Abstract
Fragrant species of the genus Salvia have been attributed many medicinal properties, which include anticancer activity. In the present study, cytotoxic properties of total methanol extract of Salvia chloroleuca Rech. f. & Aellen and its fractions were investigated on MCF- 7, a breast carcinoma cell line. Malignant and non-malignant cells were cultured in RPMI medium and incubated with different concentrations of plant extracts. Cell viability was quantitated by 3-(4,5-dimethylthiazol-2-yl) -5-(3-carboxymethoxyphenyl) -2-(4-sulphophenyl) -2H-tetrazolium (MTS) assay. Apoptotic cells were determined using propidium iodide (PI) staining of DNA fragmentation by flow cytometry (sub-G1 peak). S. chloroleuca inhibited the growth of malignant cells in a dose-dependent manner. Among solvent fractions of S. chloroleuca, the n-hexane and methylene chloride fractions were found to be more toxic compared to other fractions. S. chloroleuca-induced a sub-G1 peak in flow cytometry histogram of treated cells compared to control and DNA fragmentation suggested the induction of apoptosis. Administration of N-acetyl cysteine and vitamin C two ROS scavengers also resulted in significant inhibition of cytotoxicity induced by S. chloroleuca. These results support a mechanism whereby S. chloroleuca induces apoptosis of MCF-7 human breast cells through a ROS-mediated pathway.
Key Words: Salvia chloroleuca, Lamiaceae, Cytotoxicity, Apoptosis, ROS
Introduction
The genus Salvia (Lamiaceae) with about 900 different species, divided into five subgenera (Sclarea, Audibertia, Jungia, Leonia and Salvia), present in diverse areas as the Mediterranean, Central Asia, Pacific Islands, tropical Africa and America (1) of which 17 species are endemic to Iran (2). The known antioxidant, aromatic and antimicrobial properties of different members of the genus Salvia made it popular in the cosmetic industry, medicine and as food flavouring and preservation products since ancient times (3, 4).
The genus Salvia has widely used in traditional folk medicine. Different parts of the plant especially root (5, 6) are a rich source of different phytochemicals including terpenoids, polyphenols, as well as essential oil (7, 8). Salvia officinalis, S. miltiorrhiza, S. plebeia and S. menthaefolia are some species of the genus that have been recognized to possess anticancer and anti-proliferative activity on tumor cells. Salvia menthaefolia extract has been shown to take marked anti-proliferative activity against glioblastoma (DBTRG-05MG, T98G, U-87MG), colorectal adenocarcinoma (WiDr and HT-29), prostate adenocarcinoma (MDA Pca2b), choriocarcinoma (JEG-3), endometrium adenocarcinoma (HEC-1A) and B lymphoblast (CIR) tumor cell lines. In the same experiment S. spinosa, S. sclarea and S. dominica extracts have been shown to have a degree of cytotoxic activity dependent on the cell line type (9). In another study S. miltiorrhiza, has been exerted strong cytotoxic effects, and inhibited the proliferation of HepG2 cells clearly (10). Different diterpenoid quinones from S. officinalis L. have been reported to act cytotoxically and damaged DNA of human colon hepatoma cells (11). The characteristic apoptotic symptoms such as DNA fragmentation and chromatin condensation have been confirmed in the K562 cells treated with CH2Cl2 extract from S. plebeian (12).
Apoptosis or programmed cell death is an essential physiological process to maintain homeostasis in healthy tissues. Increasing evidences suggest that the process of neoplastic transformation, progression and metastasis involve alteration of normal apoptotic pathways (13). Because of the important role of apoptosis induction on the anticancer therapy most cancer-therapeutics are apoptosis inducing agent (14).
On the basis of these findings, Salvia species could be considered as a source of potential antitumor agents. The aim of this study was to evaluate the anti-proliferative activity of S. chloroleuca, one of the Iranian species of Salvia. Although the antimicrobial effect of the S. chloroleuca (15) has been reported previously but there is not any reported literature on S. chloroleuca. However, different reports have verified the cytotoxic and antitumor properties of some species belonging to this genus. Therefore, this study was designed to explore the cytotoxic and proapoptotic effect of S. chloroleuca on MCF-7 a human breast cancer cell line, extensively used in the study of breast cancer.
Experimental
Reagents and chemicals
RPMI-1640 medium and fetal bovine serum were purchased from Gibco (London, UK); 3-(4, 5-dimethylthiazol-2-yl) -5-(3-carboxymethoxyphenyl) -2-(4-sulphophenyl) -2H-tetrazolium (MTS), from Promega (Madison, WI, USA); ethidium bromide, RNase A and Proteinase K from Fermentas (Ontario, Canada).
Plant materials
The roots of S. chloroleuca were collected from Hosseinabad valley (2100 m height) in Pivejan on July 2011, a village at 65 km south-west of Mashhad, Razavi Khorasan province, northeast of Iran. The plant was identified by Mr M.R. Joharchi, from Ferdowsi University of Mashhad Herbarium (FUMH). Voucher specimen (No.11289) was deposited in herbarium of School of Pharmacy, University of Mashhad Medical Sciences.
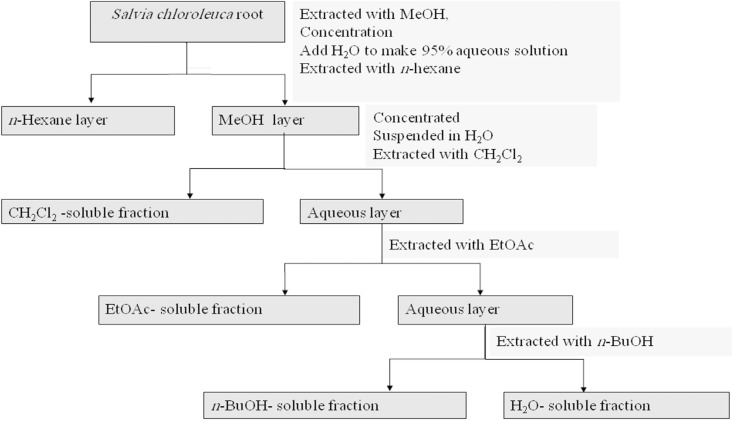
The dried root (100 g) was perculated with methanol (MeOH) at room temperature. The whole extract was filtered and the solvent was evaporated under reduced pressure at 40–45°C, to afford crude methanol extract (11.4 g). Methanol extract (10 g) was then resolved in methanol 95% and partitioned successively between n-hexane, methylen chloride (CH2Cl2), ethylacetate (EtOAc), and n-butanol (n-BuOH), and finally water based on increasing polarity of the solvent. n-Hexane, CH2Cl2, and EtOAc fractions were evaporated under vacuum to yield the residues of 0.27, 2.0, and 2.0 g fraction respectively. Water fraction was freeze dried. Extracts were stored at 4°C until analysis. A partitioning scheme of S. chloroleuca methanol extract is presented in Figure 1 (16).
Figure 1.
Partitioning scheme using immiscible solvents
All of the isolated fractions were dissolved in dimethylsulfoxide (DMSO) and then were subjected to cytotoxic and apoptosis assays.
Cell culture and treatment
The human breast cancer cells (MCF-7) were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 ºC in a humidified atmosphere of 95% air and 5% CO2. The stock solution of each compound was prepared at 100 mg/mL in dimethylsulfoxide and kept at -20 °C.
Human umbilical cord blood samples (50 mL) were collected from a fresh umbilical cord attached to the placenta by gravity flow in sterile 50 mL syringe containing citrate buffer as an anticoagulant. The sample diluted with an equal volume of Phosphate Buffered Saline (PBS), then layered over Ficoll-Hypaque density gradient separation solution (1.077 g/mL), and centrifuged at 800 g for 20 min at room temperature. The mononuclear cell layer was removed, washed twice in PBS and resuspended in RPMI 1640 medium supplemented with 10% (v/v) fetal bovine serum, and 100 U/mL penicillin and 100 mg/mL streptomycin. This study protocol was approved by the ethical committee of Mashhad University of Medical Sciences.
For MTS assay, cells were seeded at 104 cells per well onto 96-well culture plates. For assay of apoptosis, cells were seeded at 104 cell per well onto a 24-well plate. For each concentration and time course study there was a control sample that remained untreated and/ or received the equal volume of DMSO.
Cell viability
The MTS assay (17), is based on the reduction, by mitochondrial dehydrogenase in metabolically active cells, of the novel tetrazolium compound, 3-(4,5-dimethylthiazol-2-yl) -5-(3-carboxymethoxyphenyl) -2-(4-sulphophenyl) -2H-tetrazolium inner salt (MTS), to the colored, water-soluble formazan that absorbs at 490 nm. About 104 MCF-7 cells were seeded in each well of a 96-microwell plate and treated with various concentrations of each fraction of S. chloroleuca. After incubation for 48 h, CellTiter 96® aqueous one solution reagent (Promega, Madison, WI, USA), which is composed of the novel tetrazolium compound MTS and an electron coupling reagent phenazine methosulfate (PES, a redox intermediary), was added to each well according to the manufacturer’s instructions. After 3 h in culture, the cell viability was determined by measuring the absorbance at 490 nm using an ELISA microplate reader (Awareness, Palm City, FL, USA). The cytotoxicity of methanol extract of S. chloroleuca and its fractions was expressed as IC50, which was calculated using Graph Pad Software (Graph Pad prism 5 software) and presented as mean±SEM of three independent experiments with three replicates for each concentration fraction of S. chloroleuca fractions.
Cell morphology
The MCF-7 cells were plated in 96-well plates at a density of 104 cells/well and grown for 48 h in order to attach to the surface of the plates completely. The cytotoxicity of methanol extract of S. chloroleuca and its fractions were added in different concentrations (0, 7.8, 15.6, 31.2, 62.5, 125 and 250 μg/mL) to the cells and then the cells were grown at 37˚C in a humidified atmosphere with 5% CO2 for 48 h. For cell morphology experiments, the culture plates were examined and photographed by the inverted light microscope.
PI staining
Apoptotic cells were detected using PI staining of treated cells followed by flow cytometry to detect the so-called sub-G1 peak (18, 19).
It has been reported that DNA fragmentation creates small fragments of DNA that can be eluted following incubation in a hypotonic phosphate-citrate buffer. When stained with a quantitative DNA-binding dye such as PI, cells that have lost DNA will take up less stain and will appear to the left of the G1 peak. Briefly, 106 MCF-7 cells were seeded in each well of a 24-well plate and treated with CH2Cl2 fraction of S. chloroleuca in different concentrations (0, 25, 50 and 100 μg/mL) for 48 h. Floating and adherent cells were then harvested and incubated at 4°C overnight in the dark with 750 μL of a hypotonic buffer (50 μg/mL PI in 0.1% sodium citrate plus 0.1% Triton X-100) before flow cytometric analysis using a FACScan flow cytometer (Becton Dickinson). 104 events were acquired with FACS.
DNA fragmentation
Isolation of apoptotic DNA fragments was performed, based on the modified method previously described (20). In brief; the MCF-7 cells were incubated with CH2Cl2 fraction of S. chloroleuca in different concentrations (0, 12.5, 50, 100 and 200 μg/mL) for 48 h.
The formation of high molecular weight and oligonucleosomal DNA fragments was examined by agarose gel electrophoresis. Cells (106 cells) were seeded onto 6 well plates and treated for 48 h. The cells were collected by centrifugation at 800 g for 7 min. The DNA from treated and untreated cells was extracted as explained below: cells were incubated with 50 μL of lysis buffer (20 mM Tris, 20 mM EDTA, 200 mM NaCl and 1% SDS) and 2 μL RNase A (500 μg/mL) for 1 h at 37ºC. The cells were further incubated at 50ºC for 1 h after adding 2.5 μL of 10 mg/mL Proteinase K which had been preheated in 37ºC for 30 min. The lysate was mixed with 10 mL of loading solution (30% Ficoll, and 1% bromophenol blue in TBE), The DNA samples were separated in 2% agarose gel electrophoresis at 120 V, 30 min and visualized with ethidium bromide.
Determination of ROS
An increased reactive oxygen species generation can induce apoptosis (21). MCF-7 cells were cultured on 96-well microplate to 1×104 cells/well. Cells were treated with 250 mM CH2Cl2 fraction of S. chloroleuca for 24 h, washed, then incubated with 20 mM ROS-specific dye, CM-H2DCFDA that is specific for hydrogen peroxide (H2O2) in HBSS for 30 min at 37°C. NAC (3 and 6 μM) or Vit C (3 and 6 μM) was added 1 h before and during CH2Cl2 fraction of S. chloroleuca treatment. The fluorescence intensity was analyzed with an excitation wavelength at 485 nm and emission at 530 nm (22, 23).
Statistical analysis
One way analysis of variance (ANOVA) and Bonferroni’s posthoc were used for data analysis. All results were expressed as mean ± SEM and p values below 0.05 were considered statistically significant.
Results
Inhibition of cell viability
Inhibition of cell viability caused by total methanol extract of S. chloroleuca and its fractions was examined using MTS assay.
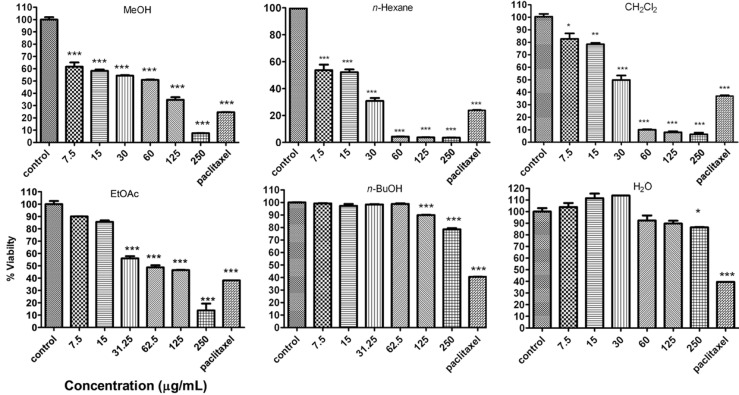
In order to compare the cytotoxicity of total methanol extract of S. chloroleuca and its fractions the MCF-7 cells were incubated with different concentrations for 48 h. The results showed methanol extract of S. chloroleuca and n-hexane and CH2Cl2 fractions decreased cell viability of cells in a concentration-dependent manner (Figure 2). This toxicity was coupled with morphological changes including decrease in cell volume and rounding of the cells. The substantial morphological changes observed in treated MCF-7 cells were examined and photographed by the inverted light microscope. Damaged cells became round and shrunken, while the untreated cells remained normal in size and shape (Figure 2). The doses inducing 50% cell growth inhibition (IC50) against MCF-7 cells for total methanol extract of S. chloroleuca and n-hexane, CH2Cl2, EtOAc, n-BuOH, and H2O fractions are presented in Table 1. In comparison, the cytotoxic effect of total methanol extract of S. chloroleuca and n-hexane, CH2Cl2, EtOAc, n-BuOH, and H2O fractions on normal lymphocyte isolated from peripheral blood was minimal (Figure 2). We used 700 nM of Paclitaxel as a positive control.
Figure 2.
A: Dose-dependent growth inhibition of MCF-7 cells by solvent fractions of S. chloroleuca (0, 7.8, 15.6, 31.2, 62.5, 125 and 250 μg/mL) after 48 h. Viability was quantitated by MTS assay. The dose inducing IC50 against MCF-7 by MeOH, n-hexane, CH2Cl2, EtOAc, n-BuOH, and H2O solvent fractions of S. chloroleuca were calculated 60.25, 28.06, 25.49, 63.14, >250, and >250 respectively.
B: The cytotoxic effect of total methanol extract of S. chloroleuca and n-hexane, CH2Cl2, EtOAc, n-BuOH, and H2O fractions on normal lymphocyte proliferation isolated from peripheral blood. Paclitaxel (700 nM) was used as a positive control. Results are mean ± SEM (n = 3). *p <0.05, **p < 0.01 and ***p < 0.001 compared to control.
C: Morphological changes of MCF-7 cells after treatment with MeOH, n-hexane, and CH2Cl2 solvent fractions of S. chloroleuca (0, 7.8, 15.6, 31.2, 62.5, 125 and 250 μg/mL) after 48 h. Control cells remained untreated and received an equal volume of the solvent.
Table 1.
IC50 values (μg/mL) for different solvent fractions of S. chloroleuca in MCF-7 cell line
| IC 50 range | IC 50 | |
|---|---|---|
| to 72.31 48.29 | 60.25 | MeOH |
| to 33.09 23.80 | 28.06 | n-hexane |
| to 30.99 20.96 | 25.49 | CH2Cl2 |
| to 85.71 46.51 | 63.14 | EtOAc |
| 250< | n-BuOH | |
| … | 250< | H2o |
Apoptosis induction by CH 2 Cl 2 fraction in MCF-7 cells
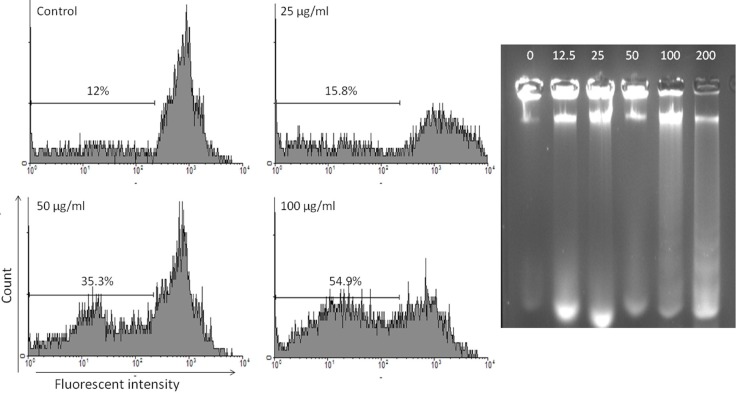
Apoptosis following treatment with CH2Cl2 solvent fraction of S. chloroleuca was measured with PI staining and flow cytometry aiming to detect the sub-G1 peak resulting from DNA fragmentation. The MCF-7 cells treated with 12.5, 25, 50 μg/mL CH2Cl2 solvent fraction of S. chloroleuca for 48 h induced a sub-G1 peak in flow cytometry histogram compared to untreated control cells (Figure 3A).
Figure 3.
A: Flow cytometry histograms of apoptosis assays by PI method in MCF-7 cells. Cells were treated with different concentration of CH2Cl2 solvent fractions of S. chloroleuca (0, 25, 50 and 100 μg/mL) for 48 h. Sub-G1 peak as an indicative of apoptotic cells, was induced in CH2Cl2 solvent fractions of S. chloroleuca treated but not in control cells. CH2Cl2 fraction-treated cells exhibited a sub-G1 peak in MCF-7 cells in a concentration dependent manner that indicates the involvement of an apoptotic process in CH2Cl2 fraction-induced cell death.
B: Internucleosomal fragmentation of CH2Cl2 fraction treated MCF-7 cells. Cells were treated with different concentration of CH2Cl2 fraction (lane 1, 2, 3, 4, 5 and 6 are 0, 12.5, 25, 50, 100 and 200 μg/mL respectively) for 48 h. After harvesting the cells, isolated DNA was analyzed by agarose gel electrophoresis.
Another characteristic event of cell apoptosis is the fragmentation of genomic DNA into integer multiples of 180-200 bp units producing a characteristic ladder on agarose gel electrophoresis. This event was observed in the MCF-7 cells within 48 h after treatment with CH2Cl2 solvent fraction of S. chloroleuca at 12.5, 50, 100 and 200 μg/mL (Figure 3B).
CH 2 Cl 2 solvent fraction of S. chloroleuca increased the production of ROS
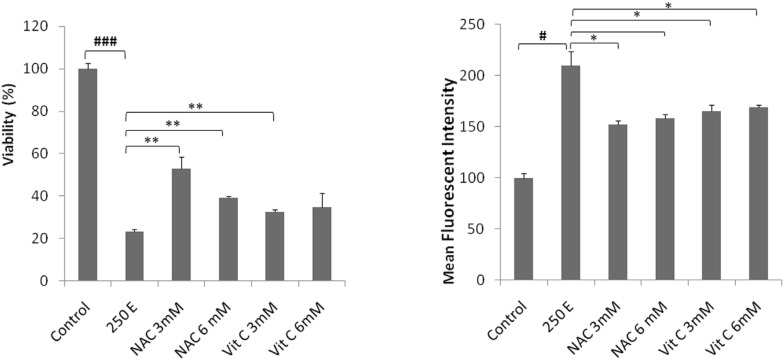
The intracellular level of ROS was measured, to determine whether CH2Cl2 solvent fraction of S. chloroleuca-induced apoptosis is mediated by oxidative stress. As shown in Figure 4A, treatment of cells with CH2Cl2 solvent fraction of S. chloroleuca increased the production of ROS significantly. For each time point, the 520 nm fluorescence level was measured in control and CH2Cl2 solvent fraction of S. chloroleuca treated cells. The ratio obviously indicates a ROS production in CH2Cl2 solvent fraction of S. chloroleuca-treated MCF-7 cells (Figure 4). In addition, the co-treatment of cells with CH2Cl2 solvent fraction of S. chloroleuca and either NAC (3 and 6 mM) or Vit C (3 and 6 mM) antioxidant inhibited the CH2Cl2 solvent fraction of S. chloroleuca -induced ROS production significantly (Figure 4B) indicating that the fluorescence augmentation was related to ROS production.
Figure 4.
Effects of ROS generation on CH2Cl2 solvent fractions of S. chloroleuca induced cytotoxicity in MCF-7 cells. A: CH2Cl2 solvent fractions of S. chloroleuca induce intracellular ROS accumulation in MCF-7 cells. MCF-7 cells were treated, or not (control), for 24 h with 250 μM CH2Cl2 solvent fractions of S. chloroleuca in the presence, or absence, of either 3 and 6 mM NAC, or 3 and 6 mM NAC Vit C before incubation with 10 μM of CM-H2DCFDA probe for 30 min and the fluorescence was analyzed with an excitation wavelength at 485 nm and emission at 530 nm. B: ROS accumulation contributes to the cytotoxicity induced by CH2Cl2 solvent fractions of S. chloroleuca in MCF-7 cells. Cell viability was measured by the MTS assay. Results are mean±SEM (n = 3). *p <0.05, **p <0.01 and ****p <0.001 <0.001 compared to control.
Discussion
Cytotoxic properties of total methanol extract of S. chloroleuca and its fractions were investigated on MCF-7, a breast carcinoma cell line as the most common neoplasm in women around the world (24). Extracts were tested for cytotoxic activity at a range of 0-250 μg/mL after 48 h of treatment.
There are few reports on biological activity of the S. chloroleuca. Yousefzadi et al., reported the in-vitro antimicrobial activity of the essential oil of S. chloroleuca against seven Gram-positive and Gram-negative bacteria and three fungi (25). The anti-proliferative and cytotoxic effect of many Salvia species on several cancer cell lines have been reported previously (9, 10, 12, 26). In a previous study Badisa et al., were evaluated eight crude extracts of five Salvia species for cytotoxic activities against brine shrimps and four human cancer cell lines (HCA, HepG2, MCF-7, HPC) (27).
In another study in-vitro anti-proliferative screening investigation of crude methanol extracts of six Salvia species: S. dominica L. leaves, S. lanigera Desf. aerial parts, S. menthaefolia Ten. roots, S. palaestina Benth. aerial parts, S. sclarea L. roots and S. spinosa L. aerial parts, revealed growth inhibitory activity with IC50 values ranged from 90 to 400 μg/mL (9).
It was also reported that CH2Cl2 extract from S. plebeia R. Br. may inhibit the cancer cell proliferation by inducing cell apoptosis (12). However there was not any similar investigation on S. chloroleuca.
In our study methanol extract obtained from S. chloroleuca has a cytotoxic and apoptotic activity on MCF-7 cells. In order to gain insight into the nature of the active principles responsible for the cytotoxic activity, the methanol extract was fractionated using solvents of increasing polarity. The results observed with the entire S. chloroleuca extract and n-hexane and CH2Cl2 fractions confirmed the presence of potent none/ semi polar phytochemicals in the plant. The entire extract of plant is generally a complicated mixture of several compounds that possess variable chemophysical properties. The major plan for extrication of these compounds is based on their chemophysical properties that can be exploited to primarily separate them into various chemical groups.
From the literature search of the related genera and families, it is possible to predict the cytotoxic compounds that might be present in S. chloroleuca extract. Extraction with solvents of increasing polarity helps to predict specific classes of compounds (28).
The IC50 values of the fractions and methanol extract are compared in Table 1. An active constituent(s) of intermediate polarity is thus likely to be responsible for the observed cytotoxicity and future bioassay guided fractionation needs to focus on the CH2Cl2 fraction. These results suggest that CH2Cl2 fraction obtained from S. chloroleuca could be used as a potential apoptosis inducing agent, and that the CH2Cl2 fraction obtained from S. chloroleuca consists of a key component for cytotoxic activity.
Apoptosis induction protects organisms against tumor development (29, 30). Ladder-like pattern of DNA fragmentation into the multiples of 180 bp has been considered as a biochemical hallmark of apoptosis (31).
Anti-cancer effect through ROS induction has been reported for many naturally occurring compounds including d-Limonene, a bioactive food component from citrus (32, 33).
To investigate the mechanism of the CH2Cl2 solvent fraction of S. chloroleuca -induced cytotoxicity, we examined the effect of CH2Cl2 solvent fraction of S. chloroleuca on ROS levels in MCF-7 cells. The results indicate that cellular ROS were strongly induced by CH2Cl2 solvent fraction of S. chloroleuca, suggesting that CH2Cl2 solvent fraction of S. chloroleuca function as pro-oxidants in cancer cells.
ROS accumulation contributes to the cytotoxicity induced by CH2Cl2 solvent fraction of S. chloroleuca. We next investigated whether the ROS accumulation is required for the potentiated cytotoxicity induced by CH2Cl2 solvent fraction of S. chloroleuca-treatment. As shown in Figure 4A, both the ROS scavengers Vit C and NAC suppressed the CH2Cl2 solvent fraction of S. chloroleuca-induced cytotoxicity.
These results suggest that induction of ROS is crucial for CH2Cl2 solvent fraction of S. chloroleuca-induced cytotoxicity in MCF-7 cells.
Our finding in this study also showed that CH2Cl2 solvent fraction of S. chloroleuca induced significant cellular ROS accumulation in cancer cells, which substantially contribute to cytotoxicity in CH2Cl2 solvent fraction of S. chloroleuca treated cell.
Regarding the sequential extraction with solvents of ascending polarity, the fractionation of S. chloroleuca with n-hexane and CH2Cl2 generated fractions that were cytotoxic for tumor cells. This can be explained by the low polarity of the solvents used in these fractions, which extract low-polar compounds that are either successfully absorbed through the cell or have cytotoxic activity. Therefore, it is presumed that most of the cytotoxic compounds or the compounds with the highest potency were concentrated in the n-hexane and CH2Cl2 fractions.
In conclusion, this study determined an anti-cancer effect of CH2Cl2 fraction obtained from S. chloroleuca mediated by the induction of apoptosis, which is associated with DNA fragmentation and induction of ROS, in MCF-7 cells. As apoptosis has become a new therapeutic target in cancer research, these results confirm the potential cytotoxic activity of n-hexane and CH2Cl2 fractions obtained from S. chloroleuca in human breast cancer cells and n-hexane and CH2Cl2 fractionation are more efficient than methanol extraction. n-Hexane and CH2Cl2 fractions can extract a compounds that has valuable cytotoxic activity. However, further investigation on the active principals of CH2Cl2 fraction obtained from S. chloroleuca is necessary to be elaborated.
Acknowledgements
The authors would like to thank Dr. H. Nasirli for her assistance in flow cytometry. This work was supported by grants (No. 89514) from Research Affairs of Mashhad University of Medical Sciences as a part of Pharm. D. thesis.
References
- 1.Hedge IC. A global survey of the biogeography of the Labiatae. In: Harley RM, Reynolds T, editors. Advances in Labiatae Science. Kew, London: Royal Botanical Gardens; 1992. pp. 7–17. [Google Scholar]
- 2.Emami SA, Aghazari F. Téhéran, Iran: Publications de I’Université de Téhéran; 2011. Les Phanerogames Endemiques de la Flore d’Iran; pp. 394–397. [Google Scholar]
- 3.Cowan MM. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gali-Muhtasib H, Hilan C, Khater C. Traditional uses of Salvia libanotica, (east Mediterranean sage) and the effects of its essential oils. J. Ethnopharmacol. 2000;71:513–520. doi: 10.1016/s0378-8741(99)00190-7. [DOI] [PubMed] [Google Scholar]
- 5.Gohari AR, Ebrahimi H, Saeidnia S, Foruzani M, Ebrahimi P, Ajani Y. Flavones and flavone glycosides from Salvia macrosiphon Boiss. Iran. J. Pharm. Res. 2011;10:247–251. [PMC free article] [PubMed] [Google Scholar]
- 6.Fronza M, Murillo R, Ślusarczyk S, Adams M, Hamburger M, Heinzmann B, Laufer S, Merfort I. In-vitro cytotoxic activity of abietane diterpenes from Peltodon longipes as well as Salvia miltiorrhiza and Salvia sahendica. Bioorg. Med. Chem. 2011;19:4876–81. doi: 10.1016/j.bmc.2011.06.067. [DOI] [PubMed] [Google Scholar]
- 7.Farjam MH, Rustaiyan A, Ezzatzadeh E, Jassbi AR. Labdane-type diterpene and two flavones from Salvia sharifii Rech. f. & Esfand. and their biological activities. Iran. J. Pharm. Res. 2013;12:395–399. [PMC free article] [PubMed] [Google Scholar]
- 8.Lu Y, Foo LY. Polyphenolics of Salvia-a review. Phytochemistry. 2002;59:117–140. doi: 10.1016/s0031-9422(01)00415-0. [DOI] [PubMed] [Google Scholar]
- 9.Fiore G, Nencini C, Cavallo F, Capasso A, Bader A, Giorgi G, Micheli L. In-vitro antiproliferative effect of six Salvia species on human tumor cell lines. Phytother. Res. 2006;20:701–703. doi: 10.1002/ptr.1911. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Shen HM, Ong CN. Salvia miltiorrhiza inhibits cell growth and induces apoptosis in human hepatoma HepG2 cells. Cancer Lett. 2000;153:85–93. doi: 10.1016/s0304-3835(00)00391-8. [DOI] [PubMed] [Google Scholar]
- 11.Slamenˇova´ D, Masˇterova´ I, La´baj J, Horva´thova´ E, Kubala P, Jakubı´kova´ J, Wso´lova´ L. Cytotoxic and DNA-damaging effects of diterpenoid quinones fromthe roots of Salvia officinalis L. on colonic and hepatic human cells cultured in-vitro. Basic Clin. Pharmacol. Toxicol. 2004;94:282–290. doi: 10.1111/j.1742-7843.2004.pto940605.x. [DOI] [PubMed] [Google Scholar]
- 12.Jie R, Sha-Sha P, Xu-Zhang L, Min Z, Kun H. Antitumor activity of dichloromethane extract from Salvia plebeia and induction of apoptosis on K562 cells. Chin. Herbal Med. 2010;3:36–40. [Google Scholar]
- 13.Bold RJ, Termuhlen PM, McConkey DJ. Apoptosis, cancer and cancer therapy. Surg. Oncol. 1997;6:133–142. doi: 10.1016/s0960-7404(97)00015-7. [DOI] [PubMed] [Google Scholar]
- 14.Kamesaki H. Mechanism involved in chemotherapy induced apoptosis and their implications in cancer chemotherapy. Int. J. Hematol. 1998;68:29–43. doi: 10.1016/s0925-5710(98)00038-3. [DOI] [PubMed] [Google Scholar]
- 15.Yousefzadi M, Sonboli A, Ebrahimi SN, Hashemi SH. Antimicrobial activity of essential oil and major constituents of Salvia chloroleuca. Z. Naturforsch. C. 2008;63:337–40. doi: 10.1515/znc-2008-5-605. [DOI] [PubMed] [Google Scholar]
- 16.Otsuka H. Purification by solvent extraction using partition coefficient. In: Sarker SD, Latif Z, Gray AL, editors. Natural Products Isolation. 2nd ed. New Jersey: Humana Press; 2006. pp. 269–73. [Google Scholar]
- 17.Malich G, Markovic B, Winder C. The sensitivity and specificity of the MTS tetrazolium assay for detecting the in-vitro cytotoxicity of 20 chemicals using human cell lines. Toxicology. 1997;124:179–192. doi: 10.1016/s0300-483x(97)00151-0. [DOI] [PubMed] [Google Scholar]
- 18.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 19.Tayarani-Najaran Z, Mousavi SH, Asili J, Emami SA. Growth inhibitory effect of Scutellaria lindbergii in human cancer cell lines. Food Chem. Toxicol. 2010;48:599–604. doi: 10.1016/j.fct.2009.11.038. [DOI] [PubMed] [Google Scholar]
- 20.Park DJ, Patek PQ. Detergent and enzyme treatment of apoptotic cells for the observation of DNA fragmentation. Biotechniques. 1998;24:558–560. doi: 10.2144/98244bm07. [DOI] [PubMed] [Google Scholar]
- 21.Buttke TM, Sandstrom PA. Oxidative stress as a mediator of apoptosis. Immunol. Today. 1994;15:7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 22.Rosenkranz AR, Schmaldienst S, Stuhlmeier KM, Chen W, Knapp W, Zlabinger GJ. A microplate assay for the detection of oxidative products using 2›, 7›-dichlorofluorescin-diacetate. J. Immunol. Methods. 1992;156:39–45. doi: 10.1016/0022-1759(92)90008-h. [DOI] [PubMed] [Google Scholar]
- 23.Mansat-de Mas V, Bezombes C, Quillet-Mary A, Bettaieb A, D›orgeix AD, Laurent G, Ja¡rezou JP. Implication of radical oxygen species in ceramide generation, c-Jun N-terminal kinase activation and apoptosis induced by daunorubicin. Mol. Pharmacol. 1999;56:867–874. doi: 10.1124/mol.56.5.867. [DOI] [PubMed] [Google Scholar]
- 24.Baselga J, Mendelsohn J. The epidermal growth factor receptor as a target for therapy in breast carcinoma. Breast Cancer Res. Treat. 1994;29:127–138. doi: 10.1007/BF00666188. [DOI] [PubMed] [Google Scholar]
- 25.Rózalski M, Kuźma L, Krajewska U, Wysokińska H. Cytotoxic and proapoptotic activity of diterpenoids from in-vitro cultivated Salvia sclarea roots studies on the leukemia cell lines. Z. Naturforsch. C. 2006;61:483–8. doi: 10.1515/znc-2006-7-804. [DOI] [PubMed] [Google Scholar]
- 26.Parsaee H, Asili J, Mousavi SH, Soofi H, Emami SA, Tayarani-Najaran Z. Apoptosis induction of Salvia chorassanica root extract on human cervical cancer cell line. Iran. J. Pharm. Res. 2013;12:75–83. [PMC free article] [PubMed] [Google Scholar]
- 27.Badisa RB, Tzakou O, Couladis M, Pilarinou E. Cytotoxic activities of Salvia of the Labiatae family. Pharm. Biol. 2005;42:640–645. [Google Scholar]
- 28.Tayarani-Najaran Z, Emami SA, Asili J, Mirzaei A, Mousavi SH. Analyzing cytotoxic and apoptogenic properties of Scutellaria litwinowii root extract on cancer cell lines. Evidence Based Complement. Alternat. Med. 2011;2011:1–9. doi: 10.1093/ecam/nep214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hickman JA. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 1992;11:121–129. doi: 10.1007/BF00048059. [DOI] [PubMed] [Google Scholar]
- 30.Barry MA, Behnke CA, Eastman A. Activation of programmed cell death. (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem. Pharmacol. 1990;40:2353–2362. doi: 10.1016/0006-2952(90)90733-2. [DOI] [PubMed] [Google Scholar]
- 31.Cohen GM, Sun XM, Snowden RT, Dinsdale D, Skilleter DN. Key morphological features of apoptosis may occur in the absence of internucleosomal DNA fragmentation. Biochem. J. 1992;286:331–334. doi: 10.1042/bj2860331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loo G. Redox-sensitive mechanisms of phytochemical-mediated inhibition of cancer cell proliferation. J. Nutr. Biochem. 2003;14:64–73. doi: 10.1016/s0955-2863(02)00251-6. [DOI] [PubMed] [Google Scholar]
- 33.Rabi T, Bishayee A. d-Limonene sensitizes docetaxel-induced cytotoxicity in human prostate cancer cells, generation of reactive oxygen species and induction of apoptosis. J. Carcinog. 2009;8 doi: 10.4103/1477-3163.51368. [DOI] [PMC free article] [PubMed] [Google Scholar]