Abstract
Purpose
To clarify the functional and molecular mechanisms inducing hyper-excitability of C-fiber bladder afferent pathways after spinal cord injury we examined changes in the electrophysiological properties of bladder afferent neurons, focusing especially on voltage-gated K channels.
Materials and Methods
Freshly dissociated L6-S1 dorsal root ganglion neurons were prepared from female spinal intact and spinal transected (T9-T10 transection) Sprague Dawley® rats. Whole cell patch clamp recordings were performed on individual bladder afferent neurons. Kv1.2 and Kv1.4 α-subunit expression levels were also evaluated by immunohistochemical and real-time polymerase chain reaction methods.
Results
Capsaicin sensitive bladder afferent neurons from spinal transected rats showed increased cell excitability, as evidenced by lower spike activation thresholds and a tonic firing pattern. The peak density of transient A-type K+ currents in capsaicin sensitive bladder afferent neurons from spinal transected rats was significantly less than that from spinal intact rats. Also, the KA current inactivation curve was displaced to more hyperpolarized levels after spinal transection. The protein and mRNA expression of Kv1.4 α-subunits, which can form transient A-type K+ channels, was decreased in bladder afferent neurons after spinal transection.
Conclusions
Results indicate that the excitability of capsaicin sensitive C-fiber bladder afferent neurons is increased in association with reductions in transient A-type K+ current density and Kv1.4 α-subunit expression in injured rats. Thus, the Kv1.4 α-subunit could be a molecular target for treating overactive bladder due to neurogenic detrusor overactivity.
Keywords: urinary bladder, overactive, spinal cord injuries, potassium channels, voltage-gated, afferent pathways, nerve fibers, unmyelinated
Spinal cord injury above the lumbosacral level eliminates voluntary and supraspinal control of voiding, leading initially to areflexic bladder and urinary retention, followed by the slow development of automatic micturition and NDO mediated by spinal micturition reflex pathways.1 Electrophysiological studies in animal models revealed that excitability of the afferent limb of the micturition reflex is increased after SCI, which was proposed as an important pathophysiological basis of NDO with SCI.1
Bladder afferent pathways consist of 2 types of axons, ie Aδ and C fibers. In the normal condition Aδ fibers, which are mechanosensitive and respond to bladder distention, initiate the micturition reflex. In chronic SCI rats capsaicin sensitive C-fiber afferents show increased excitability2 and must be responsible for initiating NDO because desensitizing C-fiber afferents by systemic capsaicin administration suppresses nonvoiding contractions in SCI rats.1
Mechanisms underlying the hyperexcitability of C-fiber bladder afferent pathways after SCI were previously investigated using whole cell patch clamp recordings in DRG neurons innervating the rat bladder.2 The density of TTX sensitive Na+ currents in bladder afferent neurons significantly increased, while TTX resistant Na+ current density decreased after SCI. This indicated that SCI induces a switch in the expression of Na+ channels from the TTX resistant to the TTX sensitive type. Since TTX sensitive Na+ currents have a lower threshold for action potential activation than TTX resistant currents, it is assumed that these changes in the expression of Na+ channels in bladder afferent neurons after SCI contribute to a low threshold for spike activation in these neurons.
Although Na+ channels are a major determinant of neuronal excitability, Kv channel activation is also an important factor for spike threshold and firing frequency control. Kv currents in sensory neurons are divided into 2 major categories, ie sustained KDR and transient KA currents.3 A reduction in KDR and/or KA currents is involved in the hyperexcitability of afferent pathways under various pathological conditions,4 including chronic bladder inflammation, which increases the excitability of capsaicin sensitive bladder DRG neurons by decreasing KA currents without affecting KDR currents.5,6
However, little is known about functional changes in Kv currents after SCI. In addition, to our knowledge the molecular mechanism responsible for changes in Kv currents after SCI remains to be elucidated. Therefore, we sought to clarify the mechanisms inducing bladder afferent neuron hyperexcitability in SCI rats, especially focusing on Kv channels.
MATERIALS AND METHODS
Animal Preparation
Experiments were performed in spinal intact and spinal transected adult female Sprague Dawley rats weighing 170 to 220 gm. All animal experiments were done in accordance with institutional guidelines and approved by the University of Pittsburgh institutional animal care and use committee.
Spinal cord transected rats were prepared by complete transection of the Th8-Th9 spinal cord, as previously described.2 The population of DRG neurons that innervates the bladder was labeled by retrograde axonal transport of the fluorescent dye Fast Blue (1% weight per volume, PolyScience®) or DiI (1% weight per volume, Invitrogen™). Dye was injected in the bladder wall in isoflurane anesthetized animals 7 to 10 days before dissociation, as described in our previous study.7
Cell Dissociation and Whole Cell Patch Clamp Recordings
Four weeks after spinal cord transection dissociated L6-S1 DRG cells were prepared as previously described.7 Since 80% of C-fiber bladder afferent neurons are sensitive to capsaicin but only 5% of Aδ-fiber bladder neurons are capsaicin sensitive,8 capsaicin sensitive neurons were selected for C-fiber population evaluation. Whole cell patch clamp recordings were performed at room temperature (20C to 22C) on each Fast Blue positive neuron within 10 hours after dissociation. The internal solution contained 140 mM KCl, 1 mM CaCl2, 2 mM MgCl2, 11 mM EGTA, 10 mM HEPES and 2 mM Mg adenosine triphosphate, adjusted to pH 7.4 with KOH. Patch electrodes had 2 to 4 MΩ resistance when filled with the internal solution. Neurons were superfused at a flow rate of 2.0 ml per minute with an external solution containing 150 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2, 10 mM HEPES and 10 mM D-glucose, adjusted to pH 7.4 with NaOH.
To isolate K+ currents after evaluating action potential characteristics we changed the external solution to one containing 150 mM choline Cl, 5 mM KOH, 0.03 mM CaCl2, 10 mM HEPES, 3 mM Mg(OH)2 and 10 mM D-glucose, adjusted to pH 7.4 with tris base. Slow KA current inactivation and activation characteristics were examined using the same membrane voltage paradigm described in our previous study.7
Immunohistochemistry
Spinal intact and transected rats were anesthetized with pentobarbital (80 mg/kg) intraperitoneally. They were perfused through the left ventricle with 300 ml cold oxygenated PBS, followed by fixative solution consisting of 4% paraformaldehyde in 0.1 M PBS. L6 DRGs were then removed and postfixed for 12 hours in the same fixative solution. Tissues were placed in PBS containing increasing concentrations of sucrose (10%, 20% and 30%) at 4C for cryoprotection, frozen in mounting medium and sectioned at 10 μm. After mounting on slides the sections were washed and incubated with antibodies for Kv1.2 or Kv1.4 α-subunits (Alomone Labs, Jerusalem, Israel) for 18 hours at 4C, followed by incubation with anti-rabbit IgG antibody conjugated to FITC for 1.5 hours at room temperature. Images were obtained with a fluorescence microscope. We confirmed that there was no positive staining above background when primary antibody was omitted (data not shown).
In 6 randomly selected DRG sections from each of 3 rats we measured FITC fluorescence intensity in individual neurons using ImageJ (http://rsbweb.nih.gov/ij/). The mean labeling intensity of Kv α-subunits was calculated in dye labeled bladder afferent neurons and in unlabeled DRG neurons. We determined the ratio of mean labeling intensity of bladder afferent neurons to that of unlabeled neurons in each DRG section. The staining density ratio (dye labeled vs unlabeled cells) per section was then averaged in randomly selected DRG sections from each rat. The mean ratio in each rat was again averaged in the spinal intact or spinal transected groups. These analytical methods of Kv α-subunit staining were used to minimize variations in staining intensity between different DRG sections, which might occur due to different staining conditions and nonlinear fluorescent signal decay among sections.
Laser Capture Microdissection and Real-Time PCR
Using isoflurane anesthesia, L6 DRGs were removed in a separate group of 5 spinal intact and 5 transected rats that received DiI injection in the bladder wall 1 week earlier. L6 DRGs were embedded in Tissue-Tek® O.C.T.™ Compound and stored at −80C until use. Samples were sectioned at 8 μm and sections were mounted on PEN membrane slides (Leica Microsystems, Wetzlar, Germany). The tissue was air-dried. Laser capture microdissection was performed using an LMD6000 (Leica Microsystems) to separately dissect DiI labeled and unlabeled bladder afferent neurons. Excised cells were individually captured in the caps of 0.5 ml Eppendorf tubes and lysed. RNA isolation, reverse transcription and real-time PCR were performed using a Cells Direct™ One-Step qRT-PCR Kit.
Gene specific primers and TaqMan® probes crossing exon-exon junctions were designed for the Kv1.2 and Kv1.4 α-subunits using Primer3 (http://primer3.sourceforge.net/) (table 1). Probes contained FAM fluorophore and TAMRA quencher. Primer-probe combinations were optimized within suitable ranges for efficiency and correlation coefficients using standard curve dilutions. Data output was done on a StepOnePlus™ thermocycler. cDNA was amplified under the conditions of 1 cycle at 50C for 45 minutes and 95C for 2 minutes, followed by 50 cycles at 95C for 15 seconds and 60C for 1 minute. Reactions were analyzed in triplicate and normalized relative to GAPDH.
Table 1.
Primer and TaqMan probe sequences
| Kv1.2 | Kv1.4 | GAPDH | |
|---|---|---|---|
| Primer: | |||
| 5′ | CCTGTCTATCACCCAGGAACA | TGACCTACTGCCACAGGATG | AGACAGCCGCATCTTCTTGT |
| 3′ | TTGAGGAGAGTGGAGCTTGG | GGTTTCGAAGCGTAGACCAG | GATACGGCCAAATCCGTTC |
| Probe | CATCTGCAAGGGCAACGTCACA | CAGTGACTGTTGTGAACGCGTGGT | GCAGTGCCAGCCTCGTCTCA |
| % Efficacy | 99.8 | 104.4 | 104.0 |
Real-time PCR data were analyzed by the difference in CPs (crossing points) method using the equation, R = 2(CP sample – CP control), where R represents the expression ratio of each target gene relative to that of GAPDH. We also determined specificity to cDNA using real-time PCR to verify that our primer-probe sets did not amplify genomic DNA. All primer-probe combinations showed 100% to 104% efficiency (table 1).
Statistical Analysis
Data are shown as the mean ± SEM. The unpaired Student t-test was used to determine statistical differences between 2 groups with p <0.05 considered significant.
RESULTS
Spinal Transected Rats
Increased excitability of bladder afferent neurons
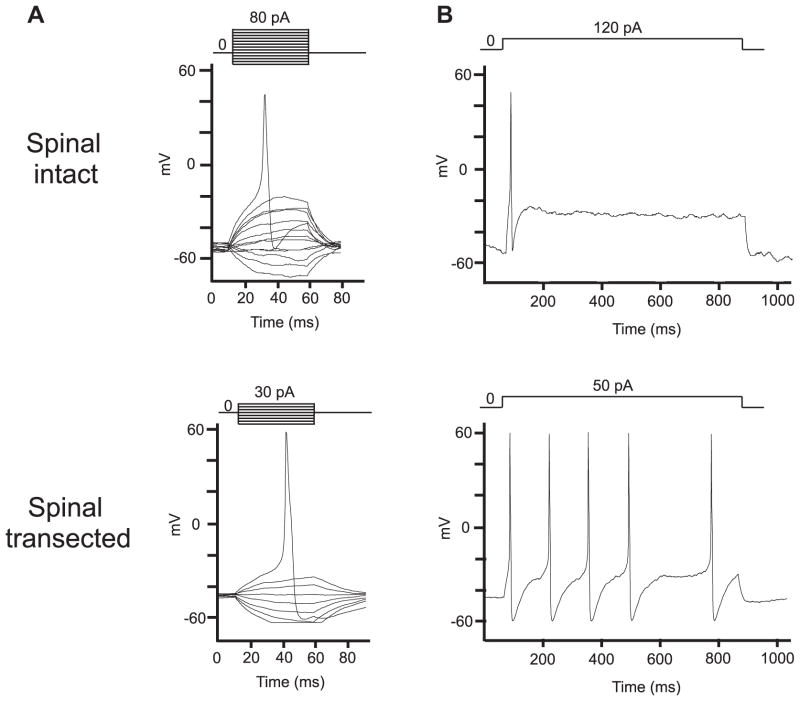
Figure 1 shows representative recordings of action potentials in capsaicin sensitive bladder afferent neurons from the 2 groups of rats. The resting membrane potential of capsaicin sensitive bladder afferent neurons did not differ between spinal intact and transected rats (table 2). However, the mean threshold for eliciting action potentials from spinal transected rats was significantly lower than that from spinal intact rats. Also, the number of action potentials during 800-millisecond membrane depolarization in capsaicin sensitive bladder afferent neurons from spinal transected rats was significantly greater than that in neurons from spinal intact rats when current intensity was set to the value just above the threshold for inducing spike activation with a 50-millisecond pulse (table 2). These results indicate that capsaicin sensitive bladder afferent neurons became hyperexcitable in SCI rats. In addition, the diameter and cell input capacitance of capsaicin sensitive bladder afferent neurons from spinal transected rats were significantly greater than those from spinal intact rats, indicating that spinal transection induced somal hypertrophy of bladder afferent neurons, as previously noted.2,9
Figure 1.
Representative action potential recordings in capsaicin sensitive bladder afferent neurons from spinal intact and transected rats. A, action potentials evoked by 50-millisecond (ms) depolarizing current pulses injected through patch pipettes during current clamp recording. B, firing patterns during sustained 800-millisecond membrane depolarization.
Table 2.
Electrophysiological properties of capsaicin sensitive bladder afferent neurons
| Control | SCI | |
|---|---|---|
| No. cells/rats | 17/13 | 19/13 |
| Mean ± SEM diameter (μm) | 25.8 ± 0.6 | 32.5 ± 0.9* |
| Mean ± SEM input capacitance (pF) | 23.2 ± 1.4 | 45.0 ± 3.4* |
| Mean ± SEM membrane potentials (mV): | ||
| Resting | −47.8 ± 1.5 | −46.8 ± 1.0 |
| Spike threshold | −21.8 ± 0.9 | −26.4 ± 1.3* |
| Peak | 38.0 ± 2.1 | 40.8 ± 2.1 |
| Mean ± SEM spike duration (msecs) | 4.3 ± 0.2 | 4.2 ± 0.3 |
| Mean ± SEM No. 800-msec depolarization action potentials | 1.3 ± 0.1 | 4.7 ± 0.7* |
| Density: | ||
| No. cells/rats | 20/12 | 17/10 |
| Mean ± SEM intensity KA (pA/pF) | 68.6 ± 6.3 | 38.1 ± 4.6* |
| Mean ± SEM intensity KDR (pA/pF) | 54.0 ± 7.0 | 48.6 ± 6.6 |
p <0.01 vs control.
Slow KA current reduction
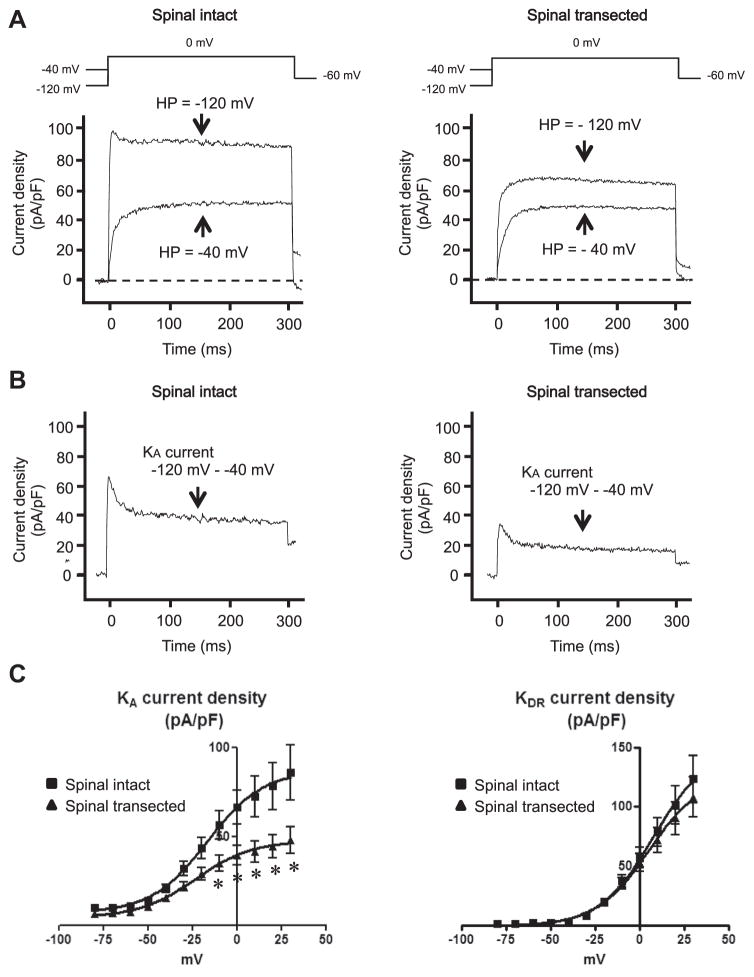
We estimated the density of slow KA currents by measuring the difference in currents activated by a depolarizing voltage pulse from −120 and −40 mV holding potentials (fig. 2, A and B). This method is useful because our previous studies showed that slow KA currents in C-fiber bladder afferent neurons were activated by depolarizing voltage steps from hyperpolarized membrane potentials but they were almost completely inactivated when membrane potential was maintained at a depolarized level of greater than −40 mV.5,10
Figure 2.
Changes in capsaicin sensitive bladder afferent neuron K+ currents in rats without vs with spinal cord transection. A, representative recordings show superimposed outward K+ currents evoked by voltage steps to 0 mV from −120 and −40 mV holding potentials (HP). B, KA currents were obtained by subtracting K+ currents evoked by depolarization to 0 mV from −40 and −120 mV holding potentials. C, mean ± SEM KA and KDR current-voltage relationships in 20 cells from 12 spinal intact rats and 17 from 10 spinal transected rats. Asterisk indicates p <0.05 vs spinal intact.
We calculated the peak density of slow KA currents evoked by depolarization to 0 mV from the difference in K+ currents activated from holding potentials of −40 and −120 mV, and sustained KDR currents evoked by depolarization to 0 mV from a holding potential of −40 mV. Density was increased during membrane depolarization in capsaicin sensitive bladder afferent neurons from spinal intact and transected rats (fig. 2, C ). However, slow KA peak current density was lower in neurons from spinal transected rats than those from spinal intact rats. We detected significant differences in current density at depolarizing pulses greater than −10 mV. However, the peak current density of sustained KDR currents in capsaicin sensitive bladder afferent neurons did not differ between spinal intact and transected rats.
Bladder Afferent Neurons
Steady-state activation and inactivation characteristics of KA currents in spinal intact and spinal transected rats
KA current in spinal intact rats started to inactivate at membrane potentials positive to −120 mV and were almost totally inactivated by depolarizing prepulses to −40 mV. Data were well fitted by the modified Boltzmann equation with a Vh of −87.1 mV (8 cells from 6 rats). This inactivation curve indicated that 10% to 15% of maximum current could be elicited at membrane potentials in the range of −60 to −50 mV, equivalent to resting membrane potential (fig. 3, A). In contrast, KA currents in spinal transected rats were almost negligible when holding membrane potential was in the range of −60 to −50 mV. In contrast to the inactivation curve in neurons from spinal intact rats, the inactivation curve for spinal transected rats was displaced to more hyperpolarized levels by approximately 10 mV. The Vh for KA current inactivation in spinal transected rats was −98.3 mV (7 cells from 7 rats) (fig. 3, A).
Figure 3.
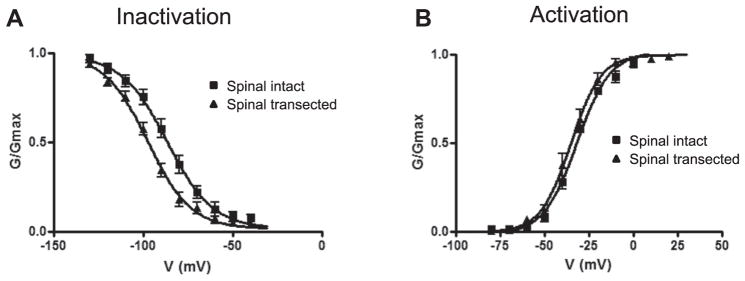
Mean ± SEM steady-state activation and inactivation characteristics of KA currents in capsaicin sensitive bladder afferent neurons, shown as relative peak conductance of KA currents normalized to maximal KA current conductance (G/Gmax) plotted against membrane potentials. A, inactivation characteristics in 8 cells from 6 spinal intact rats and 7 from 7 spinal transected rats. B, activation characteristics in 7 cells from 7 spinal intact rats and 12 from 10 spinal transected rats.
In contrast to the difference in inactivation characteristics, the voltage dependence of KA current activation did not differ between spinal intact and transected rats. KA current in bladder afferent neurons from spinal intact rats was elicited by membrane depolarizations higher than −60 mV with Vh occurring at the membrane potential of −32.2 mV according to the modified Boltzmann equation (7 cells from 7 rats). Similarly, the Vh of KA current activation of bladder afferent neurons from spinal transected rats was −35.4 mV (12 cells from 10 rats) (fig. 3, B).
Reduction of Kv1.4 α-subunit expression in spinal transected rats
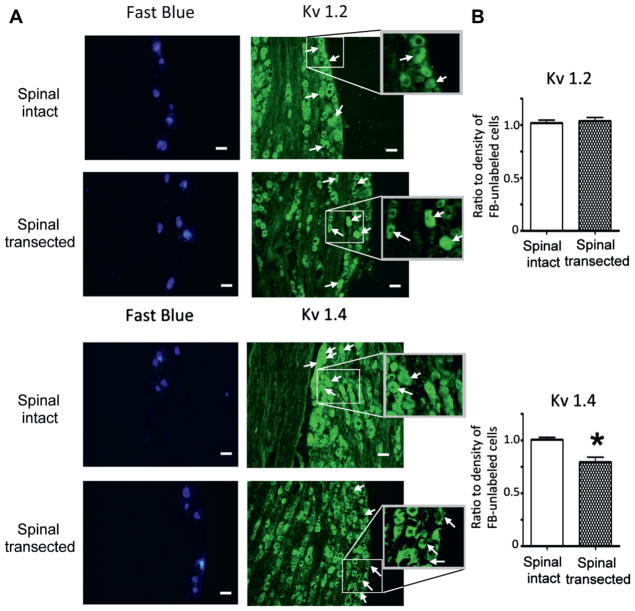
The ratio of Kv1.4 α-subunit staining density in bladder afferent and unlabeled DRG neurons was significantly lower in spinal transected than in spinal intact rats (112 and 132 cells from 3 rats each, 0.79 vs 1.01) (fig. 4, A) in which there was similar Kv1.4 α-subunit staining in Fast Blue labeled and unlabeled neurons. In contrast, in each group we noted similar Kv1.2 α-subunit staining density in Fast Blue labeled and unlabeled neurons (121 and 114 cells from 3 spinal intact and 3 spinal transected rats, respectively) (fig. 4, B).
Figure 4.
Kv α-subunit immunoreactivity in L6 DRGs after spinal cord transection. A, photomicrographs show same Fast Blue, Kv1.2 and Kv1.4 stained sections from spinal intact and transected rats under fluorescence illumination with ultraviolet (blue areas) and FITC (green areas) filters. Arrows indicate neuronal profiles identified by Fast Blue labeling. Rectangles show insets at higher magnification. Scale bars indicate 50 μm. B, mean ± SEM expression shown as ratio of FITC immunoreactivity intensity in Fast Blue labeled vs unlabeled cells, including Kv1.2 in 121 cells from 3 spinal intact rats and 114 from 3 spinal transected rats, and Kv1.4 in 132 cells from 3 spinal intact rats and 112 from 3 spinal transected rats. Asterisk indicates p <0.05 vs spinal intact.
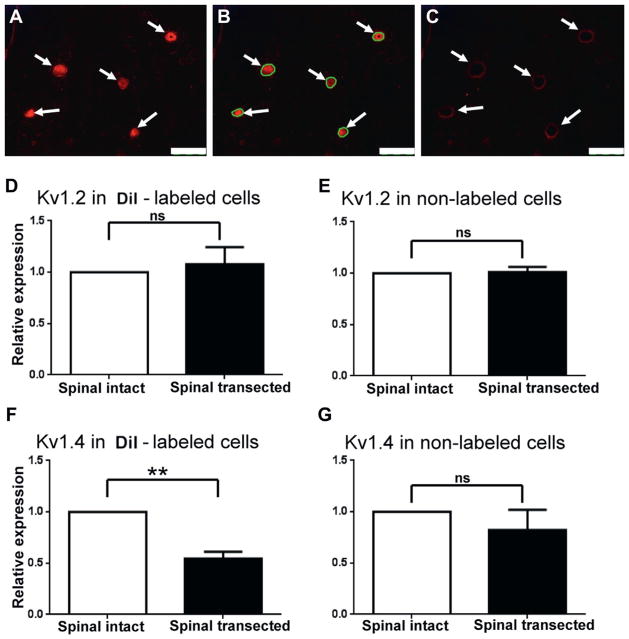
Changes in mRNA levels of Kv1.2 and Kv1.4 α-subunits were also examined in laser captured DRG neurons. The relative expression level of Kv1.2 in DiI labeled and unlabeled neurons (30 cells per rat) did not differ between 5 spinal intact and 5 spinal transected rats (fig. 5, D and E ). However, relative Kv1.4 expression was significantly lower (0.53) in DiI labeled bladder afferent neurons (30 cells per rat) from 5 spinal transected rats compared to those from 5 spinal intact rats (fig. 5, F ). There was no significant difference between the 2 groups in Kv1.4 mRNA levels in unlabeled neurons (fig. 5, G).
Figure 5.
Kv α-subunit mRNA expression after spinal cord transection. A to C, photomicrographs show single L6 DRG section during laser capture microdissection of DiI labeled bladder afferent neurons. Arrows indicate neurons positively stained with DiI. Scale bars represent 100 μm. A, before microdissection. B, before microdissection. Green circles indicate laser captured areas. C, after microdissection. D to G, mean ± SEM levels in 30 neurons from each of 5 spinal intact and 5 spinal transected rats. ns, not significant. Asterisks indicate p <0.01 vs spinal intact.
DISCUSSION
Our results indicate that 1) capsaicin sensitive bladder afferent neurons in spinal transected rats show hyperexcitability, as evidenced by lower spike activation thresholds and tonic firing pattern, 2) slow KA current density was decreased and the KA current inactivation curve of spinal transected rats was displaced to more hyperpolarized levels compared to those of spinal intact rats and 3) protein and mRNA expression of the Kv1.4 but not the Kv1.2 α-subunit was decreased in bladder afferent neurons after SCI. To our knowledge this is the first report of a direct association of functional and molecular changes in the Kv channels responsible for the hyperexcitability of bladder afferent neurons in SCI rats.
Kv currents in sensory neurons are divided into 2 major categories, ie sustained KDR and transient KA currents.3 Transient KA currents in sensory neurons, including DRG cells, can be further subdivided into at least 2 subtypes based on inactivation kinetics, ie fast and slow decaying KA currents.4 The slow decaying KA current is preferentially expressed in small, capsaicin sensitive bladder afferent neurons. A reduction in slow KA currents by the application of the KA channel blocker 4-aminopyridine increased the excitability of these neurons, as evidenced by lower spike activation thresholds and tonic firing.5,10
In this study we confirmed that the current density of slow KA currents was decreased in bladder afferent neurons from spinal transected rats compared to that in spinal intact rats (fig. 2). In addition, the inactivation curve of spinal transected rats was displaced by about 10 mV to more hyperpolarized levels compared to the inactivation curve in spinal intact rats. These results suggest that a reduction in slow decaying KA currents is a key event resulting in the hyperexcitability of capsaicin sensitive bladder afferent neurons after SCI in rats.
A reduction in slow decaying KA channel activity was associated with decreased expression of Kv1.4 α-subunit protein and mRNA in bladder afferent neurons. Kv channels are composed of homotetramers or heterotetramers of α-subunits that form K+ ion conducting pores.11 Previous reports indicated that Kv1 α-subunits, including Kv1.1, Kv1.2 and Kv1.4, could be major components of Kv channels in DRG neurons.12 The homotetramer of Kv1.4 α-subunits shows rapid, prominent inactivation processes but it is insensitive to DTX, a specific inhibitor of Kv1.1 and Kv1.2.13 The heteromeric channels containing Kv1.4 and DTX sensitive Kv1.1 and Kv1.2 α-subunits show inactivation that is much slower than the Kv1.4 homomeric channels and similar to slow decaying KA in DRG neurons.14
We previously reported that DTX partially suppressed slow decaying KA currents in capsaicin sensitive, small DRG neurons.15 Therefore, it seems reasonable that the assembly of Kv1.4 with other DTX sensitive Kv α-subunits, such as Kv1.1 and/or Kv1.2, contributes to the formation of slow decaying KA channels. Also, decreased Kv1.4 α-subunit expression after SCI might be a molecular mechanism responsible for the reduction in slow decaying KA currents, leading to hyperexcitability of capsaicin sensitive C-fiber bladder afferent neurons.
We previously reported that SCI induces a switch in the expression of Na+ channels from the TTX resistant to the TTX sensitive type.2 In contrast to neurons from spinal intact rats, in which approximately 70% of bladder afferent neurons show high threshold TTX resistant action potentials, 60% of bladder afferent neurons from SCI rats show low threshold TTX sensitive action potentials.2 We also previously reported that applying the KA channel blocker 4-aminopyridine significantly decreased the spike threshold and increased the firing number during sustained membrane depolarization.5 These results suggest that changes in Na+ channel property contribute to a lower spike activation threshold and changes in K+ channel property contribute to a lower threshold for spike activation and tonic firing pattern in these neurons. Therefore, it is reasonable that changes in the expression of Na+ channels and K+ channels in bladder afferent neurons after SCI contribute to the hyperexcitability of C-fiber afferent pathways.
CONCLUSIONS
The current study provides direct evidence that the excitability of capsaicin sensitive C-fiber bladder afferent neurons is increased in association with decreases in KA current density and Kv1.4 α-subunit expression in SCI rats. Thus, the Kv1.4 α-subunit could be a molecular target for treating overactive bladder due to neurogenic detrusor overactivity.
Acknowledgments
Supported by National Institutes of Health Grants P01DK093424, DK088836, DOD W81XWH-11-1-0763 and PVA 2793.
Abbreviations and Acronyms
- DiI
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- DRG
dorsal root ganglion
- DTX
α-dendrotoxin
- FITC
fluorescein isothiocyanate
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- KA
A-type K+
- KDR
delayed rectifier-type K+
- Kv
voltage-gated K+
- NDO
neurogenic detrusor overactivity
- PBS
phosphate buffered saline
- PCR
polymerase chain reaction
- SCI
spinal cord injury
- TTX
tetrodotoxin
- Vh
half-maximal conductance
Footnotes
Study received University of Pittsburgh institutional animal care and use committee approval.
References
- 1.de Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog Brain Res. 2006;152:59. doi: 10.1016/S0079-6123(05)52005-3. [DOI] [PubMed] [Google Scholar]
- 2.Yoshimura N, de Groat WC. Plasticity of Na+ channels in afferent neurons innervating rat urinary bladder following spinal cord injury. J Physiol. 1997;503:269. doi: 10.1111/j.1469-7793.1997.269bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gold MS, Shuster MJ, Levine JD. Characterization of six voltage-gated K+ currents in adult rat sensory neurons. J Neurophysiol. 1996;75:2629. doi: 10.1152/jn.1996.75.6.2629. [DOI] [PubMed] [Google Scholar]
- 4.Everill B, Kocsis JD. Reduction in potassium currents in identified cutaneous afferent dorsal root ganglion neurons after axotomy. J Neurophysiol. 1999;82:700. doi: 10.1152/jn.1999.82.2.700. [DOI] [PubMed] [Google Scholar]
- 5.Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci. 1999;19:4644. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayashi Y, Takimoto K, Chancellor MB, et al. Bladder hyperactivity and increased excitability of bladder afferent neurons associated with reduced expression of Kv1. 4 α-subunit in rats with cystitis. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1661. doi: 10.1152/ajpregu.91054.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshimura N, Bennett NE, Hayashi Y, et al. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci. 2006;26:10847. doi: 10.1523/JNEUROSCI.3023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshimura N, Erdman SL, Snider MW, et al. Effects of spinal cord injury on neurofilament immunoreactivity and capsaicin sensitivity in rat dorsal root ganglion neurons innervating the urinary bladder. Neuroscience. 1998;83:633. doi: 10.1016/s0306-4522(97)00376-x. [DOI] [PubMed] [Google Scholar]
- 9.Kruse MN, Bray LA, de Groat WC. Influence of spinal cord injury on the morphology of bladder afferent and efferent neurons. J Auton Nerv Syst. 1995;54:215. doi: 10.1016/0165-1838(95)00011-l. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimura N, White G, Weight FF, et al. Different types of Na+ and A-type K+ currents in dorsal root ganglion neurons innervating the rat urinary bladder. J Physiol. 1996;494:1. doi: 10.1113/jphysiol.1996.sp021471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991;350:232. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- 12.Rasband MN, Park EW, Vanderah TW, et al. Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci U S A. 2001;98:13373. doi: 10.1073/pnas.231376298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hopkins WF. Toxin and subunit specificity of blocking affinity of three peptide toxins for heteromultimeric, voltage-gated potassium channels expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1998;285:1051. [PubMed] [Google Scholar]
- 14.Po S, Roberds S, Snyders DJ, et al. Heteromultimeric assembly of human potassium channels: molecular basis of a transient outward current? Circ Res. 1993;72:1326. doi: 10.1161/01.res.72.6.1326. [DOI] [PubMed] [Google Scholar]
- 15.Yang EK, Takimoto K, Hayashi Y, et al. Altered expression of potassium channel subunit mRNA and α-dendrotoxin sensitivity of potassium currents in rat dorsal root ganglion neurons after axotomy. Neuroscience. 2004;123:867. doi: 10.1016/j.neuroscience.2003.11.014. [DOI] [PubMed] [Google Scholar]