Abstract
Cough and swallow are highly coordinated reflex behaviors whose common purpose is to protect the airway. The pharynx is the common tube for air and food/liquid movement from the mouth into the thorax, has been largely overlooked, and is potentially seen as just a passive space. The thyropharyngeus muscle responds to cough inducing stimuli to prepare a transient holding area for material that has been removed from the subglottic airway. The cricopharyngeus muscle participates with the larynx to ensure regulation of pressure when a bolus/air is moving from the upper airway through to the thorax (i.e inspiration or swallow) or the reverse (i.e expiration reflex or vomiting).These vital mechanisms have not been evaluated in clinical conditions, but could be impaired in many neurodegenerative diseases leading to aspiration pneumonia. These newly described airway protective mechanisms need further study, especially in healthy and pathologic human populations.
Keywords: pharynx, cricopharyngeus, thyropharyngeus, cough, swallow
Aspiration: Relationships between Cough and Swallow
Cough is the most audible of aspiration responses, and can be stimulated by rapidly adapting receptors (cough receptors) [1, 2] and C-fibers in the laryngeal and tracheal mucosa (especially the area of the carina), which terminate on second order neurons in the nucleus tract solitarius, then project to pontine and medullary respiratory neuron populations as well as recruited neurons within the medial reticular formation [3-5]. Following the inspiratory phase, there is rapid vocal fold adduction and contraction of the expiratory muscles, including all abdominal muscles with a majority of force production from the internal and external oblique muscles [6, 7]. The material in lower airway is sheared and/or aerosolized to be removed via the mouth or deposited into the pharynx.
Smith-Hammond first described the relationship between cough and swallow, in a population of patients following stroke [8, 9]. Pitts et al [10] also examined this relationship in a cohort of patients with Parkinson’s disease (PD). In this study penetration/aspiration during swallow was associated with an impaired voluntary cough. Additionally, patients with penetration/aspiration had significantly longer cough duration (time for completion of the three cough phases) and a decrease in the expiratory phase peak flow when compared to patients with PD who did not exhibit penetration/ aspiration. Furthermore, prolongation of the compression phase, decreases in the expiratory phase peak flow and cough volume acceleration have been shown to detect and/or predict dysphagia in PD [9, 11]. These and other clinical studies have highlighted commonalities between cough and swallow. Recent studies in animals [12, 13] are beginning to explain peripheral and central interactions between cough and swallow.
Pharyngeal participation in swallow
Swallowing is made up of three distinct phases: oral, pharyngeal, and esophageal. The pharyngeal phase of swallow, especially in an upright human, presents particular risk for aspiration. The human mouth the pharynx connect at a 90 degree angle and with the addition of gravity, there is risk for aspiration at any age [14-16]. The proper movement of the bolus through the pharynx, around the larynx, and adequate use of the pyriform and valleculae sinuses is necessary to reduce aspiration risk.
The pharyngeal phase of swallow is a patterned behavior [17]. There are several actions that take place during this phase. First, the tongue base retracts and then moves superior and posterior, which in turn directs the bolus toward the pharynx. During the tongue movement there is closure of the velopharyngeal port. Velopharyngeal closure is important because it allows for a build-up of pressure in the pharynx to help propel the bolus toward the esophagus, and the contact of the soft palate with the back pharyngeal wall prevents the bolus from moving into the nasopharynx [18, 19]. The pharynx then has two basic movements: there is elevation of the entire pharynx and then a descending activation of various parts of the pharyngeal musculature to act as a peristaltic wave to move the bolus along. The submental muscles contract to move the hyoid bone and larynx superior and anterior into position under the tongue base [19]. During the movement of the larynx, the vocal folds and aryepiglottic folds adduct preventing material from entering the lower airway. Additionally, the epiglottis folds over the glottal space to act as another layer of protection from material entering the lower airway. The movement of the larynx also pulls opens the superior portion of the esophageal sphincter. Following the contraction of the inferior pharyngeal muscle there is a relaxation of the muscles making up the upper esophageal sphincter. The bolus is then passed into the esophagus.
Anatomy of the pharynx
The posterior and lateral portions of the pharyngeal wall are made up of encircling striated muscle fibers and its embryonic division is from splanchnic mesoblast that is found surrounding the foregut [20]. Humans, in contrast with smaller animals, have longitudinal muscle fibers and have the addition of the stylopharyngeus muscle which works to suspend the pharynx for vertical movement which is necessary during the pharyngeal phase of swallowing [20, 21].
Overlying the muscle is pharyngeal mucosa e.g. [22]. The innervation of the pharyngeal mucosa is complex. This complex innervation is referred to as the pharyngeal plexus [22]. Mu and Sanders [23] stained and dissected the afferent innervation in the mucosa of the lateral and posterior pharyngeal walls in a human cadaver and found that the oropharynx is innervated by the pharyngeal branch of the glossopharyngeal and vagus nerves, and the laryngopharynx is innervated by the inferior branch of the superior laryngeal nerve. Afferent fiber density, in humans, varied across the pharyngeal complex, with the naso-pharynx having the least dense innervation and the lateral pharyngeal walls having the highest density.
Hypopharynx (thyropharyngeus and cricopharyngeus muscles)
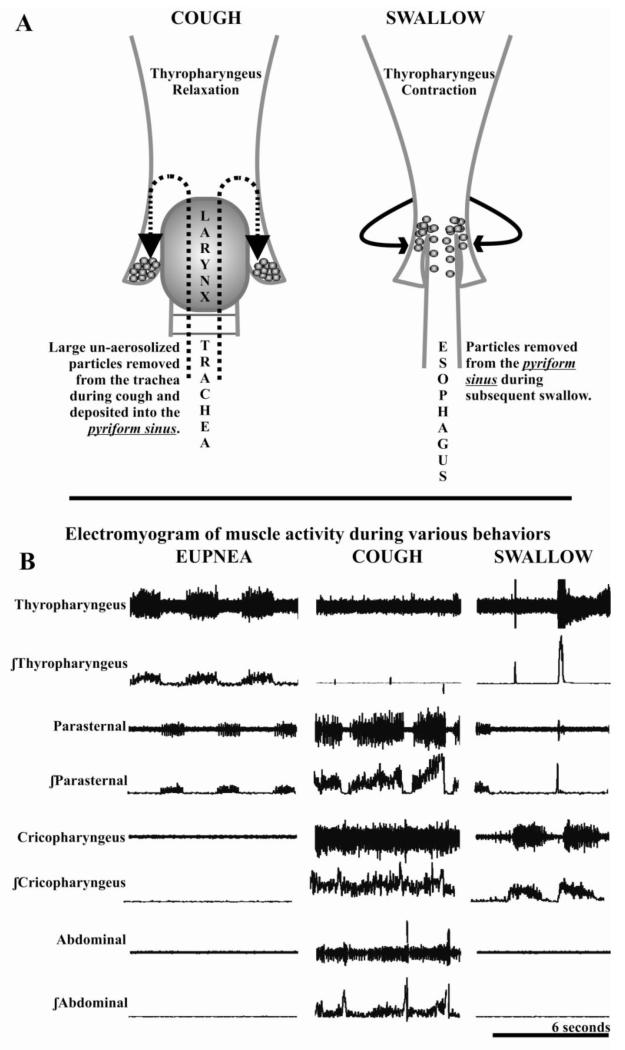
The pharyngeal response to aspiration has not been well described, and its importance may be underappreciated. The hypopharynx (lower pharynx) can be described in anatomical (thyropharyngeus and cricopharyngeus) or functional terms (inferior pharyngeal constrictor and upper esophageal sphincter). There are physiological differences between these two muscles, and in our experiments, conducted in anesthetized cats, we have employed electromyographic methods that show the thyropharyngeus acts as the inferior pharyngeal constrictor and the cricopharyngeus acts as the upper esophageal sphincter (Figure 1).
Figure 1a.
Control of the pyriform sinus by the thyropharyngeus acting as the inferior pharyngeal constrictor. Following the expiratory phase of cough the pyriform sinus can act as a holding reservoir for material which can then be passed into the esophagus during the subsequent swallow. 1b. Electromyogram activity (raw and integrated) of the thyropharyngeus, parasternal (inspiratory chest-wall muscle), cricopharyngeus (acting as the upper esophageal sphincter), and abdominal muscles during eupnea, cough and swallow. During eupnea (resting breathing) the thyropharyngeus is expiratory phasic, its activity is suppressed beginning at the mechanical stimulation of the trachea and continuing through the cough efforts. It activity is markedly increased during swallow.
The thyropharyngeus is a fan shaped muscle beginning at the thyroid cartilage, of the larynx, and extending around to the midline raphe. Efferent innervation is from the vagus and afferent information is carried by vagal and possibly the glossopharyngeal nerves [24]. The thyropharyngeus is composed of predominantly type II muscle fibers (fast contracting, glycolytic, and highly fatigable), and the motor end plates terminate in the belly of the muscle [25].
The thyropharyngeus is intrinsically involved in pharyngeal clearance and has active control of the diameter of the pyriform sinus (Figure 1) [13]. The pharynx has upper (valleculae sinus) and lower (pyriform sinus) cavities that act as bilateral reservoirs for collection of material [26-28]. Dua and colleagues [29] have also demonstrated that there are volume sensitive receptors, in the pyriform sinus, which can also trigger a swallow in humans. Additionally, when a swallow is produced during sequential cough, the swallow related thyropharyngeus activity increases 14% on average in cats [13]. Beyond its participation in the pharyngeal phase of swallow; the pyriform sinus is a location for safely holding material that has been removed from the lower airways by a cough bout. Figures 1a is a schematic of the pharynx during eupnea (breathing), cough, and swallow. The thyropharyngeus, in the cat and human, is expiratory phasic during breathing [13], and is suppressed during cough [13] (Figure 1b). This suppression of the thyropharyngeus EMG activity during cough is a newly described airway protective mechanism, and we propose that it is used to collect particulates that were too large to be aerosolized or did not get expectorated. Theoretically, material is then collected to be removed during the subsequent swallow.
Conversely, the cricopharyngeus is made up of two distinct components the oblique and horizontal [24]. The oblique follows a similar path as the thyropharyngeus, except it arises from the cricoid cartilage of the larynx; however the horizontal portion is a “loop of tissue” which connects to the lower third of the cricoid cartilage. The horizontal portion is agreed to be a part of the upper esophageal sphincter (UES), however other portions of the thyropharyngeus may also be involved in sphincter activity. Efferent innervation is by the vagus, specifically the pharyngoesophageal nerve, and afferent is carried by the vagus [24]. As opposed to the thyropharyngeus, the cricopharyngeus is mostly made of type I muscle fibers (slow contracting, oxidative, and resistant to fatigue), and the motor endplates are diffuse throughout the muscle [25].
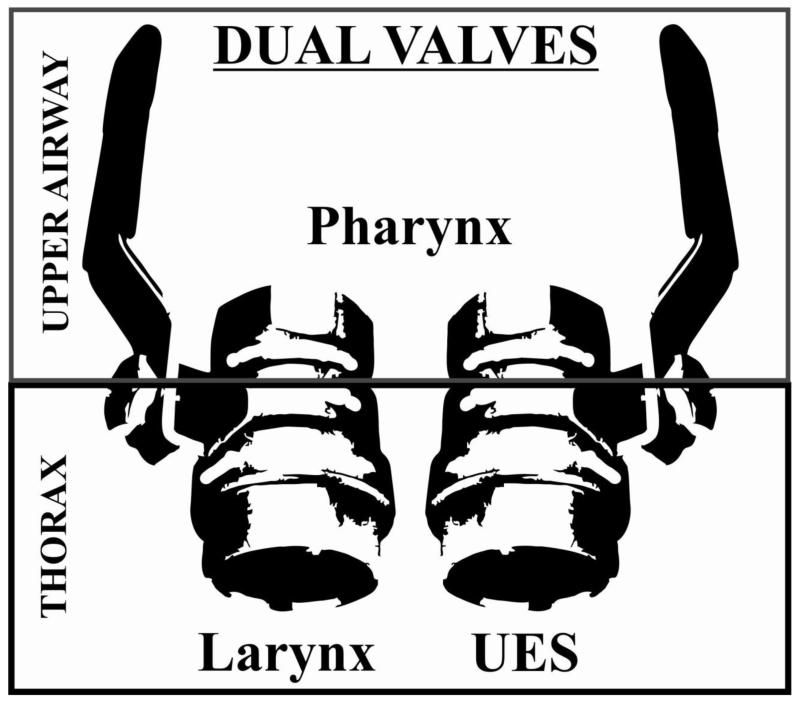
Dual valve hypothesis
The larynx has been described as a valve for many years [30-33]. Recent data [13], however, supports the hypothesis that the UES works in concert with the larynx creating a dual-valve system (Figure 2). During the pharyngeal phase of swallow the larynx is maximally adducted, reciprocally the UES is maximally relaxed. However, during the compression phase of cough (to maintain intra-thoracic pressure and ensure cough effectiveness) both the larynx is adducted and the UES is maximally contracted [13]. Cricopharyngeus EMG activity is also highly responsive to mechanical stimulation of the trachea, resulting in a significant increase in tone [13]. This could be a result of a feedforward mechanism in the brainstem control network to prepare the pharynx for cough production.
Figure 2.
The larynx and upper esophageal sphincter act as “dual valves.” They work in concert to control the path of pressure for movement of air/liquid/bolus from the upper airway into the thorax (breathing and swallowing) and from the thorax into the upper airway (cough and vomiting).
A common condition that arises during neurodegenerative disease progression is cricopharyngeal bar [34, 35]. Increased cricopharyngeal tone leads to “bunching” of sphincter tissue, which in turn blocks the entrance of the esophagus leading to “food getting stuck” and potentially aspiration of material into the lungs. A common treatment of this condition is injection of botulium neurotoxin A (botox) directly into the muscle [36-38]. This leads to an interesting clinical condition, where the upper esophageal sphincter can no longer participate in pressure regulation. There are no studies available examining cough production pre and post botox injections to the cricopharyngeus; according to the dual valve hypothesis, this would lead to significant cough impairment.
Summary
The pharynx is the common space for which air travels for breathing and food/liquid for sustenance. However, it is not a passive tube, but an active participant in airway protection. The thyropharyngeus is responsive to cough inducing stimulation to prepare a transient reservoir for removed material. The cricopharyngeus participates with the larynx to ensure regulation of pressure when a bolus/air moves from the upper airway through to the thorax (i.e inspiration or swallow) or the reverse (i.e expiration reflex or vomiting). These vital mechanisms have not been evaluated in clinical conditions, but could be impaired in many neurodegenerative diseases leading to aspiration pneumonia. Clinical studies are needed to evaluate their regulation in healthy and disease conditions.
Acknowledgments
NIH Institute of Heart Lung and Blood HL111215
References
- 1.Canning BJ. Anatomy and neurophysiology of the cough reflex. Chest. 2006;129(1 suppl):33. doi: 10.1378/chest.129.1_suppl.33S. [DOI] [PubMed] [Google Scholar]
- 2.Canning BJ. Encoding of the cough reflex. Pulm Pharmacol Ther. 2007;20(4):396–401. doi: 10.1016/j.pupt.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pantaleo T, Bongianni F, Mutolo D. Central nervous mechanisms of cough. Pulm Pharmacol Ther. 2002;15(3):227–233. doi: 10.1006/pupt.2002.0358. [DOI] [PubMed] [Google Scholar]
- 4.Shannon R, Baekey D, Morris K, Lindsey B. Brainstem respiratory networks and cough. Pulmonary Pharmacology. 1996;9(5-6):343–347. doi: 10.1006/pulp.1996.0045. [DOI] [PubMed] [Google Scholar]
- 5.Shannon R, Baekey D, Morris K, Nuding S, Segers L, Lindsey B. Production of reflex cough by brainstem respiratory networks. Pulmonary Pharmacology and Therapeutics. 2004;17(6):369–376. doi: 10.1016/j.pupt.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 6.Fontana GA, Lavorini F. Cough motor mechanisms. Respiratory Physiology and Neurobiology. 2006;152(3):266–281. doi: 10.1016/j.resp.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Fontana GA, Pantaleo T, Lavorini F, Benvenuti F, Gangemi S. Defective motor control of coughing in Parkinson’s disease. Americal Journal of Respiratory Critical Care Medicine. 1998;158(2):458–464. doi: 10.1164/ajrccm.158.2.9705094. [DOI] [PubMed] [Google Scholar]
- 8.Smith Hammond CA, Goldstein LB, Zajac DJ, Gray L, Davenport PW, Bolser DC. Assessment of aspiration risk in stroke patients with quantification of voluntary cough. Neurology. 2001;56(4):502–506. doi: 10.1212/wnl.56.4.502. [DOI] [PubMed] [Google Scholar]
- 9.Smith Hammond CA, Goldstein LB, Horner RD, Ying J, Gray L, Gonzalez-Rothi L, Bolser DC. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest. 2009;135(3):769–777. doi: 10.1378/chest.08-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitts T, Bolser D, Rosenbek J, Troche M, Sapienza C. Voluntary cough production and swallow dysfunction in Parkinson’s disease. Dysphagia. 2008;23(3):297–301. doi: 10.1007/s00455-007-9144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitts T, Troche MS, Carnaby-Mann G, Rosenbek JC, Okun MS, Sapienza CM. Utilizing voluntary cough to detect penetration and aspiration during oropharyngeal swallowing in Parkinson’s disease. Chest. 2010 doi: 10.1378/chest.10-0342. [DOI] [PubMed] [Google Scholar]
- 12.Tsujimura T, Udemgba C, Inoue M, Canning BJ. Laryngeal and tracheal afferent nerve stimulation evokes swallowing in anaesthetized guinea pigs. The Journal of Physiology. 2013 doi: 10.1113/jphysiol.2013.256024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitts T, Rose MJ, Mortensen AN, Poliacek I, Sapienza CM, Lindsey BG, Morris KF, Davenport PW, Bolser DC. Coordination of cough and swallow: A meta-behavioral response to aspiration. Respiratory physiology & neurobiology. 2013 doi: 10.1016/j.resp.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falk D. Comparative anatomy of the larynx in man and the chimpanzee: implications for language in Neanderthal. American Journal of Physical Anthropology. 1975;43(1):123–132. doi: 10.1002/ajpa.1330430116. [DOI] [PubMed] [Google Scholar]
- 15.Lieberman P, Laitman JT, Reidenberg JS, Gannon PJ. The anatomy, physiology, acoustics and perception of speech: essential elements in analysis of the evolution of human speech. Journal of Human Evolution. 1992;23(6):447–467. [Google Scholar]
- 16.B Leder, S., Karas DE. Fiberoptic endoscopic evaluation of swallowing in the pediatric population. The Laryngoscope. 2000;110(7):1132–1136. doi: 10.1097/00005537-200007000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Miller The neurobiology of swallowing and dysphagia. Dev Disabil Res Rev. 2008;14(2):77–86. doi: 10.1002/ddrr.12. [DOI] [PubMed] [Google Scholar]
- 18.Logemann JA, Rademaker AW, Pauloski BR, Ohmae Y, Kahrilas PJ. Normal swallowing physiology as viewed by videofluoroscopy and videoendoscopy. Folia Phoniatr Logop. 1998;50(6):311–319. doi: 10.1159/000021473. [DOI] [PubMed] [Google Scholar]
- 19.Logemann Evaluation and Treatment of Swallowing Disorders. ProEd. 1998 [Google Scholar]
- 20.Bosma JF. Deglutition: pharyngeal stage. Physiol Rev. 1957;37(3):275–300. doi: 10.1152/physrev.1957.37.3.275. [DOI] [PubMed] [Google Scholar]
- 21.Edgeworth FH. Quartly Journal of Microscopic science. 1916;6 I:383. [Google Scholar]
- 22.Kitagawa J, Shingai T, Takahashi Y, Yamada Y. Pharyngeal branch of the glossopharyngeal nerve plays a major role in reflex swallowing from the pharynx. Am J Physiol Regul Integr Comp Physiol. 2002;282(5):R1342–1347. doi: 10.1152/ajpregu.00556.2001. [DOI] [PubMed] [Google Scholar]
- 23.Mu L, Sanders I. Neuromuscular specializations of the pharyngeal dilator muscles: II. Compartmentalization of the canine genioglossus muscle. Anat Rec. 2000;260(3):308–325. doi: 10.1002/1097-0185(20001101)260:3<308::AID-AR70>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 24.Goyal RK, Cobb BW. Motility of the pharynx, esophagus, and esophageal sphincters. Physiology of the gastrointestinal tract. 1981;1:359–391. [Google Scholar]
- 25.Hyodo M, Aibara R, Kawakita S, Yumoto E. Histochemical study of the canine inferior pharyngeal constrictor muscle: implications for its function. Acta Oto-Laryngologica. 1998;118(2):272–279. doi: 10.1080/00016489850155017. [DOI] [PubMed] [Google Scholar]
- 26.Dodds WJ, Logemann JA, Stewart ET. Radiologic assessment of abnormal oral and pharyngeal phases of swallowing. AJR. American journal of roentgenology. 1990;154(5):965–974. doi: 10.2214/ajr.154.5.2108570. [DOI] [PubMed] [Google Scholar]
- 27.Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA, Kahrilas PJ, Smith CH. Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. Journal of Speech, Language and Hearing Research. 2000;43(5):1264. doi: 10.1044/jslhr.4305.1264. [DOI] [PubMed] [Google Scholar]
- 28.Dodds WJ, Stewart ET, Logemann JA. Physiology and radiology of the normal oral and pharyngeal phases of swallowing. AJR. American journal of roentgenology. 1990;154(5):953–963. doi: 10.2214/ajr.154.5.2108569. [DOI] [PubMed] [Google Scholar]
- 29.Dua K, Surapaneni SN, Kuribayashi S, Hafeezullah M, Shaker R. Pharyngeal airway protective reflexes are triggered before the maximum volume of fluid that the hypopharynx can safely hold is exceeded. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2011;301(2):G197–G202. doi: 10.1152/ajpgi.00046.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batson OV. The Valsalva maneuver and the vertebral vein system. Angiology. 1960;11(5):443–447. doi: 10.1177/000331976001100512. [DOI] [PubMed] [Google Scholar]
- 31.Bartlett D. Respiratory functions of the larynx. Physiological Reviews. 1989;69(1):33–57. doi: 10.1152/physrev.1989.69.1.33. [DOI] [PubMed] [Google Scholar]
- 32.Merwin GE, Goldstein LP, Rothman HB. A comparison of speech using artificial larynx and tracheoesophageal puncture with valve in the same speaker. The Laryngoscope. 1985;95(6):730–734. doi: 10.1288/00005537-198506000-00017. [DOI] [PubMed] [Google Scholar]
- 33.Morris I. Functional anatomy of the upper airway. Emergency medicine clinics of North America. 1988;6(4):639. [PubMed] [Google Scholar]
- 34.Wang AY, Kadkade R, Kahrilas PJ, Hirano I. Effectiveness of esophageal dilation for symptomatic cricopharyngeal bar. Gastrointestinal endoscopy. 2005;61(1):148–152. doi: 10.1016/s0016-5107(04)02447-2. [DOI] [PubMed] [Google Scholar]
- 35.Goyal RK, Martin SB, Shapiro J, Spechler SJ. The role of cricopharyngeus muscle in pharyngoesophageal disorders. Dysphagia. 1993;8(3):252–258. doi: 10.1007/BF01354547. [DOI] [PubMed] [Google Scholar]
- 36.Fan X, Scott L, Underbrink M, Hersey M. AMERICAN JOURNAL OF GASTROENTEROLOGY. NATURE PUBLISHING GROUP; 75 VARICK ST, 9TH FLR, NEW YORK, NY 10013-1917 USA: 2008. Resolution of cricopharyngeal bar with botox injection combined with esophageal dilation. [Google Scholar]
- 37.Natt R, McCormick M, Clayton J, Ryall C. Percutaneous chemical myotomy using botulium neurtoxin A under local anaesthesia in the treatment of cricopharyngeal dysphagia following laryngectomy. Auris Nasus Larynx. 2010;37(4):500–503. doi: 10.1016/j.anl.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Williams RB, Wallace KL, Ali GN, Cook IJ. Biomechanics of failed deglutitive upper esophageal sphincter relaxation in neurogenic dysphagia. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2002;283(1):G16–G26. doi: 10.1152/ajpgi.00189.2001. [DOI] [PubMed] [Google Scholar]