydrophobic amino acid (Ile) could cause marA overexpression (23). However, the finding that C6 could not overexpress marA reveals that substitution of hydrophobic amino acid (Met) with hydrophilic one (Thr) at codon 74 may not provide suitable change for dissociation of MarR from DNA at first and further induction with Tc is needed for complete depression.
In the parallel studies it was found W26 and its clone C14 had a mutation in acrR gene encoding AcrR repressor of acrAB operon (submitted for publication). This mutation caused alteration of Arg to Cys which was shown to increase the expression of acrB (11). However, W26 and C14 did not enhance the expression of acrA (submitted for publication). This is confirmed by another finding that W26 and C14 could not tolerate cyclohexane (17 & submitted for publication). It was shown that overexpression of acrAB-tolC is necessary for cyclohexane tolerance (24).
Moreover, overexpression of acrAB and micF was reported either in mutants with higher MIC for Tc or in the presence of higher concentration of Tc. It was shown that providing these situations caused more marA overexpression (21).
Taken together, it is suggested that overexpression of acrAB operon and micF is not just dependent on overexpression of marA. The level of marA overexpression is also important for activation of acrAB operon and micF. This is consistent with the findings of previous work that showed the extent to which genes of the marA regulon are activated is a function of MarA concentration (25). Higher levels of marA overexpression might gain in clones with the same genetic background and higher MIC for tetracycline.
Acknowledgements
This work was financially supported by the University of Shahrekord. We thank Prof. R. G. Lloyd for kind gift of MG1655.
References
- 1.Rhee S, Martin RG, Rosner JL, Davis DR. A novel DNA binding motif in MarA: the first structure for an AraC family transcriptional activator. Proc. Natl. Acad. Sci. USA. 1998;95:1043–1048. doi: 10.1073/pnas.95.18.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alekshum MN, Levy SB. The Escherichia coli mar locus antibiotic resistance and more. ASM News. 2004;70:451–456. [Google Scholar]
- 3.Grkovic S, Brown MH, Skurray RA. Regulation of bacterial drug export systems. Microbiol and Mol. Biol. Rev. 2002;66:671–701. doi: 10.1128/MMBR.66.4.671-701.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin RG, Jair KW, Wolf RE, Rosner JL. Autoactivation of the marRAB multiple antibiotic resistance operon by the MarA transcriptional activator in Escherichia coli. J. Bacteriol. 1996;178:2216–2223. doi: 10.1128/jb.178.8.2216-2223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin RG, Gillette WK, Rhee S, Rosner JL. Structure requirements for marbox function in transcriptional activation of mar/sox/rob regulon promoters in Escherichia coli: sequence, orientation and spatial relationship to the core promoter. Mol. Microbiol. 1999;34:431–441. doi: 10.1046/j.1365-2958.1999.01599.x. [DOI] [PubMed] [Google Scholar]
- 6.Vogel J, Papenfort K. Small non-coding RNAs and the bacterial outer membrane. Curr. Opin. Microbiol. 2006;9:605–611. doi: 10.1016/j.mib.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Valentin-Hansen P, Johansen J, Rasmussen AA. Small RNAs controlling outer membrane porins. Curr. Opin. Microbiol. 2007;10:152–155. doi: 10.1016/j.mib.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Peterson U, Schemke T, Kuhlmann J. In: Quinolone antibacterials. Dalhof A, Zeiler HJ, editors. Vol. 127. Berlin, Germany: Springer-Verlag; 1998. pp. 63–118. [Google Scholar]
- 9.Davis R, Markham A, Balfour JA. Ciprofloxacin An updated review of its pharmacology, therapeutic efficacy and tolerability. Drugs. 1996;6:1019–1074. doi: 10.2165/00003495-199651060-00010. [DOI] [PubMed] [Google Scholar]
- 10.Piddock LJV. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clinic Microbiol Rev. 2006;19:382–402. doi: 10.1128/CMR.19.2.382-402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webber MA, Talukder A, Piddock LJV. Contribution of mutation at amino acid 45 of AcrR to acrB expression and ciprofloxacin resistance in clinical and veterinary Escherichia coli isolates. Antimicrobial. Agents Chemother. 2005;49:4390–4392. doi: 10.1128/AAC.49.10.4390-4392.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dzwokai M, Alberti M, Lynch C, Nikaido H, Hearst JE. The local repressor AcrR plays a modulating role in the regulation of acrAB genes of Escherichia coli by global stress signals. Mol. Microbiol. 1996;19:101–112. doi: 10.1046/j.1365-2958.1996.357881.x. [DOI] [PubMed] [Google Scholar]
- 13.Swick MC, Morgan-Linnell SK, Carlson KM, Zechiedrich L. Expression of multidrug efflux pump genes acrAB-tolC, mdfA and norE in Escherichia coli clinical isolates as a function of fluoroquinolone and multidrug resistance. Antimicrob. Agents Chemother. 2011;55:921–924. doi: 10.1128/AAC.00996-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruiz C, Levy SB. Many chromosomal genes modulate MarA-mediated multidrug resistance in Escherichia coli. Antimicrob. Agents Chemother. 2010;54:2125–2134. doi: 10.1128/AAC.01420-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen SP, Hachler H, Levy SB. Genetic and functional analysis of the multiple antibiotic resistance (mar) locus in Escherichia coli. J. Bacteriol. 1993;175:1484–1492. doi: 10.1128/jb.175.5.1484-1492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perera IC, Grove A. Molecular mechanisms of ligand-mediated attenuation of DNA binding by MarR family transcriptional regulators. J. Mol. Cell Biol. 2010;2:243–254. doi: 10.1093/jmcb/mjq021. [DOI] [PubMed] [Google Scholar]
- 17.Pourahmad Jaktaji R, Ebadi R, Karimi M. Study of organic solvent tolerance and increased antibiotic resistance properties in E coli gyrA mutants. Iran. J. Pharm. Res. 2011;11:595–600. [PMC free article] [PubMed] [Google Scholar]
- 18.Pourahmad Jaktaji R, Mohiti E. Study of mutations in the DNA gyrase gyrA gene of Escherichia coli. Iran. J. Pharm. Res. 2010;9:43–45. [PMC free article] [PubMed] [Google Scholar]
- 19.Kishii R, Takei M. Relationship between the expression of OmpF and quinolone resitance in Escherichia coli. J. Infect. Chemother. 2009;15:361–366. doi: 10.1007/s10156-009-0716-6. [DOI] [PubMed] [Google Scholar]
- 20.George AM, Levy SB. Amplifiable resistance to tetracycline, chloramphenicol and other antibiotics in Escherichia coli: involvement of a non-plasmid determined efflux of tetracycline. J. Bacteriol. 1983;155:531–540. doi: 10.1128/jb.155.2.531-540.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viveiros M, Dupont M, Rodrigues L, Couto I, Davin-Regli A, Martins M, Pages J M, Amaral L. Antibiotic stress, genetic response and altered permeability of E. coli. PLoS. 2007;4:e365. doi: 10.1371/journal.pone.0000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST ©) for group wise comparison and statistical analysis of relative expression results in real time PCR. Nuc. Acids Res. 2002;30:1–10. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alekshun MN, Yang SK, Levy SB. Mutational analysis of MarR, the negative regulator of marRAB expression in Escherichia coli, suggests the presence of two regions required for DNA binding. Mol. Microbiol. 2000;35:1394–1404. doi: 10.1046/j.1365-2958.2000.01802.x. [DOI] [PubMed] [Google Scholar]
- 24.Aono R, Tsukagoshi N, Yamamoto M. Involvement of outer membrane protein TolC a possible member of the mar-sox regulon in maintenance and improvement of organic solvent tolerance of Escherichia coli K-12. J. Bacteriol. 1998;180:938–944. doi: 10.1128/jb.180.4.938-944.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin RG, Bartlett ES, Rosner JL, Wall ME. Activation of the E coli marA/soxS/rob regulon in response to transcriptional activator concentration. J. Mol. Biol. 2008;380:278–284. doi: 10.1016/j.jmb.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]