Abstract
The uses of non-steroidal anti-inflammatory drugs (NSAIDs) are limited by a variety of side effects. So research on preparing new analgesic agents is important. According to some reports about the analgesic activity of hydrazide and hydrazine derivatives a new series of these compounds were synthesized in order to obtain new analgesic compounds. The final compounds 10a-10e and 15a-15d were prepared by condensation of corresponding hydrazides 7,8 and 11-14 with different aldehydes 9a-9e. The structures of all synthesized compounds were confirmed by means of FT-IR, 1H-NMR and Mass spectra. All compounds were evaluated for their analgesic activities by abdominal constriction test (writhing test). Most of the synthesized compounds induced significant reduction in the writhing response when compared to control and compound 15 was more potent than mefenamic acid in the writhing test.
Key Words: Hydrazide, Hydrazone, Analgesic activity, Fenamate
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are extremely used in the treatment of pain and inflammation. These compounds non selectively inhibit the two isoforms of the cyclooxygenase (COX-1 and COX-2) and so prevent the metabolism of cellular arachidonic acid (AA) and the upregulation of prostaglandin formation, which in other respects lead to an increase of vascular permeability, edema, hyperalgesia, pyrexia and inflammation (1-5).
Additionally, Leukotrienes, particularly LTB4 which are products of 5-LO together with decrease in prostaglandin formation are incriminated in the acute ulceration induced by NSAID’s. Accordingly, compounds that obtain dual inhibition of enzymes COX and 5-LO can show safer profile of activity and enhanced efficacy in the battle of pain in inflammatory diseases.
Some reports suggest that the hydrazone moiety present in phenylhydrazone derivative 1 (Figure 1) is a pharmacophore group for the inhibition of COX and hydrazone type compounds such as compound 2 were depicted as dual COX/5-LO inhibitors (Figure 1) (3, 4).
Figure 1.
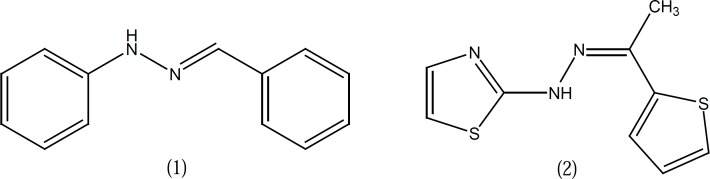
Structure of compounds 1, 2
2-phenoxyphenyl ring system and it’s analogues has attracted some interests in medicinal chemistry and this scaffold have demonstrated a wide range of biological effects which include anticonvulsant, antimycobacterial, analgesic and anti-inflammatory activities (6- 11).
According to the above and on the basis of molecular hybridization approach which is based on the combination of different pharmacophores to produce a new hybrid molecule with improved activity, we aimed at the synthesis of new hybrid compounds containing both of hydrazone and 2-phenoxyphenyl structures in the hope of obtaining new potent analgesic agents with potential dual COX/5-LO inhibitory activity and lower side effects (12-15).
Experimental
Chemistry
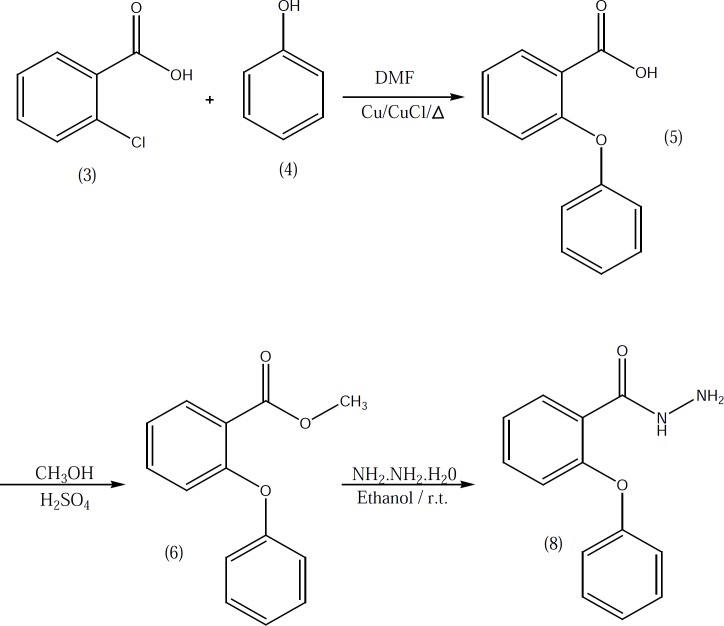
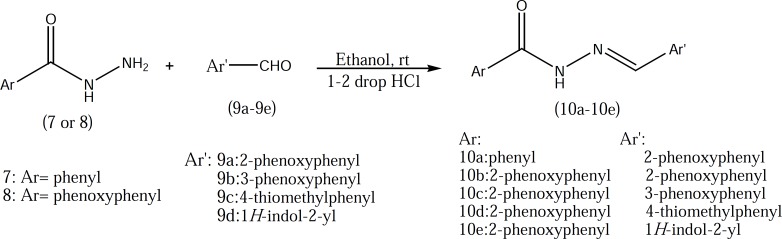
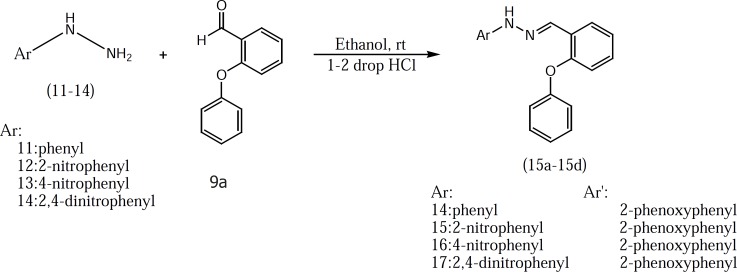
2-phenoxybenzoic acid hydrazide 8 which used in the preparation of compounds 10b-10e was synthesized according to our previously described method (Figure 2) (3). Target compounds were synthesized in one step shown in Figures 3 and 4. The designed compounds 10a-10e and 15a-15d were synthesized by acid-catalyzed condensation of hydrazides 7 and 8 or hydrazines 11-14 with corresponding aldehydes 9a-9d. The structures of the synthesized compounds were assigned on the basis of IR, 1H-NMR and Mass spectra.
Figure 2.
Synthesis of 2-phenoxybenzoic acid hydrazide (8
Figure 3.
Synthesis of target compounds (hydrazide derivatives).
Figure 4.
Synthesis of target compounds (hydrazine derivatives).
Chemicals were purchased from Merck Chemical company (Tehran, Iran) and ACROS company (Renningen, Germany). The melting points were determined in open capillary tubes and presented uncorrected. 1H-NMR spectra were obtained using a Bruker FT-400 spectrometer (Bruker, Rheinstetten, Germany). Tetramethylsilane was used as an internal standard. Mass Spectra were obtained using a Finnigan Mat TSQ-70 spectrometer at 70 eV (Finnigan Mat, Bremen, Germany). The IR spectra were obtained using a Nicolet FT-IR Magna 550 Spectrographs (KBr disks) (Nicolet, Madision, WI, USA). The target compounds were synthesized according to the Figures 3 and 4.
Table1.
Physical data of synthesized compounds
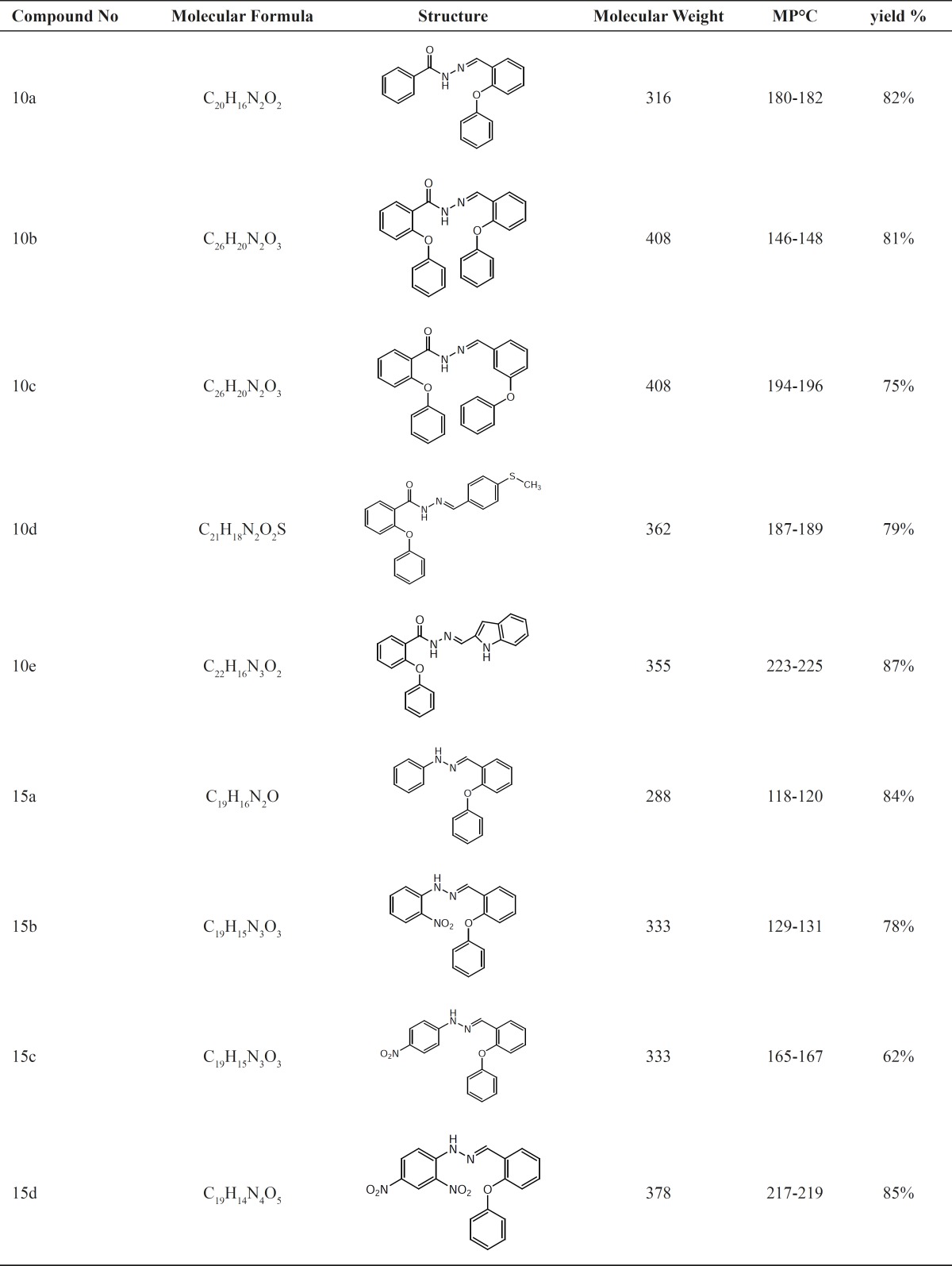
|
General procedure for the synthesis of hydrazides (10b-10e) and hydrazines (15a-15d)
A mixture of hydrazides 7-8 or hydrazines 11-14 (2mmol) and corresponding aldehydes 9a-9d (2.1mmol) in absolute ethanol (20ml) were stirred at room temperature for 1-5 hr in the presence of two drops of hydrochloric acid as a catalyst. The end of the reaction was observed by TLC, and then neutralized by 10% aqueous solution of sodium bicarbonate. The resulting precipitate was filtered, washed with 20 ml water and dried and crystallized by ethanol.
Spectral data of selected compounds
N’-(2-phenoxybenzylidene)benzohydrazide (10a)
1H-NMR (DMSO, d6) : 11.93 (s, 1H, NH), 8.78 (s, 1H, CH=N), 7.90 (d, J=6.8Hz, 2H, aromatic), 7.58-7.16 (m, 9H, aromatic), 7.01(d, J=7.6Hz, 2H, aromatic), 6.96(d, J=7.6Hz, 1H, aromatic). IR (KBr) : ν cm-1, 3310 (NH), 1682 (C=O), 1643(C=N). Mass (m/z): 316(M+ ,78), 121(100), 105 (64), 93 (55), 77 (95); Anal. Calcd. for C20H16N2O2: C, 75.93; H, 5.10; N, 8.86. Found: C, 75.64; H, 5.32; N, 8.72.
N ’ - ( 2 - p h e n o x y b e n z y l i d e n e ) - 2 - phenoxybenzohydrazide (10b)
1H-NMR (DMSO, d6): 11.94 (s, 1H, NH), 8.58 (s, 1H, N=CH), 7.99(d, J=7.6Hz, 1H, aromatic), 7.85 (d, J=7.2Hz, 1H, aromatic), 7.66-6.84 (m, 16H, aromatic). IR (KBr): ν cm-1, 3290(NH), 1678(C=O), 1638(C=N). Mass (m/z): 408(M+ ,100), 197(74), 169(38), 93(61), 77(84); Anal. Calcd. for C26H20N2O3: C, 76.45; H, 4.94; N, 6.86. Found: C, 76.20; H, 5.22; N, 6.69.
N’-(3-phenoxybenzylidene)-2-phenoxybenzohydrazide (10c)
1H-NMR (DMSO, d6): 11.72 (s, 1H, NH), 8.27 (s, 1H, N=CH), 7.95(d, J=8.0Hz, 1H, aromatic), 7.69-6.94 (m, 17H, aromatic). IR (KBr): ν cm-1, 3320(NH), 1680 (C=O), 1640 (C=N). Mass (m/z): 408(M+ ,100), 213(81), 197(80), 169(51), 93 (78), 77(85); Anal. Calcd. for C26H20N2O3: C, 76.45; H, 4.94; N, 6.86. Found: C, 76.38; H, 5.17; N, 6.73.
N’-(4-(methylthio)benzylidene)-2-phenoxybenzohydrazide (10d)
1H-NMR (DMSO, d6): 11.81(s, 1H, NH), 7.93(d, J=7.8Hz, 1H, aromatic), 7.22, 7.60 (m, 7H, aromatic), 7.15-6.93(m, 5H, aromatic), 2.51(s, 3H, CH3). IR (KBr): ν cm-1, 3295(NH), 1685(C=O), 1646(C=N). Mass (m/z): 362(M+ ,100), 197(88), 120(49), 93(70), 77(65); Anal. Calcd. for C21H18N2O2S: C, 69.59; H, 5.01; N, 7.73. Found: C, 69.72; H, 5.24; N, 7.41.
N’-((1H-indol-2-yl)methylene)-2-phenoxybenzohydrazide(10e)
1H-NMR (DMSO , d6) : 13.46(s, 1H, NH), 12.1(s, 1H, NH), 8.21(s, 1H, N=CH), 7.90(d, J=7.6Hz, 1H, aromatic), 7.71-7.37(m, 9H, aromatic), 7.19-6.90(m, 4H, aromatic), IR (KBr) : ν cm-1, 3375, 3324(NH), 1685(C=O), 1645(C=N). Mass (m/z): 355(M+ , 58), 135(100), 120(87), 104(65), 77(82); Anal. Calcd. for C22H17N3O2: C, 74.35; H, 4.82; N, 11.82. Found: C, 74.01; H, 4.99; N, 11.51.
2-(2-phenoxybenzylidene)-1-phenylhydrazine (15a)
1H-NMR (DMSO, d6): 11.20(s, 1H, NH), 8.15(s, 1H, N=CH), 7.70-7.20(m, 11H, aromatic), 7.15-6.91(m, 3H, aromatic). IR (KBr): ν cm-1, 3335(NH), 1635(C=N). Mass (m/z): 288(M+ , 100), 93(79), 92(68), 77(95).; Anal. Calcd. for C19H16N2O: C, 79.14; H, 5.59; N, 9.72. Found: C, 79.43; H, 5.39; N, 9.88.
2-(2-phenoxybenzylidene)-1-(2-nitrophenyl) hydrazine (15b)
1H-NMR (DMSO, d6): 11.33(s, 1H, NH), 8.79(bs, 1H, aromatic), 8.10(s, 1H, CH=N), 7.97(d, J=8.0Hz, 1H, aromatic), 7.65-6.92(m, 11H, aromatic).
IR (KBr): ν cm-1, 3322(NH), 1630(C=N), 1359, 1548(NO2). Mass (m/z): 333(M+ , 100), 137(48), 93(80), 77(53); Anal. Calcd. for C19H15N3O3: C, 68.46; H, 4.54; N, 12.61. Found: C, 68.39; H, 4.31; N, 12.69.
2-(2-phenoxybenzylidene)-1-(4-nitrophenyl) hydrazine (15c)
1H-NMR (DMSO, d6): 11.42(s, 1H, NH), 8.71(d, J = 8.8Hz, 2H, aromatic), 8.15(s, 1H, CH = N), 7.79-7.18(m, 8H, aromatic), 7.11-6.95(m, 3H, aromatic). IR (KBr): ν cm-1, 3342(NH), 1639(C=N), 1352, 1543(NO2). Mass (m/z): 333(M+ , 100), 137(57), 93(74), 77(61); Anal. Calcd. for C19H15N3O3: C, 68.46; H, 4.54; N, 12.61. Found: C, 68.37; H, 4.542; N, 12.08.
2-(2-phenoxybenzylidene)-1-(2,4-dinitrophenyl)hydrazine (15d)
1H-NMR (DMSO , d6) : 11.54(s, 1H, NH), 9.10(s, 1H, aromatic), 8.78(d, J=8.0Hz, 1H, aromatic), 8.63(s, 1H, CH=N), 7.92(d, J=8.0Hz, 1H, aromatic), 7.77-7.20(m, 6H, aromatic), 7.15-6.93(m, 3H, aromatic). IR (KBr): ν cm-1, 3322(NH), 1634(C=N), 1359, 1548(NO2). Mass (m/z): 378(M+ , 100), 182(91), 167(50), 77(94); Anal. Calcd. for C19H14N4O5: C, 60.32; H, 3.73; N, 14.81. Found: C, 60.16; H, 3.38; N, 14.56.
Pharmacological evaluation
Male NMRI mice weighting 20-25 g (from animals house of Faculty of Pharmacy, TUMS) were used for abdominal constriction test (writhing test). The animal were housed in colony cages and conditions of constant temperature (22 ± 2°C) and a 12 h hlight/dark schedule and allowed free access to standard diet and tap water except during the experiment. The animals were allowed to habituate to the laboratory environment for 2h before the experiments were initiated. All ethical manners for use of laboratory animals were considered carefully and the protocol of study was approved by TUMS ethical committee. The compounds were administered intraperitoneally (IP) (30 mg/kg; 0.2 ml/20g) as a suspension in saline and tween 80 (4%w/ν). Mefenamic acid (Hakim Pharmaceutical Co) (30 mg/kg, IP) was used as standard drug under the same conditions. The control group received vehicle (0.2 ml/20g, IP) alone
Analgesic activity
The analgesic activity was determined in vivo by the abdominal constriction test induced by acetic acid(0.6%; 0.1 mL/10 g) in mice (4). An acetic acid solution was administered IP 30 minutes after administration of compounds. Antinociception was recorded by counting the number of writhings immediately after injection of acetic acid during 30 minutes. The analgesic activity was expressed as the percentage of inhibition of constrictions when compared with the vehicle control group Table 2 (16). Percentage of analgesic activity (PA) was calculated according to the formula: PA= (1-T/C)× 100 where C and T are number of writhes in control and drug treated group, respectively.
Table 2.
Effects of Compounds 10a-10e and 15a-15d and mefenamic acid in the abdominal constrictions induced by acetic acid in mice
| Compound |
Dose
(mg/kg)1 |
Constriction No.
(mean ± SEM) |
Inhibition
(%)2 |
p-value |
|---|---|---|---|---|
| Control | - | 63.5 ± 16.77 | - | - |
| mefenamic acid | 30 | 8.17 ± 1.35 | 87.13 | p < 0.001 |
| 10a | 30 | 49.50 ± 9.731 | 22.04 | p > 0.05 |
| 10b | 30 | 12.33 ± 2.58 | 80.58 | p < 0.001 |
| 10c | 30 | 22.33 ± 3.02 | 54.85 | p < 0.001 |
| 10d | 30 | 50.67 ± 5.48 | 4.4 | p > 0.05 |
| 10e | 30 | 43.33 ± 1.78 | 26.25 | p < 0.01 |
| 15a | 30 | 38.54 ± 1.18 | 32.8 | p < 0.001 |
| 15b | 30 | 3.83 ± 0.91 | 91.33 | p < 0.001 |
| 15c | 30 | 19.17 ± 0.79 | 67.71 | p < 0.001 |
| 15d | 30 | 43.67 ± 1.11 | 31.22 | p < 0.01 |
1 number of animals in each group n= 6; 2 % inhibition obtained by comparison with vehicle control group.
Statistical analysis
Ststistical analysis was done by one-way analysis of variance (ANOVA) and followed by Tukey multiple comparison test. Differences with p < 0.05 between experimental groups were considered statistically significant.
Results and Discussion
In this study, a new series of hydrazide and hydrazine derivatives were synthesized and evaluated for analgesic activity using the acetic acid induced mice abdominal constriction test and the results are shown in Table 2. Except compounds 10a and 10d all of them induced significant reduction in the writhing response in comparison to control and among them compound 15b showed higher inhibitory effect in comparison to mefenamic acid.
As shown in Table 2, the best derivatives were 10b, 10c, 15b and 15c which belong to both hydrazide and hydrazine compounds.
So withdrawing of carbonyl moiety doesn’t have a deleterious effect on the analgesic potency. It has been observed that attachment of phenoxy group in both 2 and 3 positions of phenyl ring can result good compounds. Since compounds 10a, 10d and 10e showed weak activity, we can conclude that in hydrazide derivatives existence of phenoxy moiety in both side of the molecule is essential. Attachment of electronwithrawing groups like Nitro substitution on ortho or para position of phenyl ring in hydrazines can improve the activity but substitution of both positions by these groups has a decrescent effect on analgesic activity. In conclusion several synthesized compounds showed comparable or better analgesic activity than the reference drug.
Conclusion
New hydrazide and hydrazone derivatives were synthesized and their structures were confirmed by spectral data. Their analgesic activity was tested by writhing test and most of them were active analgesic agents. Since in vivo activity depend on highly complex physiological interactions, therefore the SAR’s which were presented earlier are just probable and their validity can’t be claimed absolutely.
Acknowledgment
This work was supported by a grant from Iran National Science Foundation (INSF).
References
- 1.Sawant R, Sarode V. Synthesis, Spectral Characterization and Analgesic Activity of 2-Methylthio-1,4-Dihydropyrimidines. Iran. J. Pharm. Res. 2011;10:733–739. [PMC free article] [PubMed] [Google Scholar]
- 2.Zarghi A, Arfaei S. Selective COX-2 Inhibitors: A Review of Their Structure-Activity Relationships. Iran. J. Pharm. Res. 2011;10:655–683. [PMC free article] [PubMed] [Google Scholar]
- 3.Almasirad A, Hosseini R, Jalalizadeh H, Rahimi-Moghaddam Z, Abaeian N, Janafrooz M, Abbaspour M, Ziaee V, Dalvandi A, Shafiee A. Synthesis and analgesic activity of 2-Phenoxybenzoic Acid and N-Phenylanthranilic Acid Hydrazides. Biol. Pharm. Bull. 2006;29:1180–1185. doi: 10.1248/bpb.29.1180. [DOI] [PubMed] [Google Scholar]
- 4.Almasirad A, Tajik M, Bakhtiari D, Shafiee A, Abdollahi M, Zamani MJ, Esmaily H. Synthesis and analgesic activity of N-arylhydrazone derivatives of mefenamic acid. J. Pharm. Pharmaceutic. Sci. 2005;8:419–425. [PubMed] [Google Scholar]
- 5.Almasirad A, Shafiee A, Abdollahi M, Neoparast A, Shahrokhinejad N, Vousooghi N, Tabatabai SA, Khorasani R. Synthesis and analgesic activity of 1,3,4-oxadiazoles and 1,2,4-triazoles. Med. Chem. Res. 2011;20:435–442. [Google Scholar]
- 6.Mahdavi M, Akbarzadeh T, Sheibani V, Abbasi M, Firoozpour L, Tabatabai SA, Shafiee A, Foroumadi A. Synthesis of Two Novel 3-Amino-5-[4-chloro-2-phenoxyphenyl]-4H-1,2,4-triazoles with Anticonvulsant Activity. Iran. J. Pharm. Res. 2010;9:265–269. [PMC free article] [PubMed] [Google Scholar]
- 7.Shekarchi M, Navidpour L, Rajabi Khorami A, shekarchi M, Partoazar A, Shafaroodi H, Rahmanipour N, Shafiee A, Shekarchi M. Synthesis of N-arylidene-2-(2-Phenoxyphenyl) Acetohydrazides as anti-inflammatory Agents. Iran. J. Pharm. Res. 2011;10:369–377. [PMC free article] [PubMed] [Google Scholar]
- 8.Faizi M, Sheikhha M, Ahangar N, Tabatabaei Ghomi H, Shafaghi B, Shafiee A, Tabatabai SA. Design, Synthesis and Pharmacological Evaluation of Novel 2-[2-(2-Chlorophenoxy) phenyl]-1,3,4-oxadiazole Derivatives as Benzodiazepine Receptor Agonists. Iran. J. Pharm. Res. 2012;11:83–90. [PMC free article] [PubMed] [Google Scholar]
- 9.Almasirad A, Samiee-Sadr S, Shafiee A. Synthesis and Antimycobacterial Activity of 2-(Phenylthio) benzoylarylhydrazone Derivatives. Iran. J. Pharm. Res. 2011;10:727–731. [PMC free article] [PubMed] [Google Scholar]
- 10.Shafiee A, Rineh A, Kebriaeezadeh A, Foroumadi A, Sheibani V, Afarinesh MR. Synthesis and anticonvulsant activity of 4-(2-phenoxyphenyl)semicarbazones. Med. Chem. Res. 2009;18:758–769. [Google Scholar]
- 11.Almasirad A, Tabatabai SA, Faizi M, Kebriaeezadeh A, Mehrabi N, Dalvandi A, Shafiee A. Synthesis and anticonvulsant activity of new 2-substituted-5- [2-(2-fluoro phenoxy)phenyl]-1,3,4-oxadiazoles and 1,2,4-triazoles. Bioorg. Med. Chem. Lett. 2004;14:6057–6059. doi: 10.1016/j.bmcl.2004.09.072. [DOI] [PubMed] [Google Scholar]
- 12.Lima PC, Lima LM, da Silva KCM, Leda PHO, de Miranda ALP, Fraga CAM, Barreiro EJ. Synthesis and analgesic activity of novel Nacylarylhydrazones and isosters, derived from natura lsafrole. Eur. J. Med. Chem. 2000;35:187–203. doi: 10.1016/s0223-5234(00)00120-3. [DOI] [PubMed] [Google Scholar]
- 13.Figueiredo JM, Camara CA, Amarante EG, Miranda ALP, Santos FM, Radrigues CR, Fraga CAM, Barreiro EJ. Design and synthesis of novel potent antinociceptive Agents: Methyl-imidazolyl NAcylharazone derivatives. Bioorg. Med .Chem. 2000;8:2243–2248. doi: 10.1016/s0968-0896(00)00152-8. [DOI] [PubMed] [Google Scholar]
- 14.Boschelli DH, Connor DT, Bornemeier DA, Dyer RD, Kennedy JA, Kuipers PJ, Okonkwo G C, Schrier DJ, Wright CD. 1,3,4-oxadiazole, 1, 3, 4-thiadiazole, and 1, 2, 4-triazole analogs of the fenamates: In-vitro inhibition of cyclooxygenase and 5-lipoxygenase activities. J. Med. Chem. 1993;36:1802–1810. doi: 10.1021/jm00065a002. [DOI] [PubMed] [Google Scholar]
- 15.Boschelli D, Connor DI, Hoefle M, Bornemeier DA, Dyere RD. Conversion of NSAIDS into balanced dual inhibitors of cyclooxygense and 5-lipoxygenase. Bioorg. Med. Chem. Lett. 1992;2:69–72. [Google Scholar]
- 16.Rineh A, Mahmoodi N, Abdollahi M, Foroumadi A, Sorkhi M, Shafee A. Synthesis and analgesic and anti-inflammatory activity of 4-(s-phenoxyphenyl)semicarbazones, Arch. Pharm. Chem. Life. Sci. 2007;340:409–415. doi: 10.1002/ardp.200700045. [DOI] [PubMed] [Google Scholar]