Abstract
Compounds containing triazene ring structure are cytotoxic agents and clinically used as antitumor alkylating agents. In this study, a series of triazene derivatives holding alkyl and aryl moieties were synthesized and proved to be potent cytotoxic agents in-vitro particularly against eight cancer cell lines (PC3, HT29, Hela, HL60, Jurkat, K562, MCF7, HepG2) and a non-cancerous cell line (HUVEC). The cytotoxic activity was assessed using two methods, LDH assay, and trypan blue exclusion. Some of the triazene derivatives showed cytotoxic activity more than temozolomide (TMZ) as the reference drug. The synthesized triazenes showed marked cytotoxicity effects on all eight cancer cell lines. Among the compounds synthesized, 1,3-bis(2-ethoxyphenyl)triazene C had unique efficacy and selectivity so that it had IC50 between 0.560-3.33 μM on cancer cell lines and 12.61 μM on normal cell line (HUVEC). 1-(4-nitrophenyl)-3-(2-hydroxyethyl)triazene E shows weaker effect on cancer cell lines than the other compounds having IC50 between 3-15.54 μM.
Key Words: Cytotoxic activity, Triazene derivatives, Cancer cell lines, LDH assay, Trypan blue exclusion
Introduction
Cancer is a prevalent cause of death worldwide. Every year, several natural and synthetic compounds are tested for various anti-cancer activities. Medicinal chemistry deals with synthesis of new agents for treating cancer by higher selectivity and lower side effects (1). Triazene compounds are a group of antitumor alkylating agents. Dacarbazine (DTIC) and temozolomide (TMZ; Figure 1) are two members of triazenes used in the clinical treatment of metastatic melanomas, soft tissue sarcoma, Hodgkin’s and non-Hodgkin’s lymphoma. Studies show that the antitumor activities of the desired drugs are dependent on three adjacent nitrogen atoms (2-6).
Figure 1.
Structures of dacarbazine and temozolomide used in clinical treatment
Methylation of O6-methylguanine is the main mechanism responsible for the cytotoxicity and methyldiazonium cation is a highly reactive derivative of these compounds that can react with DNA O6-methylguanine (7-11). Recently, the inhibition of tyrosine kinase receptor was suggested as a novel mechanism for triazene agents (12-14). The uses of these compounds are limited because they have highly toxic adverse effects such as skin toxicity, myelosuppression, cardiac and hepatic toxicity, severe nausea, and vomiting (2, 11, 15, 16). Many attempts have been made for increasing the selectivity and decreasing these adverse effects including the use of aryl and heteroaryl rings in the structure of the mentioned triazenes (17-18). In the present study we have replaced the imidazole ring with different aryl groups and investigated the cytotoxicity of the synthesized triazenes against eight cancer cell lines (PC3, HT29, Hela, HL60, Jurkat, K562, MCF7, and HepG2) and a non-cancerous cell line (HUVEC).
Experimental
Chemistry
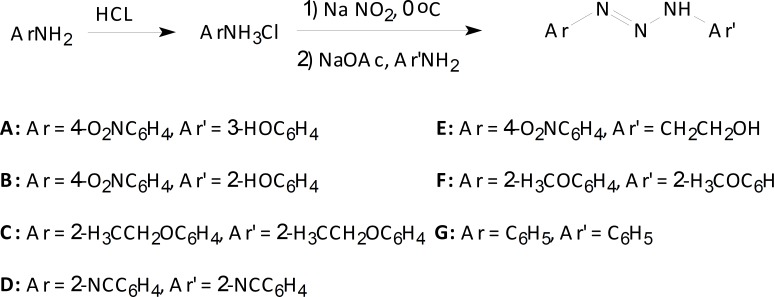
All chemicals were purchased from Merck and Fluka chemical companies. The products were characterized by their spectral data. 1H NMR and 13C NMR spectra were recorded on Bruker DRX 300 Avance spectrophotometer in DMSO-d6 as the solvent and all chemical shifts are reported in units downfield from TMS as internal standard. 1,3-Bis(2-methoxyphenyl)triazene F, 1, 3-bis(2-cyanophenyl) triazene D, and 1, 3-bis(2-ethoxyphenyl) triazene C were synthesized according to reported literatures (19-20), 1,3-diphenyltriazene G was commercially available. Temozolomide (TMZ) was used as the reference drug. Elemental analysis was performed on a Thermo Finnigan Flash EA 1112 (Thermo Fisher Scientific Inc, USA) instrument. Typical procedure for the synthesis of 1, 3-bis (2-cyanophenyl) triazene (D)
1, 3-Bis (2-cyanophenyl) triazene was prepared by the drop wise addition of NaNO2 (6.9 g) in 20 mL distilled water to a beaker containing of 2-aminobenzonitril (5.9 g, 0.05 mol) and of HCl solution (6.48 mL, 0.81 mol, d = 1.18 g/mL) placed in a coldwater bath. After 30 min stirring, CH3COONa.3H2O (13.6 g) dissolved in 20 mL distilled water was added to the solution. The resulting colored precipitate appeared after 15 min, was evaporated to dryness in the air and then crystallized from diethyl ether in a freezer in three days (21). 1, 3-Bis (2-cyanophenyl) triazene as sharp yellow powder was obtained with 88% yield, mp 129-131 °C (mp 128-130 °C Lit).
Typical procedure for the synthesis of 1-(4-nitrophenyl)-3-(2-hydroxyethyl)triazene (E)
2-Aminoethanol (0.05 mol, 3.054 g) was added at room temperature to a stirred suspension of 4-nitrobenzenediazonium chloride (0.05 mol) in water (20 mL) and stirring was continued for 20 min in a cold water bath and 10 min at RT. CH3COONa.3H2O (13.6 g) was dissolved in 20 mL distilled water and added to the solution. After 10 min a clear faintly pale yellow solution was obtained. Crystals were collected by filtration, washed with water and recrystallized from diethyl ether to afford 1-(4-nitrophenyl)-3-(2-hydroxyethyl) triazene E with 89% yield. 1H NMR (DMSO-d6, 200 MHz) δ (ppm) 3.56 (s, 1H), 4.42 (t, 2H), 5.05 (t, 2H), 7.20-7.74 (dd, 4H, J = 8.1 Hz), 7.76 (s, 1H); 13C NMR (DMSO-d6, 50 MHz) δ (ppm) 175.5, 155.3, 129.7, 125.5, 56.7, 49.4; IR (KBr, cm-1) 3500 (OH, str), 1630 (NH, bend), 1600 (C=C, str), 1500 (C=C, str), 1450 (CH, bend), 1400 (CH, bend), 1300 (N=O, str), 1200 (C-O, str), 1150 (C-N, str), 900 (C-H, OOP), 700 (N-H, OOP), 750 (O-H, OOP); Anal. Calcd. For C8H10N4O3: C, 45.71; H, 4.76; N, 26.67. Found: C, 45.52, H, 4.58, N, 26.45.
1-(4-nitrophenyl)-3-(3-hydroxyphenyl)triazene (A)
Red crystals, 85% yeild, 1H NMR (DMSO-d6, 200 MHz) δ (ppm) 3.89 (s, 1H), 5.54 (s, 1H), 6.53-6.61 (m, 4H), 7.90-7.98 (dd, 4H, J = 8 Hz); 13C NMR (DMSO-d6, 50 MHz) δ (ppm) 159.5, 154.8, 145.5, 144.1, 137.8, 126.7, 122.2, 113.3, 106.1, 102.2; IR (KBr, cm-1) 3300, 3400, 2800, 2700, 1650, 1600, 1450, 1300, 1200, 1150, 850; Anal. Calcd. For C12H10N4O3: C, 55.81; H, 3.86; N, 21.71. Found: C, 55.65, H, 3.67, N, 21.55.
1-(4-nitrophenyl)-3-(2-hydroxyphenyl)triazene (B)
Red crystals, 87% yield, 1H NMR (DMSO-d6, 200 MHz) δ (ppm) 3.27 (s, 1H), 5.19 (s, 1H), 6.65-6.75 (m, 4H), 8.05-8.15 (dd, 4H, J = 8.2 Hz); 13C NMR (DMSO-d6, 50 MHz) δ (ppm) 157.7, 145.5, 144.1, 126.2, 122.5, 121.0, 120.9, 118.9, 117.1; IR (KBr, cm-1) 3500, 3200, 2800, 2700, 1650, 1600, 1450, 1300, 1100, 1150, 850; Anal. Calcd. For C12H10N4O3: C, 55.81; H, 3.86; N, 21.71. Found: C, 55.60, H, 3.71, N, 21.59.
Biological activity
Cell lines and culture
PC3 NCBI-C427 (Human prostate adenocarcinoma), HT29 NCBI-C466 (Human colon carcinoma), Hela NCBI-C115 (Human cervix carcinoma), K562 NCBI-C122 (Human chronic myelogenom leukemia), Jurkat E6/1 NCBI-C121 (Human Acute T-cell leukemia), HL60 NCBI-C217 (Human promylocyteic leukemia), MCF7 NCBI-C135 (Human breast adenocarcinoma), HepG2 NCBI-C144 (Human hepatocyte cancer cell line), and HUVEC NCBI-C554 (Human umbilical vein endothelial cell) were purchased from Pasture Institute of Tehran-Iran. Cell lines were grown and maintained in a humidified incubator at 37°C with 5% CO2 atmosphere. Cells were cultured in DMEM (Dulbecco’s modified Eagle’s medium) supplemented with 10% fetal bovine serum (FBS), and antibiotics (100 IU/mL penicillin and 100 μL/mL streptomycin).
Scheme 1.
Synthesis of triazene compounds.
LDH assay
After incubation of different concentrations (1, 5, 10, 25, 50, 100 μM) of the synthesized compounds with cell lines for 24 and 48 h, the medium was removed without damaging cells for LDH assay. Briefly, 100 μL of the media from each well was transferred to a new plate, and 100 μL of LDH reagent and catalyst (1:45) (Roche Diagnostics Corporation, Indianapolis, IN, USA) was added to the supernatant and incubated for 30 min in dark at room temperature. The absorbance was recorded at 490 nm with background subtraction at 630 nm using the Synergy HT multi detection Micro Plate Reader. 20 μL of 10% triton X-100 and the LDH reagent were added to untreated cells and these results were collected as a positive control. Media without cells were collected as a negative control. The results were showed as percentage of LDH release (22).
Trypan blue exclusion
To determine the effect of the synthesized compounds on viability of cells, approximately 0.75 × 105 cells/mL was transferred in a 6-well tissue culture plate and the triazenes A-G were added at different concentrations. After 48 h the cells were collected and resuspended in 0.4% Trypan blue. The percentages of viable cells were counted using an inverted microscope and the percent of viability was determined in comparison with TMZ as the control (23).
Results and Discussion
Cytotoxic activity by LDH assay
The cytotoxic activities of the synthesized triazenes were examined on eight human cancer cell lines and a non-cancerous cell line by LDH assay. LDH is an enzyme exists in all cells and is released in culture medium after cell death. In this assay, NADH/H+ reacts with tetrazolium salt in the presence of a catalyst to form a red color compound, i.e. formazan, which can be measured at 490 nm (20). The synthesized triazenes showed a marked cytotoxicity effects on all eight cancer cell lines. The IC50 values are shown in Table 1.
Table 1.
Cytotoxic activities of triazene analogs with LDH assay (IC50 in μM).
| Compound | MCF7 | HepG2 | PC3 | HT29 | Hela | HL60 | K562 | Jurkat | HUVEC | Yield (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 1.51 ± 0.26 | 1.12 ± 0.30 | 1.24 ± 0.20 | 2.82 ± 0.49 | 8.87 ± 2.1 | 2.3 ± 0.51 | 2.1 ± 0.70 | 1.2 ± 0.40 | 4.4 ± 1.31 | 85 |
| B | 1.78 ± 0.50 | 1.89 ± 0.78 | 3.29 ± 1.30 | 3.37 ± 1.65 | 4.2 ± 1.40 | 1.36 ± 0.34 | 1.13 ± 0.67 | 1.05 ± 0.98 | 3.22 ± 1.10 | 87 |
| C | 0.59 ± 0.12 | 3.33 ± 1.30 | 0.56 ± 0.20 | 2.55 ± 1.33 | 2.54 ± 1.20 | 2.62 ± 0.96 | 1.66 ± 0.8 | 1.69 ± 1.02 | 12.61 ± 2.80 | 90 |
| D | 1.45 ± 0.32 | 1.49 ± 0.42 | 1.04 ± 0.11 | 2.03 ± 1.05 | 2.39 ± 1.35 | 1.34 ± 0.44 | 1.04 ± 0.38 | 1.44 ± 0.71 | 1.14 ± 0.40 | 88 |
| E | 6.14 ± 1.89 | 4.2 ± 1.20 | 3 ± 1.01 | 8.25 ± 2.20 | 15.54 ± 3.01 | 2.81 ± 0.99 | 9.87 ± 2.08 | 4.5 ± 1.67 | 5.05 ± 1.80 | 89 |
| F | 2.17 ± 0.53 | 1.45 ± 0.65 | 7 ± 2.20 | 2.26 ± 1.52 | 6.85 ± 2.80 | 1.3 ± 0.50 | 1.66 ± 0.49 | 1.61 ± 0.90 | 4.59 ± 1.76 | 92 |
| G | 1.57 ± 0.34 | 3.94 ± 1.45 | 4.13 ± 1.80 | 9.4 ± 2.30 | 4.88 ± 1.50 | 3.4 ± 1.96 | 6.33 ± 2.41 | 5.53 ± 1.44 | 7.43 ± 2.10 | - |
| TMZ | 0.77 ± 0.22 | 2.59 ± 1.30 | 1.39 ± 0.69 | 17.24 ± 3.20 | 8.88 ± 2.2 | 3.54 ± 1.56 | 3.82 ± 1.60 | 2.07 ± 1.34 | 4.85 ± 1.78 | - |
Among the compounds synthesized, 1,3-bis(2-ethoxyphenyl)triazene C had unique efficacy and selectivity as it had IC50 between 0.560-3.33 μM on cancer cell lines and 12.61 μM on normal cell line (HUVEC). It is important to note that one of the main factors in appearance of side effects of chemotherapy for a long time are the toxic effects on normal cells. Thus for compound C, the difference between toxic dosage on cancer and normal cell line can be valuable. The other unique characteristic is the high sensitivity of cell lines PC3 and MCF7 to compound C showing IC50 values 0.590 μM and 0.56 μM respectively. TMZ as the positive control also showed high cytotoxic effects on cell lines MCF7 and PC3 having IC50 0.77 and 1.39 μM respectively; however, its IC50 against HUVEC was 4.85 μM. 1-(4-nitrophenyl)-3-(2-hydroxyethyl) triazene E showed weaker effect on cancer cell lines than the other compounds having IC50 between 3-15.54 μM. Among cancer cell lines Hela had the lowest sensitivity to compound E (IC50 = 15.54 μM). The most severe toxic effect was related to 1, 3-bis (2-cyanophenyl) triazene D showing IC50 1.14 μM on normal cell line (HUVEC). Regarding the results obtained from Table 1, it could be inferred that the presence of electron-donating groups on phenyl ring increased selectivity and potency of the triazenes while the absence of aromatic ring decreased the efficacy.
Cytotoxic activity by trypan blue exclusion
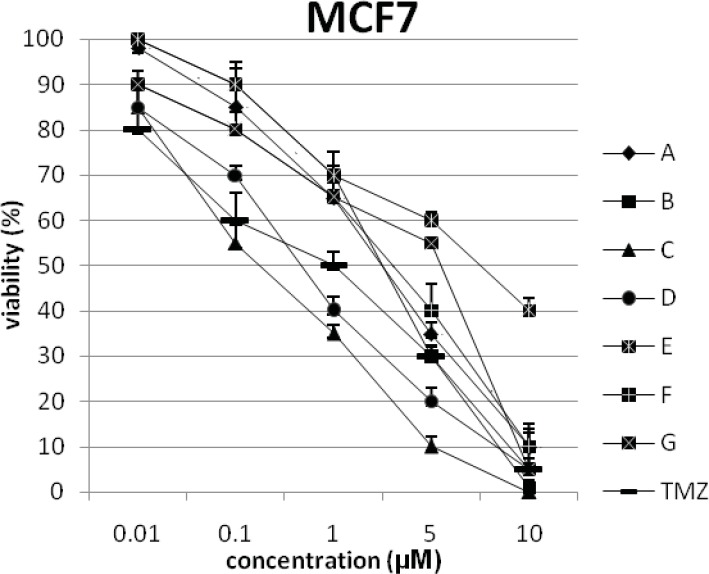
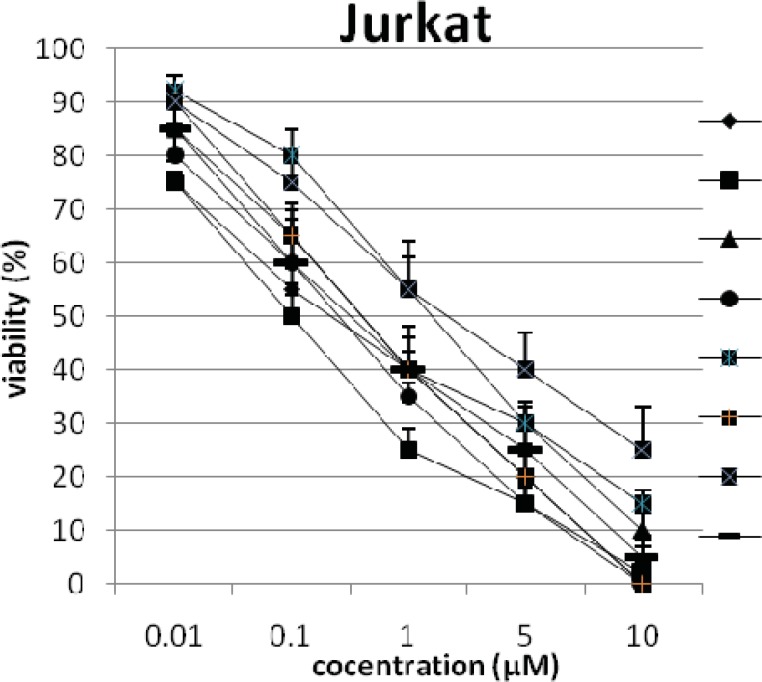
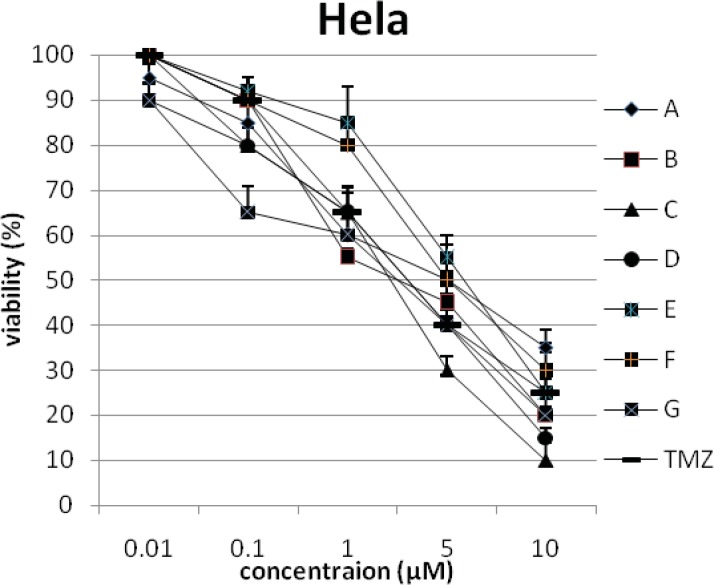
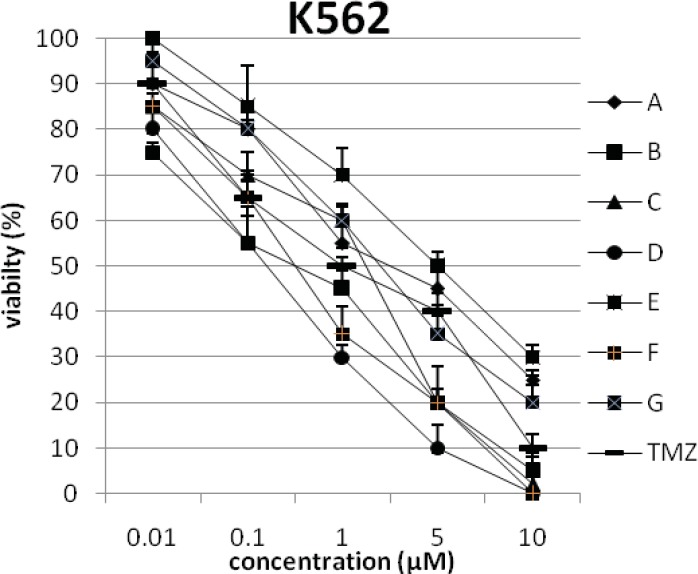
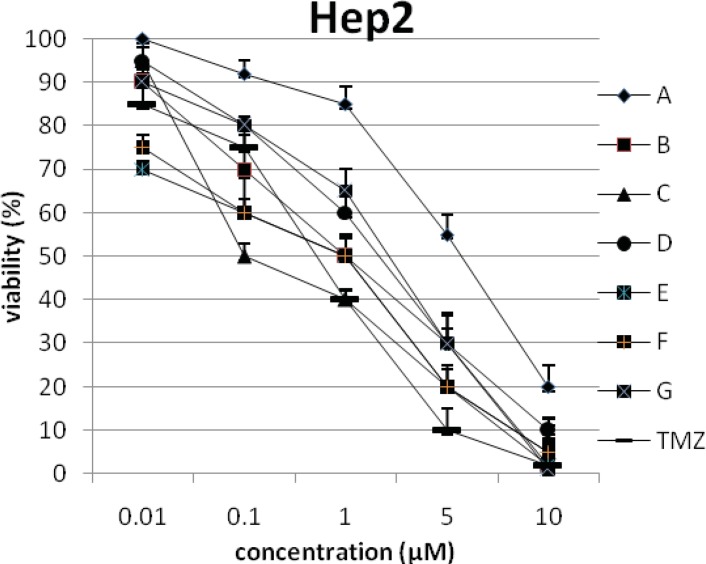
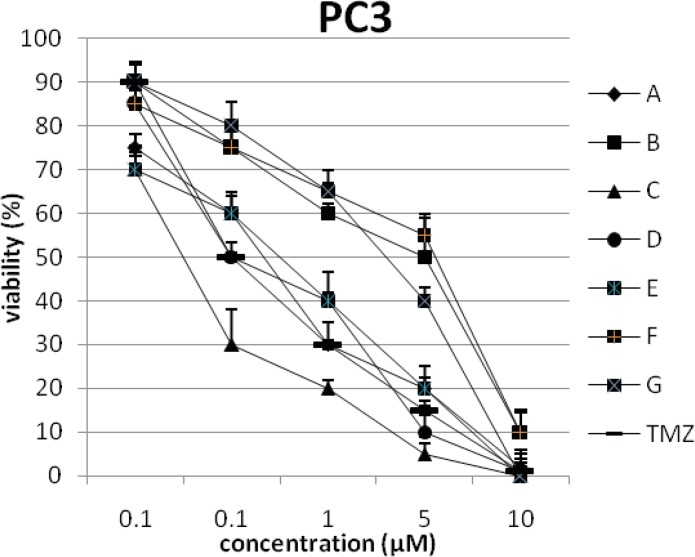
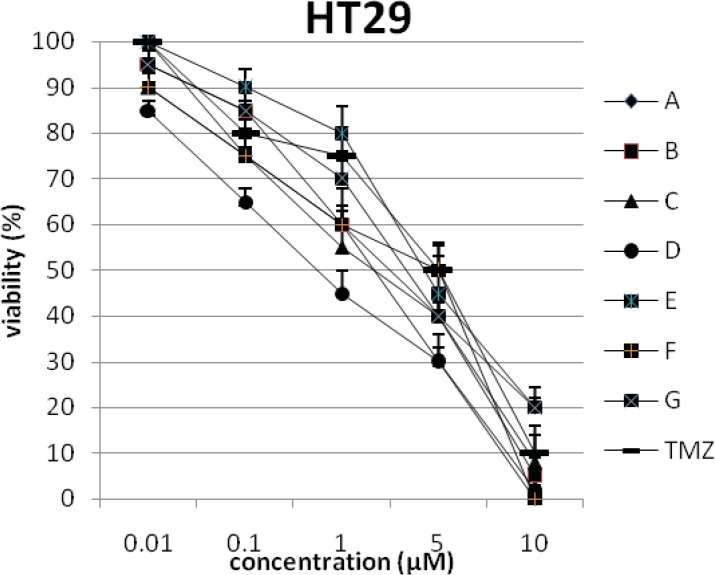
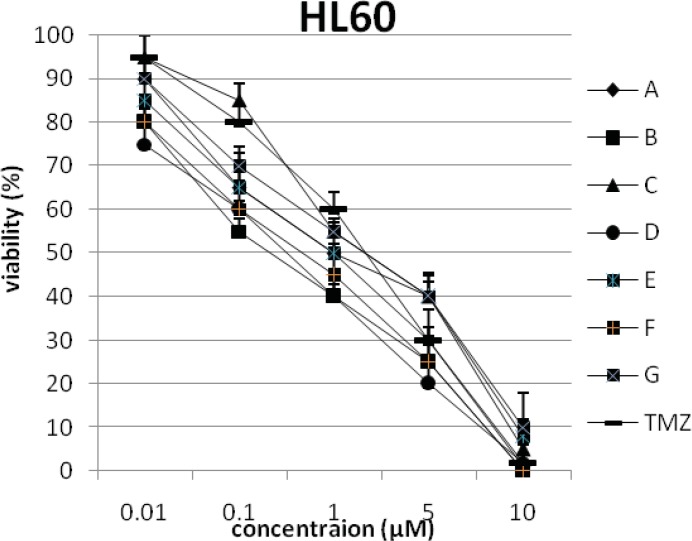
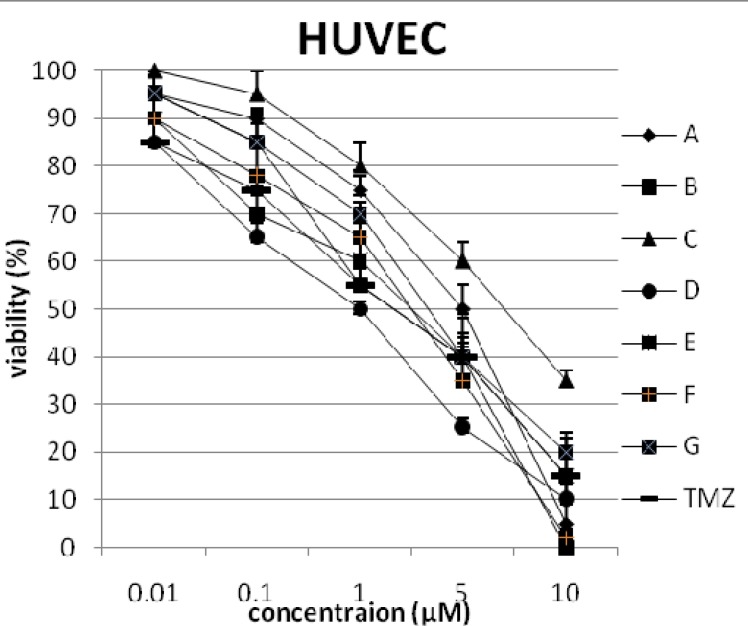
In addition, the viability of cancer cell lines was studied by using trypan blue exclusion. In this test the viable are unstained and non-viable were colored blue. The results of this assay approved the results of LDH assay. According to Figures 4 and 10, compound C showed low and high cytotoxic activity on cell lines HUVEC and PC3, respectively. 1-(4-nitrophenyl)-3-(3- hydroxyphenyl) triazene A and 1-(4-nitrophenyl)- 3-(2-hydroxyphenyl) triazene B were structural isomers, but compound B was more effective on leukemia cell lines (HL60, K562 and Jurkat) (Figures 7-9). With regards to difference in the activity of A and B, we conclude that the stereochemistry determined the efficacy of the triazene compounds.
Figure 2.
The percentage of viability versus concentration by trypan blue exclusion on cancer cell line MCF7 (human breast adenocarcinoma).
Figure 9.
The percentage of viability versus concentration by trypan blue exclusion on cancer cell line Jurkat (Human Acute T-cell leukemia).
Figure 6.
The percentage of viability versus concentration by trypan blue exclusion on cancer cell line Hela (Human cervix carcinoma).
Figure 8.
The percentage of viability versus concentration by trypan blue exclusion on cancer cell line K562 (Human chronic myelogenom leukemia).
As reported in the literature, the presumable mechanism of the cytotoxic activities of triazene compounds may be ascribed to alkylation of DNA, inhibition of DNA and RNA synthesis, and inhibition tyrosine kinase (12-14, 24).
The synthesized compounds have distinctly lower cytotoxicity against normal cell line and higher cytotoxicity against cancer cell lines and it can be expected that this is the selective action of the examined compounds. According to the results obtained from LDH and trypan blue exclusion, induction of apoptosis can be proposed as a probable mechanism for the synthesized triazenes.
Figure 3.
The percentage of viability versus concentration by trypan blue exclusion on cancer cell line HepG2 (Human Hepatocyte cancer cell line).
Figure 4.
The percentage of viability versus concentration by trypan blue exclusion on cancer cell line PC3 (Human prostate adenocarcinoma).
Figure 5.
The percentage of viability versus concentration by trypan blue exclusion on cancer cell line HT29 (Human colon carcinoma).
Figure 7.
The percentage of viability versus concentration by trypan blue exclusion on cancer cell line HL60 (Human promylocyteic leukemia).
Figure 10.
The percentage of viability versus concentration by trypan blue exclusion on non-cancerous cell line HUVEC (Human umbilical vein endothelial cell). Effects of the synthesized triazenes on studied human cancer cell lines as investigated by trypan blue exclusion after 48 h. In all experiments the reported results represent the mean ± standard deviation from triplicate tests
Conclusion
In conclusion, the triazene derivatives tested in this study demonstrated cytotoxic and antiproliferative properties which warrant further investigation as potential anticancer agents. The synthesized compounds which are triazene bioisosteres were evaluated for their cytotoxicity against several human cancer cell lines and showed moderate to high potency. Among these, the compound C had the highest efficacy and selectivity. From the structure-activity relationships we may conclude that the presence of aryl groups would be critical for their biological activities. Further studies are in progress to define the important mechanism of action of above mentioned compounds.
Acknowledgment
We gratefully acknowledge Kermanshah University of Medical Sciences Research Council for financial support. This work was performed in partial fulfillment of the requirements for a Pharm. D of Mohammad Bagher Majnooni in Faculty of Pharmacy, Kermanshah University of Medical Sciences Kermanshah, Iran.
References
- 1.Khodarahmi GA, Chen PY, Hakimelahi Gh, Chern JW. Synthesis and primary cytotoxic screening of some 3-sulfonamide substituted benzamido-benzimidazolones. Iran. J. Pharm. Res. 2005;4:43–56. [Google Scholar]
- 2.Marchesi F, Turriziani M, Tortorelli G, Avvisati G, Torino F, Vecchis LD. Triazene compounds: mechanism of action and related DNA repair systems: Review. Pharmacol. Res. 2007;56:275–287. doi: 10.1016/j.phrs.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Rachid Z, Katsoulas A, Brahimi F, Jean-Claude BJ. Synthesis of pyrimidinopyridine–triazene conjugates targeted to abl tyrosine kinase. Bioorg. Med. Chem. Lett. 2003;13:3297–3300. doi: 10.1016/s0960-894x(03)00553-5. [DOI] [PubMed] [Google Scholar]
- 4.Yahalom J, Voss R, Leizerowitz R, Fuks Z, Polliack A. Secondary leukemia following treatment of Hodgkin’s disease: Ultra structural and cytogenetic data in two cases with a review of the literature. Am. J. Clin. Pathol. 1983;80:231–236. doi: 10.1093/ajcp/80.2.231. [DOI] [PubMed] [Google Scholar]
- 5.O’Reilly SM, Newlands ES, Glaser MG, Brampton M, Rice-Edwards JM, Illingworth RD. Temozolomide: a new oral cytotoxic chemotherapeutic agent with promising activity against primary brain tumors. Eur. J. Cancer. 1993;29:940–2. doi: 10.1016/s0959-8049(05)80198-4. [DOI] [PubMed] [Google Scholar]
- 6.Ünsalan S, Çıkla P, Küçükgüzel G, Rollas S, Şahin F, Bayrak Ö. Synthesis and characterization of triazenes derived from sulfonamides. Marmara Pharmaceut. J. 2011;15:11–17. [Google Scholar]
- 7.Roos WP, Baumgartner M, Kaina B. Apoptosis triggered by DNA damage O6-methylguanine in human lymphocytes requires DNA replication and is mediated by p53 and Fas/CD95/Apo-1. Oncogene. 2004;23:359–367. doi: 10.1038/sj.onc.1207080. [DOI] [PubMed] [Google Scholar]
- 8.Roos WP, Batista LF, Naumann SC, Wick W, Weller M, Menck CF, Kaina B. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine. Oncogene. 2007;26:186–197. doi: 10.1038/sj.onc.1209785. [DOI] [PubMed] [Google Scholar]
- 9.Graziani G, Faraoni I, Grohmann U, Bianchi R, Binaglia L, Paul Margison G, Jane Watson A, Orlando L, Bonmassar E, D›Atri S. O6-Alkylguanine-DNA alkyltransferase attenuates triazene induced cytotoxicity and tumor cell immunogenicity in murine L1210 leukemia. Cancer Res. 1995;55:6231–6236. [PubMed] [Google Scholar]
- 10.Agnihotri SS, Gajadhar A, Ternamian C, Gorlia TL, Diefes KS, Mischel P. Alkylpurine–DNA–N-glycosylase confers resistance to temozolomide in xenograft models of glioblastoma multiform and is associated with poor survival in patients. Clin. Investig. 2012;122:253–266. doi: 10.1172/JCI59334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell RB, Dolan ME. Effect of temozolomide and dacarbazine on O6-alkylguanine-DNA alkyltransferase activity and sensitivity of human tumor cells and xenografts to 1,3-bis (2-chloroethyl)-1-nitrosourea. Can. Chemother. Pharmacol. 1993;32:59–63. doi: 10.1007/BF00685877. [DOI] [PubMed] [Google Scholar]
- 12.Matheson SL, McNamee J, Jean-Claude BJ. Design of a chimeric 3-methyl-1,2,3-triazene with mixed receptor tyrosine kinase and DNA damaging properties: a novel tumor targeting strategy. Pharmaco. Exp. Therap. 2001;296:832–840. [PubMed] [Google Scholar]
- 13.Katsoulas A, Rachid Z, Brahimi F, McNamee J, Jean-Claude BJ. Engineering 3-alkyltriazenes to block bcr-abl kinase: a novel strategy for the therapy of advanced bcr-abl expressing leukemia›s. Leukemia Res. 2005;29:693–700. doi: 10.1016/j.leukres.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Matheson SL, McNamee JP, Wang T, Alaoui-Jamali MA, Tari AM, Jean-Claude BJ. The combi-targeting concept: dissection of the binary mechanism of action of the combi-triazene SMA41 in-vitro and antitumor activity in-vivo. Pharmaco. Exp. Therap. 2004;311:1163–1170. doi: 10.1124/jpet.104.071977. [DOI] [PubMed] [Google Scholar]
- 15.Caporaso P, Turriziani M, Venditti A, Marchesi F, Buccisano F, Cristina Tirindelli M, Alvino E, Garbin A, Tortorelli G, Toppo L, Bonmassar E, D’Atr S, Amadori S. Novel role of triazenes in hematological malignancies: pilot study of temozolomide, lomeguatrib and IL-2 in the chemo-immunotherapy of acute leukemia. DNA Repair. 2007;6:1179–1186. doi: 10.1016/j.dnarep.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Murray L, editor. Physicians’ Desk Reference. 58th ed. Oradell: Medical Economics; 2004. pp. 857–858. [Google Scholar]
- 17.Cameron LM, La France RJ, Hemens CM, Vahghman K, Rajaraman R, Ceubb DC, Goddard PM. Triazene metabolism IV Derivatives of hydroxymethyltriazenes: potential prodrugs for the active metabolites of the antitumor triazene, DTIC. Anti-Cancer Drugs. 1985;1:27–36. [PubMed] [Google Scholar]
- 18.Gadjeva VG. Two spin labeled triazenes: relationship between biochemical and biological activities. Int. J. Pharm. 2002;247:39–45. doi: 10.1016/s0378-5173(02)00360-5. [DOI] [PubMed] [Google Scholar]
- 19.Rofouei MK, Melardi MR, Salemi Y, Kazemi SR. 1,3-Bis(2-ethoxybenzene)triazene. Acta Cryst. 2009;65:719. doi: 10.1107/S1600536809008034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gholivand MB, Mohammadi M, Khodadadian M, Rofouei MK. Novel platinum(II) selective membrane electrode based on 1,3-bis(2-cyanobenzene)triazene. Talanta. 2009;78:922–928. doi: 10.1016/j.talanta.2008.12.070. [DOI] [PubMed] [Google Scholar]
- 21.Rofouei MK, Shamsipur M, Payehghadr M. Crystal Structure of Triazene-1,3-di(2-methoxyphenyl) Anal. Sci. 2006;22:79–80. [Google Scholar]
- 22.Schmitt A, Schmitt J, Mun´ch G, Gasic-Milenkovic J. Characterization of advanced glycation end products for biochemical studies: Side chain modifications and fluorescence characteristics. Anal. Biochem. 2005;338:201–215. doi: 10.1016/j.ab.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Chandrappa S, Kavitha CV, Shahabuddin MS, Vinaya K, Ananda KCS, Ranganatha SR, et al. Synthesis of 2-(5-((5-(4-chlorophenyl)furan-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid derivatives and evaluation of their cytotoxicity and induction of apoptosis in human leukemia cells. Bioorg. Med. Chem. 2009;17:2576–2584. doi: 10.1016/j.bmc.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Mokhtari S, Mosaddegh M, Hamzelo Moghadam M, Soleymani Z, Ghafari S, Kobarfard F. Synthesis and cytotoxic evaluation of novel 3-substituted derivatives 0f 2-indolinone. Iran. J. Pharm. Res. 2012;11:411–421. [PMC free article] [PubMed] [Google Scholar]