Abstract
The loss of ovarian function during the menopausal transition has a profound impact on female skeletal health. Currently it is estimated that one in every two Caucasian women will experience an osteoporotic fracture during her lifetime,1 contributing to considerable morbidity and an enormous economic burden within the aging female population. However, most studies have been conducted in postmenopausal women, with fewer investigations focusing specifically on perimenopausal bone health. The Study of Women’s Health Across the Nation (SWAN) is the largest prospective cohort to date where changes in bone mineral density and bone turnover have been examined in relation to ovarian aging among women followed across the menopause transition.2–3 As defined by bleeding pattern in SWAN, early perimenopause is characterized by increasing menstrual irregularity but less than 3 months of amenorrhea, late perimenopause by amenorrhea lasting greater than 3 months but less than 1 year, and postmenopause by the absence of menstrual bleeding for twelve consecutive months or more.3–4 A recent multi-study collaboration has further recommended that the early menopause transition be defined by a persistent 7+ day difference in consecutive cycle lengths and the late menopause transition by at least 60 days of amenorrhea.5–6 A serum follicle-stimulating hormone (FSH) level of 40 IU/L or greater has also been found to be an independent marker of the transition that may facilitate predicting the time to the final menstrual period.6–7
Keywords: osteoporosis, perimenopause, menopause, bone, fractures
Conducted in a large multi-ethnic population of more than 2000 women across five clinical centers in the United States, the SWAN bone study has contributed greatly to our understanding of both early and late changes in bone metabolism during perimenopause, associated clinical and race/ethnic differences, and the implication of these findings for optimization of postmenopausal bone health. This review will focus on bone loss during the menopausal transition, changes surrounding the final menstrual period, and the role of endogenous hormones and ethnic variation in predicting bone density and bone loss. Specific findings from SWAN and other studies, data on perimenopausal fractures, fracture risk and implications for clinical management will also be discussed.
Bone Loss During the Menopausal Transition
Changes in Bone Mineral Density (BMD)
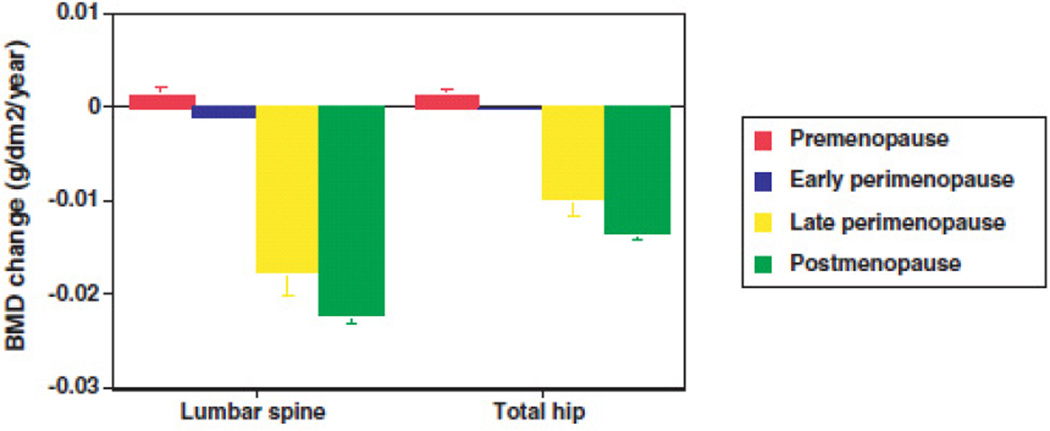
While there is a large body of literature examining bone mineral density among postmenopausal women, and a moderate body of literature examining bone mineral density among premenopausal women, fewer studies have monitored bone mineral density (BMD) serially in a cohort of women who were initially premenopausal and continued monitoring BMD until women experienced their final menstrual period.8–11 Some of these earlier studies reported that BMD remained stable in premenopausal women while others found that BMD begins to decline well before the final menstrual period.3, 12–17 However, many of the early studies lacked clear definitions of menopausal status, and prior studies often examined bone density as a function of chronologic age instead of menopause stage or time from the final menstrual period. SWAN investigators examined changes in BMD of the lumbar spine and total hip across six annual visits in nearly 2000 participants with carefully characterized perimenopausal stage.3 There was little change in BMD during the pre- or early perimenopausal period. Bone loss accelerated dramatically during late perimenopause and continued through the early postmenopause.3 This acceleration in bone loss during the late perimenopause (Figure 1) was characterized by a 1.8- 2.3% annual rate of bone loss in the lumbar spine and 1.0–1.4% in the hip.3 Body weight was found to be an important predictor of the rate of bone loss, independent of differences in race/ethnicity. Compared with women in the highest tertile of body weight, rate of bone loss was 35–55% higher in women in the lowest tertile of body weight.3
Figure 1.
Annual adjusted rates of change in bone mineral density (BMD) of the lumbar spine and total hip during the menopausal transition among 1902 SWAN participants. Error bars represent 95% confidence limits. (Data from Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopausal transition in a multiethnic cohort of women. Journal of Clinical Endocrinology and Metabolism 2008; 93:861–868, with permission)
Similar rates of accelerated bone loss have also been reported in the Melbourne Women’s Midlife Health Project where an average annual rate of BMD decline of 2.5% in the lumbar spine and 1.7% in the femoral neck occurred in the time surrounding the final menstrual period.18 These findings are comparable to early data obtained in perimenopausal women residing in France where the average annual decline in vertebral BMD was in the range of 2.35%.19 The association of higher body mass or body mass index with slower rates of bone loss at various skeletal sites (including the forearm, hip and spine) has also been described in several studies, consistent with the known protective effect of body size and adiposity on bone loss and fracture risk.11, 17–18, 20
The Role of Endogenous Hormones
Bone loss in postmenopausal women has historically been attributed to estrogen deficiency. A detailed review of changes in ovarian and pituitary hormones during the menopausal transition is described elsewhere in this issue of Obstetrics and Gynecology Clinics of North America (Reproductive Hormones and Menopause). There are numerous studies in older postmenopausal women demonstrating a significant association between circulating estrogen levels, BMD and fracture risk.21–22 However, in pre- and perimenopausal women, it is difficult to extrapolate a single estradiol level, even when obtained during a well-defined portion of the menstrual cycle, to an entire menstrual cycle or series of menstrual cycles. This is because estradiol levels change from day to day and vary over a range of more than 10-fold across the course of a normal menstrual cycle.23
There is also evidence that fluctuations in estradiol may be more pronounced in the perimenopause, at least in its earlier stages.24 Thus, in contrast to the postmenopause when estradiol levels are quite stable, perimenopausal estradiol levels might not be expected to correlate well with bone density and bone loss during the perimenopause, more because of the difficulty in assessing estrogen status rather than a lack of a true relationship. Moreover, the effect of fluctuating estradiol on bone density and bone loss is not well understood. It is possible that the periods of normal-to-high estradiol followed by periods of low estradiol that are characteristic of the perimenopause may relate differently to bone than the more stable and consistent levels seen in midreproductive-aged women.
Perimenopause is characterized by an increase in bone resorption and reduction in BMD.25 These findings are accompanied by higher serum FSH levels, although estrogen levels may remain within the premenopausal range during the early transition.25 In pre- and early perimenopausal Australian women examined across the menopausal transition, estradiol level measured at the final time point was significantly associated with perimenopausal bone loss.26 In a separate examination of Swedish women undergoing prospective measurement of distal radius BMD at menopause, postmenopausal serum estradiol level was also found to correlate with changes in BMD.27
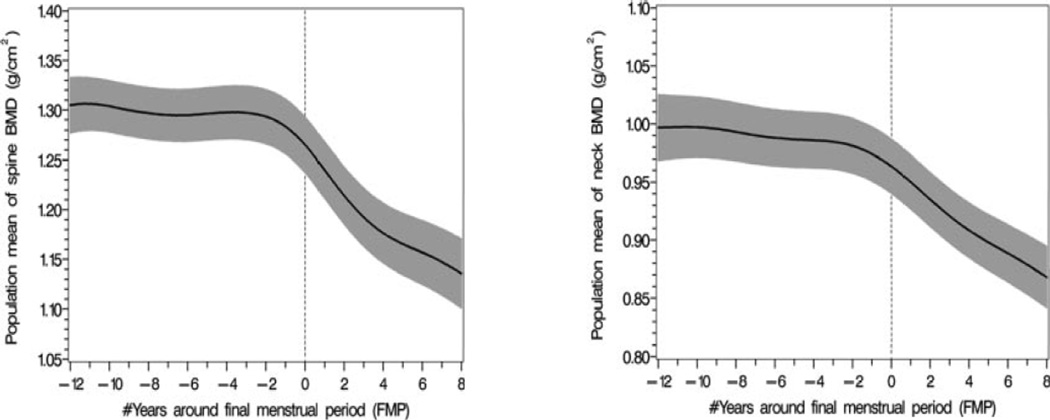
In SWAN, significant cross-sectional associations were observed between baseline serum FSH levels and BMD, but not between baseline serum estradiol levels and BMD, in pre- and early perimenopausal women.28 Baseline FSH levels and changes in FSH levels were associated with longitudinal changes during the menopause transition29 whereas annual measures of serum estradiol did not predict bone loss. Estradiol concentrations below 35 pg/mL were associated with lower BMD levels during the transition, however.29 Examination of BMD change in the context of FSH staging in the Michigan Bone Health and Metabolism Study (Figure 2) showed that bone loss in the lumbar spine and femoral neck became evident when FSH increased into the range of 34–56 IU/l, which occurred approximately two years before the final menstrual period.17 The annualized rate of bone loss in the lumbar spine increased from 1.7% in perimenopausal women with FSH levels of 34–56 IU/l to 3.3% during the two years after the final menstrual period and then declined to 1.1% per year in subsequent postmenopausal years.17
Figure 2.
The pattern of population mean lumbar spine and femoral neck bone mineral density (BMD) values in relation to the final menstrual period with 95% upper and lower confidence intervals (black solid line with shaded areas). The Michigan Bone Health and Metabolism Study. (Data from Sowers MR, Zheng H, Jannausch ML, et al. Amount of bone loss in relation to time around the final menstrual period and follicle-stimulating hormone staging of the transmenopause. Journal of Clinical Endocrinology and Metabolism 2010; 95:2155–2162, with permission)
There has also been increasing awareness of other potential hormones that may contribute to bone loss during ovarian aging.30 For example, it has been reported that mice with null mutations in the FSH receptor gene do not manifest bone loss despite the atrophic ovaries and uterus and disordered estrous cycles, suggesting that FSH is required in order for bone loss to occur in states of estrogen deficiency.31–32 However, because mice with null mutations in the FSH receptor gene have elevated testosterone levels,33 the lack of bone loss may be due to direct effects of testosterone on bone. Other gonadal peptides, such as inhibin A, inhibin B and activin have also been purported to contribute to changes in BMD with ovarian aging. Both inhibin A and inhibin B suppress osteoblast and osteoclast development and they oppose the stimulatory effects of activin and bone morphogenetic proteins on bone formation.34 Whether any of these compounds truly contributes to the regulation of bone homeostasis during the perimenopause is currently unclear. Progesterone has also been implicated in the regulation of bone mass. The decline in progesterone concentrations associated with anovulation or luteal phase deficiency35 and the reduction in inhibin secretion evident during the perimenopause may represent an additional mechanism for bone loss independent of circulating estrogen.30, 34, 36 In a subset of pre- and early perimenopausal SWAN women in whom urinary excretion of estrogen and progesterone metabolites was assessed daily for one menstrual cycle each year, no association between measures of luteal function and BMD was observed;37 however, larger studies are needed to examine the specific impact of changes in ovulatory function on BMD in midlife women. Finally, new data have led some experts to hypothesize that other intrinsic age-related factors may be important in the pathogenesis of postmenopausal osteoporosis, particularly with regard to loss of trabecular bone.30
Changes in Bone Turnover
Bone turnover markers have been used for many years in the research setting to monitor the response to specific osteoporosis therapies.38 Peptides made by osteoblasts, including bone-specific alkaline phosphatase, osteocalcin and procollagen type I N-propeptide (P1NP) are often used to assess bone formation while products of type I collagen degradation, including the cross-linked N-terminal and C-terminal telopeptides of type I collagen [N-telopeptide (NTX) or serum C-telopeptide (CTX)] are often used to assess bone matrix degradation. In cross-sectional analyses, bone resorption markers are consistently higher in untreated postmenopausal osteoporotic women than in premenopausal women whereas bone formation markers are more variable.39–42 Higher levels of bone turnover markers have been shown in many, but not all studies,40, 43–45 to predict subsequent bone loss40, 46–47 or fracture risk40–41, 44, 48 (independent of bone density changes) in postmenopausal women not receiving anti-osteoporosis treatment.
There are still, however, many gaps in knowledge that hinder the utilization of bone turnover markers in clinical practice. Few studies have measured bone turnover markers in perimenopausal women or in a racially diverse cohort of women. In a cross-sectional analysis, urinary NTX and osteocalcin were measured in 2,375 SWAN participants who were either pre-or early perimenopausal.49 Mean NTX and osteocalcin levels were slightly higher in the perimenopausal women as compared with the premenopausal women; however, these differences were not statistically significant. Another cross-sectional analysis of 2313 pre- or early perimenopausal SWAN subjects demonstrated ethnic/racial differences in osteocalcin and urinary NTX levels wherein Caucasian subjects had the highest levels of both markers, even after adjusting for anthropometric and lifestyle differences.50 The increased bone turnover observed in Caucasian subjects may explain the lower BMD, as discussed later in this chapter, seen in these subjects as compared with women from other racial groups. Additionally, only a few studies have assessed longitudinal changes in bone turnover markers during the menopause and the published reports have been limited by small cohort size. In a three year study of fifteen pre-menopausal women where six women became post-menopausal, bone resorption markers were unchanged prior to the final menses but started to increase six months after the final menstrual period.51 The mean within-subject increase in bone resorption markers was 30–50%.51 In an eight year study of 104 pre-menopausal women, 34 women became menopausal over the observation period with significant within-individual increases in bone resorption markers.52 Longitudinal changes in bone resorption markers in the SWAN study are currently being analyzed; given SWAN’s cohort size and racial diversity, these data will add substantively to our knowledge of the effect of the menopause on bone turnover markers. Because of significant within-individual variability of these markers, lack of uniform reference standards and challenges with interpretation, the use of bone turnover markers has been limited primarily to the research domain. However, with increasing knowledge of the hormonal and physiologic factors that affect bone turnover markers, a stronger clinical role for bone turnover measurements may become evident in the future.38, 40
Race/Ethnic Differences
It is well known that BMD and osteoporotic fracture risk vary by race/ethnicity, with unadjusted BMD values typically lowest in Asian women, intermediate in Caucasian women, and highest in African-American women. Fracture rates follow a different pattern, however, with the highest rates in Caucasians, intermediate rates in Asians, and the lowest rates in African-Americans. Some of the observed differences in BMD among race/ethnic groups could be due to differences in bone size (one of the limitations of dual energy x-ray absorptiometry which measures areal and not three-dimensional BMD). However, race/ethnic differences have similarly been reported in population studies utilizing quantitative computed tomography (QCT) which measures volumetric BMD53 and adjusting BMD values in SWAN for bone size did not eliminate race/ethnic differences in BMD.54
The specific sampling structure of the SWAN Bone Study across five clinical centers, each with approximately half the participants selected based on being of non-Hispanic Caucasian race and the remaining based on African-American, Chinese and Japanese race with annual BMD measurements during follow-up provides one of the few population-based studies able to examine race/ethnic differences in BMD and bone loss across the menopausal transition.2 At the baseline evaluation, the racial variation in BMD (highest in African-Americans followed by Caucasians and lowest in Chinese and Japanese women) was found to be largely due to differences in body weight.54 After adjustment for weight and other confounders, there were minimal differences in lumbar spine BMD among African-American, Chinese, or Japanese women, all of whom had higher adjusted BMD compared to Caucasian women.54 Unadjusted femoral neck BMD was highest in African-Americans, intermediate in Caucasians, and lowest in Japanese and Chinese women with mean differences of 14–24% between African-American’s and the other groups. After careful weight matching, the difference in femoral neck BMD between African-Americans and the other groups was reduced to 8–9% and the difference between Asians and Caucasians was eliminated. It is only because SWAN has large numbers of women from each of these racial/ethnic groups that weight matching and adjustment was possible, thus allowing new insights into racial/ethnic differences in BMD. Across the menopausal transition, rates of bone loss were greatest among Chinese and Japanese women, intermediate among Caucasian women and lowest among African-American women; however, like the baseline differences in BMD, these variations were also largely accounted for by differences in body weight rather than race/ethnicity per se.3 Taken together, SWAN has helped to reinforce the finding that Caucasian race is a risk factor for bone loss, and that adjusting for body weight is critical in the determination of peak bone mass as well as a woman’s risk of bone loss during the menopausal transition.
Perimenopausal Fracture Risk
Population-based studies, conducted mainly in Caucasian women, have contributed substantially to our understanding of fracture patterns across the aging lifespan. Among 10,902 middle-aged Swedish women followed for up to 11 years, the largest proportion of low-energy fractures occurred in the forearm (37%) followed by the ankle (12%), spine and proximal humerus (9% each), hands or feet (8%) and hip (8%).55 Overall, the incidence of fracture was quite low, estimated at 5.5 per 1000 person-years for forearm fractures and approximately 3 per 1000 person-years for the proximal humerus.55 Risk factors for incident fracture included older age, prior fracture, diabetes mellitus and poor health status; higher body mass index was associated with an increased risk of proximal humerus and ankle fractures but lower risk of forearm fractures.55 Among 3068 perimenopausal women aged 47–53 years residing in Finland, 8.5% sustained a fracture during a mean follow-up period of 3.6 years, with most fractures again occurring in the extremities (26% wrist, 16% ankle, 19% hands or feet, and 15% rib).56 The presence of low BMD, prior fracture history, nonuse of hormone replacement therapy, three or more chronic illnesses and smoking were found to be independent risk factors for perimenopausal fracture.56 In a similarly aged cohort of 1857 women undergoing BMD screening in Scotland, the two year incidence of self-reported fracture was 2.4%; risk factors associated with an increased fracture risk included low spine BMD, prior fracture history, family history of hip fracture and postmenopausal status.57 Among 2171 women enrolled in SWAN followed for up to eight years, 245 reported an incident fracture.58 The subset of women with diabetes (5%) underwent an earlier menopause transition and experienced a two-fold increased risk of incident fracture compared to women without diabetes.58 Fracture risk is also influenced by other predisposing conditions, including genetic factors, relevant comorbidities and exposures (e.g., rheumatoid arthritis, malabsorptive syndromes, systemic glucocorticoids, aromatase inhibitors), differences in structural bone geometry and risk of falls.1
Clinical Management Considerations
Diet and Lifestyle Factors
The dietary and lifestyle recommendations for optimal bone health and fracture prevention during perimenopause are the same as those recommended for postmenopausal women. These include a well-balanced diet, regular exercise, smoking cessation, avoidance of excessive alcohol consumption, and fall prevention measures.1, 59 Attention should also be given to changes in weight, particularly in light of the known association of weight loss with increasing rates of bone loss and subsequent fracture risk.60
Calcium and Vitamin D
Maintenance of adequate calcium and vitamin D intake remain an important component of preventive bone health. Several studies have shown that calcium and vitamin D supplementation improves BMD and reduces fracture risk in late postmenopausal women.61–65 The Women’s Health Initiative (WHI) trial found that calcium (500 mg twice daily) and vitamin D (400 IU daily) supplementation in healthy postmenopausal women increased hip BMD modestly but did not reduce hip fracture risk significantly in the cohort as a whole, though fracture risk was reduced significantly in women who adhered to study treatment.66 However, it should be noted that women in the WHI trial received a relatively low dose of vitamin D (400 IU) and more than half were concurrently receiving hormone replacement therapy.67 In early postmenopausal women (within the first five years of their final menstrual period), calcium administration slows bone loss from sites comprised largely of cortical bone but has little effect on skeletal sites comprised largely of trabecular bone.67–70 The relationship between calcium supplementation and fracture risk in perimenopausal or early postmenopausal women is less clear.67, 70
Even though the beneficial effects of calcium administration in perimenopausal women are not well established, most experts recommend that perimenopausal women should be counseled regarding optimal calcium intake. Currently, both the National Academy of Sciences and the National Osteoporosis Foundation recommend a total daily intake of 1200 mg elemental calcium (combining dietary and supplement sources) for women over age 50.1, 59, 71 Dietary calcium sources are preferred due to greater calcium absorption and, possibly, because of a lower risk of vascular disease, particularly in light of a recent meta analysis which reported that calcium supplementation increases the risk of cardiovascular events.72–73 Calcium supplements, when taken, should be in conjunction with meals to maximize gastrointestinal absorption. Select populations at higher risk for reduced dietary calcium intake include older individuals and those with lactose intolerance, vegetarian diet or poor eating habits.59, 74 A list of calcium-rich foods can be found through the Office for Dietary Supplements, National Institutes of Health (http://ods.od.nih.gov/factsheets/calcium/).
Vitamin D may reduce fracture risk through a number of mechanisms. Correcting vitamin D deficiency can improve calcium absorption and thereby treat secondary hyperparathyroidism and osteomalacia.75–76 Additionally, correcting vitamin D deficiency can decrease fracture risk by improving muscle strength and reducing the risk of falls.77 There is ongoing debate as to the minimum 25-hydroxyvitamin D level required for skeletal benefits. A meta-analysis of seven clinical trials with 9,820 subjects suggested that a daily dose of vitamin D 700–800 IU is required to achieve a 25-hydroxyvitamin D level of 40 ng/mL, which is associated with 26% and 23% reduction in hip and non-vertebral fracture risk, respectively.62 However, the findings of this meta-analysis are discordant with the 2010 National Academy of Sciences recommendations that women younger and older than 50 years should consume 600 and 800 IU of vitamin D daily, respectively, and that the minimum desired 25-hydroxyvitamin D level for skeletal benefits is 20 ng/mL.71 Even with the ongoing debate, certain populations are at increased risk for vitamin D deficiency and may require higher doses of vitamin D (1000–2000 IU per day or pharmacologic therapy). Serum 25-hydroxyvitamin D levels reflect the dietary intake of vitamin D and the synthesis of vitamin D in response to ultraviolet B (UV-B) exposure of the skin.78–79 Thus, women with pigmented skin or limited sun exposure due to use of sunscreen or occlusive clothing are at particular risk for vitamin D deficiency.80 Dietary sources of vitamin D can be found through the Office for Dietary Supplements, National Institutes of Health (http://ods.od.nih.gov/factsheets/vitaminD/).
Bone Mineral Density Screening in Perimenopausal Women
Dual energy x-ray absorptiometry is the most widely available and validated modality for measurement of BMD and continues to be the preferred method for assessing osteoporosis.81 The National Osteoporosis Foundation recommends BMD testing for women in the menopausal transition if there is a specific risk factor associated with increased fracture risk (e.g. prior fragility fracture or high-risk medication), but recognizes that BMD assessment may not be indicated if the results will not influence treatment decisions.1 When BMD measurements are performed, The World Health Organization (WHO) criteria for osteoporosis apply to postmenopausal women, using the reference range for young adult Caucasian women for calculation of BMD T score.81–82 The North American Menopause Society advises that the WHO criteria can be used for classification of perimenopausal women, but that care should be taken to interpret bone mineral density results appropriately in this setting.59 For premenopausal women, the International Society for Clinical Densitometry advises that race-adjusted Z score (instead of T score) be used, with a Z-score of −2.0 or lower defined as “below the expected range for age” and a Z-score above −2.0 defined as “above the expected range for age” for women prior to menopause.82
There are currently no recommendations for osteoporosis screening in healthy perimenopausal women. The recent 2011 U.S. Preventive Task Force recommends screening for osteoporosis in postmenopausal women below age 65 years if their fracture risk is equivalent to that of a 65 year old white woman with no other risk factors.83 This screening threshold translates to a 9.3% ten-year risk of major osteoporotic fractures calculated using the web-based World Health Organization Fracture Risk Assessment Tool FRAX, accessible at www.shef.ac.uk/frax. Using these recommendations, the majority of healthy perimenopausal women would likely not be recommended for BMD screening. Alternatively, women with low body weight comprise a higher risk subgroup where BMD testing during late perimenopause has been suggested.3, 84 As yet, there are no controlled studies examining the benefit of early detection and intervention for low bone mineral density83 with the exception of specific premenopausal patient subsets (e.g. breast cancer, chronic glucocorticoid therapy).
Fracture Risk Assessment
Few studies have examined the application of FRAX in perimenopausal and early postmenopausal women, most of whom will have relatively low fracture risk.85 For the U.S. population, early revisions to FRAX were made in 2009 based on updated U.S. fracture incidence rates, resulting in lower rates of major osteoporotic fracture, particularly at the younger ages.86 Other risk factors considered in FRAX include age, gender, race/ethnicity, parental history of hip fracture, other clinical risk factors, and femoral neck bone mineral density. A recent study conducted in France, using data from 2651 peri- and early postmenopausal women with DXA measurements and an average follow-up of 13 years, suggested that FRAX may not improve the discriminatory value of hip BMD alone for fracture risk prediction.87 At an individual level, FRAX is a useful clinical risk assessment tool that may also aid in patient counseling. For the U.S. population, the National Osteoporosis Foundation has recommended cost-effective osteoporosis treatment thresholds of 3% for 10-year risk of hip fracture or 20% for 10-year risk of major osteoporotic fracture using the WHO FRAX model in women with osteopenia.88
Treatment Considerations
There are currently no established guidelines pertaining to the treatment and prevention of osteoporosis in perimenopausal women. For perimenopausal women who have a high fracture risk or for those in whom osteoporosis treatment is indicated, the selection of therapy should be considered on an individual basis. A detailed discussion of available osteoporosis therapies and their risks and benefits is beyond the scope of this chapter. In brief, bisphosphonate drugs are considered first-line drugs for the treatment of postmenopausal osteoporosis, with evidence for reduction in risk of hip, vertebral and non-vertebral fractures.59 However, since the optimal duration of bisphosphonate treatment remains unknown, practitioners may weigh consideration of other antiresorptive therapies for postmenopausal women with osteoporosis who are relatively young, depending on osteoporosis disease severity. Use of antiresorptive agents in perimenopausal women carries a potential hazard of prenatal exposure. Although fertility is rare and rarely desired in this age group, non-contracepting, sexually active perimenopausal women require specific counseling prior to taking bisphosphonates. Raloxifene, a selective estrogen receptor modulator shown to prevent bone loss and reduce vertebral fracture risk in elderly postmenopausal women, may be an option for younger postmenopausal women with osteoporosis, although its efficacy in preventing non-vertebral fractures and hip fractures is uncertain.59 Because raloxifene administration may reduce BMD in premenopausal women,89 it should not be used for the prevention of bone loss in perimenopausal women. For perimenopausal women with menopausal symptoms, treatment with estrogen plus progestin (or estrogen alone if the woman has had a hysterectomy) can be considered, although when hormone replacement therapy is assessed solely for osteoporosis indications, the risks and benefits should be weighed in conjunction with other non-estrogen based therapies,1, 59 There are currently no recommendations with regard to estrogen therapy for prevention of postmenopausal bone loss. Once estrogen is discontinued, there does not appear to be a persisting benefit on BMD, bone loss or fracture risk.90–92
Summary
The findings from prospective examination of BMD change across the menopausal transition demonstrate an early and accelerated rate of bone loss, particularly in the lumbar spine. Bone loss begins to accelerate 1–2 years before menopause, concurrent with the prolonged amenorrhea that characterizes the late menopausal transition.3, 17 Importantly, these rates of bone loss are also influenced by body size, with greater bone loss in non-obese women and those with lower body mass, independent of differences in race/ethnicity.3, 17 The greatest reduction in BMD occurs in the year before the final menstrual period and the first two years after the final menstrual period, with lower rates of loss during the ensuing 1–7 years.17 Clinical management considerations during the perimenopause include maintenance of adequate dietary calcium and vitamin D intake, attention to modifiable risk factors and consideration of osteoporosis screening in high risk populations with assessment of fracture risk. The indication, benefits and risks of pharmacologic osteoporosis therapy should be individually assessed as there are currently no established guidelines addressing the treatment and prevention of osteoporosis in perimenopausal women.
Acknowledgements
The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health, Kaiser Permanente, the University of California or Massachusetts General Hospital.
Disclosures: Drs. Lo and Burnett-Bowie have received research funding from Amgen, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.National Osteoporosis Foundation. Washington, DC: 2010. Clinician’s Guide to Prevention and Treatment of Osteoporosis; pp. 1–36. [Google Scholar]
- 2.Neer RM. Bone loss across the menopausal transition. Ann N Y Acad Sci. 2010;1192:66–71. doi: 10.1111/j.1749-6632.2009.05233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab. 2008;93(3):861–868. doi: 10.1210/jc.2007-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lasley BL, Santoro N, Randolf JF, et al. The relationship of circulating dehydroepiandrosterone, testosterone, and estradiol to stages of the menopausal transition and ethnicity. J Clin Endocrinol Metab. 2002;87(8):3760–3767. doi: 10.1210/jcem.87.8.8741. [DOI] [PubMed] [Google Scholar]
- 5.Harlow SD, Crawford S, Dennerstein L, et al. Recommendations from a multi-study evaluation of proposed criteria for staging reproductive aging. Climacteric. 2007;10(2):112–119. doi: 10.1080/13697130701258838. [DOI] [PubMed] [Google Scholar]
- 6.Ferrell RJ, Sowers M. Longitudinal, epidemiologic studies of female reproductive aging. Ann N Y Acad Sci. 2010;1204:188–197. doi: 10.1111/j.1749-6632.2010.05525.x. [DOI] [PubMed] [Google Scholar]
- 7.Randolph JF, Jr, Crawford S, Dennerstein L, et al. The value of follicle-stimulating hormone concentration and clinical findings as markers of the late menopausal transition. J Clin Endocrinol Metab. 2006;91(8):3034–3040. doi: 10.1210/jc.2006-0243. [DOI] [PubMed] [Google Scholar]
- 8.Ahlborg HG, Johnell O, Nilsson BE, et al. Bone loss in relation to menopause: a prospective study during 16 years. Bone. 2001;28(3):327–331. doi: 10.1016/s8756-3282(00)00451-8. [DOI] [PubMed] [Google Scholar]
- 9.Falch JA, Sandvik L. Perimenopausal appendicular bone loss: a 10-year prospective study. Bone. 1990;11(6):425–428. doi: 10.1016/8756-3282(90)90138-o. [DOI] [PubMed] [Google Scholar]
- 10.Ravn P, Hetland ML, Overgaard K, et al. Premenopausal and postmenopausal changes in bone mineral density of the proximal femur measured by dual-energy X-ray absorptiometry. J Bone Miner Res. 1994;9(12):1975–1980. doi: 10.1002/jbmr.5650091218. [DOI] [PubMed] [Google Scholar]
- 11.Reeve J, Walton J, Russell LJ, et al. Determinants of the first decade of bone loss after menopause at spine, hip and radius. QJM. 1999;92(5):261–273. doi: 10.1093/qjmed/92.5.261. [DOI] [PubMed] [Google Scholar]
- 12.Bainbridge KE, Sowers MF, Crutchfield M, et al. Natural history of bone loss over 6 years among premenopausal and early postmenopausal women. Am J Epidemiol. 2002;156(5):410–417. doi: 10.1093/aje/kwf049. [DOI] [PubMed] [Google Scholar]
- 13.Recker R, Lappe J, Davies K, et al. Characterization of perimenopausal bone loss: a prospective study. J Bone Miner Res. 2000;15(10):1965–1973. doi: 10.1359/jbmr.2000.15.10.1965. [DOI] [PubMed] [Google Scholar]
- 14.Recker RR, Lappe JM, Davies KM, et al. Change in bone mass immediately before menopause. J Bone Miner Res. 1992;7(8):857–862. doi: 10.1002/jbmr.5650070802. [DOI] [PubMed] [Google Scholar]
- 15.Sowers M, Crutchfield M, Bandekar R, et al. Bone mineral density and its change in pre- and perimenopausal white women: the Michigan Bone Health Study. J Bone Miner Res. 1998;13(7):1134–1140. doi: 10.1359/jbmr.1998.13.7.1134. [DOI] [PubMed] [Google Scholar]
- 16.Slemenda C, Hui SL, Longcope C, et al. Sex steroids and bone mass. A study of changes about the time of menopause. J Clin Invest. 1987;80(5):1261–1269. doi: 10.1172/JCI113201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sowers MR, Zheng H, Jannausch ML, et al. Amount of bone loss in relation to time around the final menstrual period and follicle-stimulating hormone staging of the transmenopause. J Clin Endocrinol Metab. 2010;95(5):2155–2162. doi: 10.1210/jc.2009-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guthrie JR, Ebeling PR, Hopper JL, et al. A prospective study of bone loss in menopausal Australian-born women. Osteoporos Int. 1998;8(3):282–290. doi: 10.1007/s001980050066. [DOI] [PubMed] [Google Scholar]
- 19.Pouilles JM, Tremollieres F, Ribot C. The effects of menopause on longitudinal bone loss from the spine. Calcif Tissue Int. 1993;52(5):340–343. doi: 10.1007/BF00310195. [DOI] [PubMed] [Google Scholar]
- 20.Rannevik G, Jeppsson S, Johnell O, et al. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas. 1995;21(2):103–113. doi: 10.1016/0378-5122(94)00869-9. [DOI] [PubMed] [Google Scholar]
- 21.Cummings SR, Browner WS, Bauer D, et al. Endogenous hormones and the risk of hip and vertebral fractures among older women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1998;339(11):733–738. doi: 10.1056/NEJM199809103391104. [DOI] [PubMed] [Google Scholar]
- 22.Greendale GA, Edelstein S, Barrett-Connor E. Endogenous sex steroids and bone mineral density in older women and men: the Rancho Bernardo Study. J Bone Miner Res. 1997;12(11):1833–1843. doi: 10.1359/jbmr.1997.12.11.1833. [DOI] [PubMed] [Google Scholar]
- 23.Filicori M, Santoro N, Merriam GR, et al. Characterization of the physiological pattern of episodic gonadotropin secretion throughout the human menstrual cycle. J Clin Endocrinol Metab. 1986;62(6):1136–1144. doi: 10.1210/jcem-62-6-1136. [DOI] [PubMed] [Google Scholar]
- 24.Santoro N, Brown JR, Adel T, et al. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81(4):1495–1501. doi: 10.1210/jcem.81.4.8636357. [DOI] [PubMed] [Google Scholar]
- 25.Ebeling PR, Atley LM, Guthrie JR, et al. Bone turnover markers and bone density across the menopausal transition. J Clin Endocrinol Metab. 1996;81(9):3366–3371. doi: 10.1210/jcem.81.9.8784098. [DOI] [PubMed] [Google Scholar]
- 26.Guthrie JR, Lehert P, Dennerstein L, et al. The relative effect of endogenous estradiol and androgens on menopausal bone loss: a longitudinal study. Osteoporos Int. 2004;15(11):881–886. doi: 10.1007/s00198-004-1624-3. [DOI] [PubMed] [Google Scholar]
- 27.Ahlborg HG, Johnell O, Turner CH, et al. Bone loss and bone size after menopause. N Engl J Med. 2003;349(4):327–334. doi: 10.1056/NEJMoa022464. [DOI] [PubMed] [Google Scholar]
- 28.Sowers MR, Finkelstein JS, Ettinger B, et al. The association of endogenous hormone concentrations and bone mineral density measures in pre- and perimenopausal women of four ethnic groups: SWAN. Osteoporos Int. 2003;14(1):44–52. doi: 10.1007/s00198-002-1307-x. [DOI] [PubMed] [Google Scholar]
- 29.Sowers MR, Jannausch M, McConnell D, et al. Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab. 2006;91(4):1261–1267. doi: 10.1210/jc.2005-1836. [DOI] [PubMed] [Google Scholar]
- 30.Khosla S, Melton LJ, 3rd, Riggs BL. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: is a revision needed? J Bone Miner Res. 2011;26(3):441–451. doi: 10.1002/jbmr.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iqbal J, Sun L, Kumar TR, et al. Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation. Proc Natl Acad Sci U S A. 2006;103(40):14925–14930. doi: 10.1073/pnas.0606805103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun L, Peng Y, Sharrow AC, et al. FSH directly regulates bone mass. Cell. 2006;125(2):247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 33.Abel MH, Huhtaniemi I, Pakarinen P, et al. Age-related uterine and ovarian hypertrophy in FSH receptor knockout and FSHbeta subunit knockout mice. Reproduction. 2003;125(2):165–173. doi: 10.1530/rep.0.1250165. [DOI] [PubMed] [Google Scholar]
- 34.Nicks KM, Fowler TW, Akel NS, et al. Bone turnover across the menopause transition : The role of gonadal inhibins. Ann N Y Acad Sci. 2010;1192:153–160. doi: 10.1111/j.1749-6632.2009.05349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santoro N, Crawford SL, Lasley WL, et al. Factors related to declining luteal function in women during the menopausal transition. J Clin Endocrinol Metab. 2008;93(5):1711–1721. doi: 10.1210/jc.2007-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seifert-Klauss V, Prior JC. Progesterone and bone: actions promoting bone health in women. J Osteoporos. 2010;2010:845180. doi: 10.4061/2010/845180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grewal J, Sowers MR, Randolph JF, Jr, et al. Low bone mineral density in the early menopausal transition: role for ovulatory function. J Clin Endocrinol Metab. 2006;91(10):3780–3785. doi: 10.1210/jc.2006-0544. [DOI] [PubMed] [Google Scholar]
- 38.Vasikaran S, Eastell R, Bruyere O, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22(2):391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 39.Akesson K, Ljunghall S, Jonsson B, et al. Assessment of biochemical markers of bone metabolism in relation to the occurrence of fracture: a retrospective and prospective population-based study of women. J Bone Miner Res. 1995;10(11):1823–1829. doi: 10.1002/jbmr.5650101127. [DOI] [PubMed] [Google Scholar]
- 40.Delmas PD, Eastell R, Garnero P, et al. The use of biochemical markers of bone turnover in osteoporosis. Committee of Scientific Advisors of the International Osteoporosis Foundation. Osteoporos Int. 2000;11(Suppl 6):S2–S17. doi: 10.1007/s001980070002. [DOI] [PubMed] [Google Scholar]
- 41.Garnero P, Hausherr E, Chapuy MC, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996;11(10):1531–1538. doi: 10.1002/jbmr.5650111021. [DOI] [PubMed] [Google Scholar]
- 42.Garnero P, Shih WJ, Gineyts E, et al. Comparison of new biochemical markers of bone turnover in late postmenopausal osteoporotic women in response to alendronate treatment. J Clin Endocrinol Metab. 1994;79(6):1693–1700. doi: 10.1210/jcem.79.6.7989477. [DOI] [PubMed] [Google Scholar]
- 43.Bauer DC, Sklarin PM, Stone KL, et al. Biochemical markers of bone turnover and prediction of hip bone loss in older women: the study of osteoporotic fractures. J Bone Miner Res. 1999;14(8):1404–1410. doi: 10.1359/jbmr.1999.14.8.1404. [DOI] [PubMed] [Google Scholar]
- 44.Garnero P. Markers of bone turnover for the prediction of fracture risk. Osteoporos Int. 2000;11(Suppl 6):S55–S65. doi: 10.1007/s001980070006. [DOI] [PubMed] [Google Scholar]
- 45.Melton LJ, 3rd, Crowson CS, O'Fallon WM, et al. Relative contributions of bone density, bone turnover, and clinical risk factors to long-term fracture prediction. J Bone Miner Res. 2003;18(2):312–318. doi: 10.1359/jbmr.2003.18.2.312. [DOI] [PubMed] [Google Scholar]
- 46.Hansen MA, Overgaard K, Riis BJ, et al. Role of peak bone mass and bone loss in postmenopausal osteoporosis: 12 year study. BMJ. 1991;303(6808):961–964. doi: 10.1136/bmj.303.6808.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosen CJ, Chesnut CH, 3rd, Mallinak NJ. The predictive value of biochemical markers of bone turnover for bone mineral density in early postmenopausal women treated with hormone replacement or calcium supplementation. J Clin Endocrinol Metab. 1997;82(6):1904–1910. doi: 10.1210/jcem.82.6.4004. [DOI] [PubMed] [Google Scholar]
- 48.Riis BJ, Hansen MA, Jensen AM, et al. Low bone mass and fast rate of bone loss at menopause: equal risk factors for future fracture: a 15-year follow-up study. Bone. 1996;19(1):9–12. doi: 10.1016/8756-3282(96)00102-0. [DOI] [PubMed] [Google Scholar]
- 49.Sowers MR, Greendale GA, Bondarenko I, et al. Endogenous hormones and bone turnover markers in pre- and perimenopausal women: SWAN. Osteoporos Int. 2003;14(3):191–197. doi: 10.1007/s00198-002-1329-4. [DOI] [PubMed] [Google Scholar]
- 50.Finkelstein JS, Sowers M, Greendale GA, et al. Ethnic variation in bone turnover in pre- and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab. 2002;87(7):3051–3056. doi: 10.1210/jcem.87.7.8480. [DOI] [PubMed] [Google Scholar]
- 51.Hassager C, Colwell A, Assiri AM, et al. Effect of menopause and hormone replacement therapy on urinary excretion of pyridinium cross-links: a longitudinal and cross-sectional study. Clin Endocrinol (Oxf) 1992;37(1):45–50. doi: 10.1111/j.1365-2265.1992.tb02282.x. [DOI] [PubMed] [Google Scholar]
- 52.Nordin BE, JM WI, Clifton PM, et al. A longitudinal study of bone-related biochemical changes at the menopause. Clin Endocrinol (Oxf) 2004;61(1):123–130. doi: 10.1111/j.1365-2265.2004.02066.x. [DOI] [PubMed] [Google Scholar]
- 53.Ito M, Lang TF, Jergas M, et al. Spinal trabecular bone loss and fracture in American and Japanese women. Calcif Tissue Int. 1997;61(2):123–128. doi: 10.1007/s002239900308. [DOI] [PubMed] [Google Scholar]
- 54.Finkelstein JS, Lee ML, Sowers M, et al. Ethnic variation in bone density in premenopausal and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab. 2002;87(7):3057–3067. doi: 10.1210/jcem.87.7.8654. [DOI] [PubMed] [Google Scholar]
- 55.Holmberg AH, Johnell O, Nilsson PM, et al. Risk factors for fragility fracture in middle age. A prospective population-based study of 33,000 men and women. Osteoporos Int. 2006;17(7):1065–1077. doi: 10.1007/s00198-006-0137-7. [DOI] [PubMed] [Google Scholar]
- 56.Huopio J, Kroger H, Honkanen R, et al. Risk factors for perimenopausal fractures: a prospective study. Osteoporos Int. 2000;11(3):219–227. doi: 10.1007/s001980050284. [DOI] [PubMed] [Google Scholar]
- 57.Torgerson DJ, Campbell MK, Thomas RE, et al. Prediction of perimenopausal fractures by bone mineral density and other risk factors. J Bone Miner Res. 1996;11(2):293–297. doi: 10.1002/jbmr.5650110219. [DOI] [PubMed] [Google Scholar]
- 58.Khalil N, Sutton-Tyrrell K, Strotmeyer ES, et al. Menopausal bone changes and incident fractures in diabetic women: a cohort study. Osteoporos Int. 2011;22(5):1367–1376. doi: 10.1007/s00198-010-1357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Management of osteoporosis in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 2010;17(1):25–54. doi: 10.1097/gme.0b013e3181c617e6. quiz 55-26. [DOI] [PubMed] [Google Scholar]
- 60.Ensrud KE, Ewing SK, Stone KL, et al. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc. 2003;51(12):1740–1747. doi: 10.1046/j.1532-5415.2003.51558.x. [DOI] [PubMed] [Google Scholar]
- 61.Avenell A, Gillespie WJ, Gillespie LD, et al. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database Syst Rev. 2009;(2):CD000227. doi: 10.1002/14651858.CD000227.pub3. [DOI] [PubMed] [Google Scholar]
- 62.Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293(18):2257–2264. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 63.Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327(23):1637–1642. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- 64.Dawson-Hughes B, Harris SS, Krall EA, et al. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337(10):670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 65.Grant AM, Avenell A, Campbell MK, et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet. 2005;365(9471):1621–1628. doi: 10.1016/S0140-6736(05)63013-9. [DOI] [PubMed] [Google Scholar]
- 66.Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 67.Finkelstein JS. Calcium plus vitamin D for postmenopausal women--bone appetit? N Engl J Med. 2006;354(7):750–752. doi: 10.1056/NEJMe068007. [DOI] [PubMed] [Google Scholar]
- 68.Citron JT, Ettinger B, Genant HK. Spinal bone mineral loss in estrogen-replete, calcium-replete premenopausal women. Osteoporos Int. 1995;5(4):228–233. doi: 10.1007/BF01774011. [DOI] [PubMed] [Google Scholar]
- 69.Dawson-Hughes B, Dallal GE, Krall EA, et al. A controlled trial of the effect of calcium supplementation on bone density in postmenopausal women. N Engl J Med. 1990;323(13):878–883. doi: 10.1056/NEJM199009273231305. [DOI] [PubMed] [Google Scholar]
- 70.Reid IR, Mason B, Horne A, et al. Randomized controlled trial of calcium in healthy older women. Am J Med. 2006;119(9):777–785. doi: 10.1016/j.amjmed.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 71.Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: Institute of Medicine of the National Academies; 2011. [PubMed] [Google Scholar]
- 72.Bolland MJ, Avenell A, Baron JA, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010;341:c3691. doi: 10.1136/bmj.c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bolland MJ, Barber PA, Doughty RN, et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ. 2008;336(7638):262–266. doi: 10.1136/bmj.39440.525752.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bailey RL, Dodd KW, Goldman JA, et al. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010;140(4):817–822. doi: 10.3945/jn.109.118539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chapuy MC, Schott AM, Garnero P, et al. Healthy elderly French women living at home have secondary hyperparathyroidism and high bone turnover in winter. EPIDOS Study Group. J Clin Endocrinol Metab. 1996;81(3):1129–1133. doi: 10.1210/jcem.81.3.8772587. [DOI] [PubMed] [Google Scholar]
- 76.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 77.Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Holick MF, MacLaughlin JA, Clark MB, et al. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210(4466):203–205. doi: 10.1126/science.6251551. [DOI] [PubMed] [Google Scholar]
- 79.Norman AW. Sunlight, season, skin pigmentation, vitamin D, and 25-hydroxyvitamin D: integral components of the vitamin D endocrine system. Am J Clin Nutr. 1998;67(6):1108–1110. doi: 10.1093/ajcn/67.6.1108. [DOI] [PubMed] [Google Scholar]
- 80.Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 81.Kanis JA, McCloskey EV, Johansson H, et al. A reference standard for the description of osteoporosis. Bone. 2008;42(3):467–475. doi: 10.1016/j.bone.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 82.Baim S, Binkley N, Bilezikian JP, et al. Official Positions of the International Society for Clinical Densitometry and executive summary of the 2007 ISCD Position Development Conference. J Clin Densitom. 2008;11(1):75–91. doi: 10.1016/j.jocd.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 83.Screening for osteoporosis: U.S. preventive services task force recommendation statement. Ann Intern Med. 2011;154(5):356–364. doi: 10.7326/0003-4819-154-5-201103010-00307. [DOI] [PubMed] [Google Scholar]
- 84.Waugh EJ, Lam MA, Hawker GA, et al. Risk factors for low bone mass in healthy 40–60 year old women: a systematic review of the literature. Osteoporos Int. 2009;20(1):1–21. doi: 10.1007/s00198-008-0643-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tremollieres F, Cochet T, Cohade C, et al. Fracture risk in early postmenopausal women assessed using FRAX. Joint Bone Spine. 2010;77(4):345–348. doi: 10.1016/j.jbspin.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 86.Ettinger B, Black DM, Dawson-Hughes B, et al. Updated fracture incidence rates for the US version of FRAX. Osteoporos Int. 2010;21(1):25–33. doi: 10.1007/s00198-009-1032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tremollieres FA, Pouilles JM, Drewniak N, et al. Fracture risk prediction using BMD and clinical risk factors in early postmenopausal women: sensitivity of the WHO FRAX tool. J Bone Miner Res. 2010;25(5):1002–1009. doi: 10.1002/jbmr.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tosteson AN, Melton LJ, 3rd, Dawson-Hughes B, et al. Cost-effective osteoporosis treatment thresholds: the United States perspective. Osteoporos Int. 2008;19(4):437–447. doi: 10.1007/s00198-007-0550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eng-Wong J, Reynolds JC, Venzon D, et al. Effect of raloxifene on bone mineral density in premenopausal women at increased risk of breast cancer. J Clin Endocrinol Metab. 2006;91(10):3941–3946. doi: 10.1210/jc.2005-2827. [DOI] [PubMed] [Google Scholar]
- 90.Cauley JA, Seeley DG, Ensrud K, et al. Estrogen replacement therapy and fractures in older women. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1995;122(1):9–16. doi: 10.7326/0003-4819-122-1-199501010-00002. [DOI] [PubMed] [Google Scholar]
- 91.Cauley JA, Zmuda JM, Ensrud KE, et al. Timing of estrogen replacement therapy for optimal osteoporosis prevention. J Clin Endocrinol Metab. 2001;86(12):5700–5705. doi: 10.1210/jcem.86.12.8079. [DOI] [PubMed] [Google Scholar]
- 92.Schneider DL, Barrett-Connor EL, Morton DJ. Timing of postmenopausal estrogen for optimal bone mineral density. The Rancho Bernardo Study. JAMA. 1997;277(7):543–547. doi: 10.1001/jama.277.7.543. [DOI] [PubMed] [Google Scholar]