Abstract
Systemic lupus erythematosus (SLE) is a multisystem disease with significant morbidity and even mortality. Cytomegalovirus (CMV) is a ubiquitous herpesvirus that, similar to SLE, can also lead to significant morbidity and mortality in the immunocompromised host. The relationship between SLE and CMV is complex, with observations suggesting that CMV induces the autoimmunity of SLE in addition to occurring in the immunocompromised host with known SLE. In this article, we first consider CMV infection in the immunocompetent host, and further examine how this infection differs in the patient with SLE. We focus on disease mechanisms, CMV detection and treatment. We review the differences between CMV infection, syndrome and disease, as identifying the correct state will determine the appropriate treatment. We propose guidelines for the screening and management of CMV infection in childhood-onset SLE, and recognize that further study in this population is required to increase our understanding of the interplay between these disease entities.
Keywords: childhood, CMV, complication, Cytomegalovirus, detection, guidelines, infection, lupus, pediatric
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that can involve any organ system, and can lead to significant morbidity and even mortality. Childhood-onset SLE (cSLE) has an incidence of up to 0.9 per 100,000 children-years and a prevalence of 3.3–8.8 per 100,000 children [1]. Patients with SLE may be immunocompromised as a result of the immune dysfunction of the disease and/or as the result of the use of immunosuppressive medications including corticosteroids. Morbidity may be due to disease flares and treatment side effects, although infections are another important and frequent cause. Symptoms of infection can range from mild requiring no treatment, moderate requiring hospitalization and systemic therapy, to severe with sequelae that include organ failure and death. Viral infections, in particular the herpes family of viruses, can mimic disease flares and should be considered in any cSLE patient with a change of disease status. One ubiquitous herpesvirus, Cytomegalovirus (CMV), has been recognized in recent years to have diffuse manifestations that can resemble active SLE disease. In this review, we focus on the importance of CMV in cSLE, specifically characterizing the mechanisms and manifestations of CMV infection in the immunocompetent and immunocompromised host with cSLE. We review the diagnostic methods and propose recommendations for surveillance and treatment of CMV in cSLE.
Methods
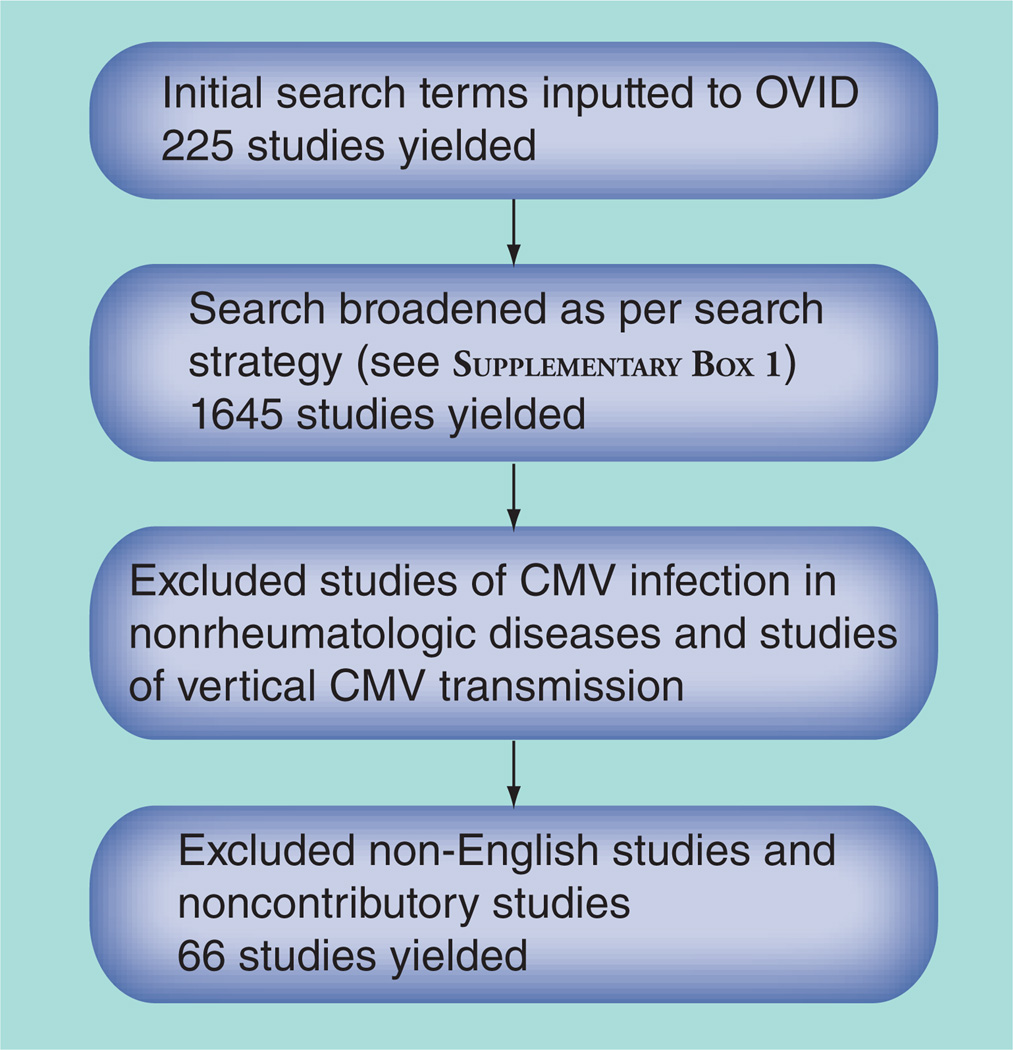
A scoping review of the topic ‘Cytomegalovirus infection in SLE’ was performed searching literature in the interface of OVID, using publications in MEDLINE and EMBASE from 1946 to April 2012. As the number of studies examining CMV in SLE was small, the search was broadened to include CMV infection in rheumatic disease to achieve a larger number of articles. This search strategy yielded 1645 unique articles, with 899 from EMBASE and 746 additional articles from MEDLINE. Articles were excluded based on criteria shown in Figure 1. See Supplementary Box 1 (online at www.futuremedicine.com/doi/suppl/10.2217/IJR.12.82) for complete search strategies.
Figure 1. Search strategy.
Summary of status of literature on CMV & cSLE
The recent literature reveals a dearth of reports specific to CMV in cSLE, with most studies including either only adult-onset SLE patients, or cSLE patients in a cohort of predominantly adult patients. The majority of the studies are case reports, lacking examination of any large pediatric cSLE cohort. There is increasing recognition of severe CMV disease in cSLE; however, the literature does not provide evidence-based guidance on surveillance, prophylaxis or treatment. In fact, only five articles exclusively reported on CMV in cSLE; four of these were case reports and the fifth was a review of childhood autoimmune conditions (including cSLE) associated with CMV.
CMV definitions
Standard definitions of CMV detection from the organ transplant literature are useful to consider in SLE, another immunocompromised population [2]. CMV infection may be primary or secondary (reactivation). Furthermore, consideration of a patient’s clinical status will determine the presence of CMV infection, CMV syndrome or CMV disease.
Primary versus secondary infection
Primary CMV infection is defined as the detection of CMV in a previously seronegative person. In organ transplant, this is strictly defined as new infection following at least 4 weeks of negative cultures during active surveillance in a previously documented CMV-positive patient. Secondary infection can occur due to de novo infection by another CMV virus, although more frequently is reactivation of latent virus [2]. A latent phase follows primary infection, whereby ongoing viral replication is controlled by the host immune system in order to prevent reactivation of the virus. Despite the lack of symptoms during the latent phase, there is persistent interaction between the virus and the host immune system. Reactivation can occur at any time from weeks to years following latency, and may lead to significant morbidity and even mortality [3]. Reactivation is most common in those with compromised T-cell immunity, which can occur, for example, in autoimmune disease as a result of treatment with immunosuppressive drugs or as a consequence of human immunodeficiency virus infection.
CMV infection, syndrome & disease
CMV is further categorized into infection, syndrome and disease. CMV infection is diagnosed when there is evidence of replicating virus in an individual. CMV disease requires symptoms of end-organ disease concurrent with CMV detection in blood, urine, bronchoalveolar lavage (BAL) or biopsy. CMV syndrome is the triad of persistent fever, CMV viremia and cytopenia (although thrombocytopenia is most common, neutropenia and anemia also occur) [2]. We will refer to these definitions throughout this review.
Epidemiology & transmission
CMV, a member of the β-herpesvirus family, is an interesting and prevalent virus with a variable mode of transmission that depends on the age of the host. CMV seropositivity rates in adults range from approximately 45 to 100% worldwide, with a higher incidence with increasing age, in women and in developing countries [4]. In the USA, between 30 and 70% of children and adolescents have evidence of prior CMV infection [5,6]. In the pediatric population CMV transmission most commonly occurs horizontally with spread through droplet contact (including urine and saliva) and less commonly via blood or bodily fluids [7]. CMV infection in the first year of life is usually contracted vertically from the mother during childbirth or via CMV-positive breast milk [8]. Due to the presence and passive transfer of anti-CMV antibodies in breast milk, infections that occur via this mode of transmission are often asymptomatic [7]. Persons requiring stem cell and/or organ transplantation are at risk of acquiring latent CMV through transplantation as the latent virus resides in cells of myeloid lineage [9], and are at risk for both primary CMV infections due to their immunocompromised state, and secondary infections due to the latent reactivation of the acquired CMV disease.
Manifestations of CMV in the immunocompetent host
The presentation of CMV infection appears to vary depending on the age of the infected individual. Most infections in childhood are either mild (flu-like illness) or asymptomatic. In neonates symptoms include jaundice, purpura, hepatosplenomegaly, microcephaly and sensorineural hearing loss. In adolescents and adults, CMV usually manifests as prolonged fever and mild hepatitis (a mononucleosis-like illness). In the immunocompetent host, CMV more rarely presents with multisystem manifestations, with symptoms ranging from fever, rash, anemia and thrombocytopenia to retinitis, encephalitis, pneumonitis, hepatitis and pancreatitis. Fatal infections are rare in immunocompetent persons, but life-threatening sequelae should not be ignored [10].
Laboratory anomalies such as anemia, thrombocytopenia, abnormal liver enzymes and elevated renal function tests may occur, depending on the organ system affected [2,11,12]. Lymphopenia (<300–700 mm3) can signal CMV reactivation due to CMV’s ability to suppress the bone marrow, and has been associated with more severe CMV infections [11,13,14]. The thrombocytopenia, however, may result from either cross-reactivity between anti-CMV antibodies and platelets, or secondary to bone marrow suppression [15]. Specific organ manifestations are likely the result of direct damage from viral invasion and replication.
CMV is a double-stranded linear DNA virus, and is the largest among the family of herpes viruses. Replication of CMV involves multiple proteins beginning with the immediate-early (IE) proteins that transcribe messenger ribonucleic acid for the early proteins to allow DNA replication to acquire the late proteins. There are multiple inhibitors that manipulate the cascade. Much of the function of the CMV proteins is unknown [16].
Normal regulation of CMV
In order to understand how CMV infection may lead to autoimmune disease it is important to first consider the normal immune response to CMV. Infection with CMV leads to the production of both T (mainly CD8+ cells) and B cells, which recognize specific antigens on CMV. In fact, up to 10% of CD4+ and CD8+ memory T cells in the immunocompetent host have CMV antigen specificity [17]. The major antigen recognized by CD8+ T cells and B cells is the phosphoprotein 65 (pp65) with a second major antigen recognized by T cells (IE-1 protein) [18]. B cells also recognize epitopes on pp65 protein and produce anti-pp65 antibodies. The initial response is limited to a few epitopes on CMV antigens, however, as the immune response matures, via epitope spreading and affinity maturation, both T and B cells will recognize multiple epitopes within pp65 and other proteins. This is a potentially important mechanism for the development of an autoimmune disease (see below). During latent infection there is a persistence of circulating T cells (both CD4+ and CD8+ cells) with specificity for multiple epitopes on pp65 and other proteins. However, the IgG anti-pp65 response is relatively restricted in healthy individuals [19].
Detection of CMV
There are several methods of CMV infection detection, including serologic testing, molecular and related testing, culture and histopathology.
Serologic
Qualitative detection can determine the presence or absence of serum anti-CMV IgG and IgM antibodies, and is frequently employed as a preliminary screen for CMV detection. However, there are several limitations to qualitative detection. A positive serum anti-CMV IgM result may not differentiate between primary infection and reactivation of latent disease [20]. In addition, qualitative testing has variable sensitivity, and reliability may vary between the commonly used diagnostic kits. Furthermore, false-positive anti-CMV IgM antibodies can occur in the presence of rheumatoid factors or heterophile antibodies [21–23]. Following the development of anti-CMV IgM antibodies, the time of conversion to IgG is variable, especially in the immunocompromised host, rendering qualitative testing less useful for monitoring the resolution of active infection [24].
Molecular testing
CMV antigenemia is detected by immunoassays that can quantify circulating CMV pp65 antigen by directly immunostaining polymorphonuclear (PMN) leukocytes with monoclonal antibodies against pp65 CMV lower-matrix protein [20,25]. CMV pp65 antigen testing is useful for diagnosis, initiation of antiviral therapy to prevent progression from CMV infection to CMV disease, and for monitoring CMV disease in SLE, transplant patients and other immunocompromised hosts [13,26]. As a general principle, the higher the viral (antigenemic) load, the more risk there is to progress to CMV disease [27]. Detecting CMV antigenemia has greater sensitivity than culture since it does not rely on viral replication to yield a positive result. Limitations of this method include the need for immediate specimen processing, the time required to process the sample, subjectivity in interpretation of results, and a requirement for an adequate number of PMNs. The assay may not be possible with an absolute count of fewer than 1000 neutrophils/µl, which, although infrequent, does occur in SLE [20].
The most frequently used method of quantitative detection determines the viral DNA copy number via PCR. PCR should be used to confirm CMV infection when qualitative CMV testing (IgM) is positive, and similar to CMV pp65 antigen testing, PCR results are particularly useful for diagnosis, determining the need for treatment initiation and for monitoring response to therapy [20]. Unfortunately, there is no absolute threshold for the number of DNA copies that can differentiate active versus latent infection, thus this test is most useful for monitoring viral burden and response to therapy over time [27,28].
Culture
CMV detection by cell culture is infrequently utilized for diagnosis as it has limited sensitivity for detection. As might be expected, culture has the highest rate of positivity in individuals with CMV disease [20]. Other limitations of culture include variable to long processing times depending on the method of culture chosen, and its nonquantitative nature limits its usefulness in guiding treatment for preventing progression to CMV disease [29].
Histopathology
Organ-specific biopsy remains the gold standard for CMV diagnosis. However, histopathologic testing should be carried out on a case-by-case basis where diagnosis is unclear as there is concomitant risk to the individual associated with obtaining the pathologic samples, and a potential diagnostic delay as the samples are processed [30]. Viral PCR testing of tissue or bone marrow specimens may have limited utility because of the possibility of false-positive results due to the detection of passenger lymphocytes. Instead, viral PCR determination in tissue samples should be considered in conjunction with peripheral blood testing.
CMV & SLE
Specific autoantigens
Infection with the herpesviruses, including CMV, have been shown to alter the immune system, and multiple studies have suggested a link between CMV infection and autoimmunity. SLE is characterized by the production of multiple autoantibodies directed against nuclear proteins including chromatin, DNA and RNA binding proteins. CMV is purported to trigger either the onset or a flare of SLE. This hypothesis is based on the induction of autoantigens on the surface of cells following CMV infection, sequence homology between CMV and autoantigens, and the induction of autoantibodies following CMV antigen exposure.
Anti-Sm and anti-RNP antibodies are closely linked autoantibodies directed against a small family of RNPs that bind to the U series of snRNAs [31]. Anti-U1RNP and anti-Sm antibodies are commonly detected in the sera of patients with cSLE. There may also be an association between these autoantibodies and CMV infection since high-titer anti-Sm antibodies are more commonly detected in individuals with latent CMV infection compared to uninfected individuals. Furthermore, in SLE, latent CMV infection is more frequent in patients with anti-RNP antibodies compared to those without anti-RNP antibodies [31,32]. Speculation that CMV suppresses the host immune system during reactivation may explain an association between autoantibody production and CMV antibody status, with lower anti-snRNP antibody levels observed in CMV IgM-positive SLE patients than in CMV IgG-positive patients [22]. We have recently noted that at the time of cSLE diagnosis, anti-RNP antibodies were more frequent in patients with symptomatic CMV infection than in patients without CMV infection [33].
Autoantibodies to another set of RNA-proteins, the Ro and La proteins (proteins that are associated with hYRNAs) are frequently found in patients with SLE and Sjorgren’s syndrome (SS). CMV infection of in vitro cell lines can lead to the expression of the La protein on the cell surface. The CMV-induced cell surface expression of the La autoantigen can then lead to the production of anti-La antibodies in genetically susceptible individuals. Another potential mechanism leading to the production of anti-La antibodies is the sequence homology of epitopes in the La protein with CMV antigens (molecular mimicry) [34,35]. The best evidence for this hypothesis is the presence of anti-La antibodies in the sera of patients with SS and SLE that cross-react with epitopes on CMV antigens [35].
The CMV immune response: pp65
As described above, following infection with CMV, the immune response is mainly directed to epitopes on the pp65 viral antigen. As the immune response matures, if one or more of the epitopes cross-reacts with nuclear proteins, a self-antigen followed by autoreactive T and/or B cells may be produced. If this response leads to a break in tolerance that is of sufficient magnitude and is sustained, then an autoimmune response becomes sustained and an autoimmune disease such as SLE can develop [32,36]. In a murine BALB/c model, mice immunized with a peptide within the c-terminus of pp65 protein (pp65336–439 peptide) produced anti-pp65336–439 antibodies. These antibodies cross-reacted with multiple antinuclear antibodies including antichromatin, anticentriole and anti-dsDNA antibodies and were shown to be pathogenic [19]. In human SLE, IgG anti-pp65 antibodies are rarely detected in healthy individuals compared with greater than 60% of patients with SLE [19]. Taken together, the murine and human data suggest that in susceptible individuals CMV infection can lead to the sustained production of pathogenic autoantibodies while in nonsusceptible individuals CMV infection leads to the production of anti-CMV antibodies that do not cause autoimmune disease.
Clinical manifestations of CMV in SLE
In patients with SLE, CMV can present with severe clinical manifestations. For example, pancreatitis, colonic perforation, pneumonia and cavitary lung lesions are rare in both CMV infection and active SLE, but have been reported to occur simultaneously [37–41]. Case reports have documented a variety of symptoms associated with CMV infection in SLE including fatigue, arthralgia, pneumonia and colitis [31,36,42]. Three additional case reports in the literature specific to children with SLE noted CMV at the time of SLE diagnosis. Two patients presented with cytopenias, one presented with leukocytosis, but all speculated on the immunodysregulation between CMV and SLE [42–44]. We recently reported a series of seven children who had CMV infection (with evidence of replicating virus) concurrent with the diagnosis of cSLE. The most frequent clinical manifestations included persistent fever despite prednisone therapy, lymphopenia, anemia and nephrotic syndrome, and a greater proportion of the patients were of non-Caucasian ethnicity than in our entire cSLE cohort [33]. A prior series of 23 adult SLE patients with CMV similarly noted a high proportion of patients with CMV infection at SLE diagnosis, with the remainder presenting with infection that was either associated with, or mimicked, a disease flare [45]. Interestingly, higher CMV IgG titers and number of viral copies by PCR are observed in SLE patients with latent CMV infection as compared with both healthy individuals and patients with other autoimmune conditions [46,47]. The significantly higher viral copy number strengthens the link between SLE and CMV, but still cannot establish if CMV contributes to onset of SLE, or if the immunocompromised state promotes the development of CMV infection (reactivation) [48,49].
Other autoimmune diseases
In addition to SLE, CMV has been linked to other autoimmune diseases that occur in childhood including juvenile idiopathic arthritis [50], antiphospholipid antibody syndrome [51] and Kawasaki’s disease [52]. In contrast to SLE, in other autoimmune diseases CMV is most often detected at the time of disease flare instead of at presentation [21,53]. In addition, anti-CMV IgG titers are consistently higher in patients with SLE as compared with patients with other systemic autoimmune diseases including SS, rheumatoid arthritis, dermatomyositis and systemic sclerosis [3,15,47,54]. These data suggest that although CMV may have a role in other autoimmune diseases, its role in SLE appears to be unique. However, development of a comorbid CMV infection in an individual with any autoimmune disease can lead to adverse outcomes. For example, CMV infection has been associated with worsening of the vasculitis in a patient with Henoch Schonlein Purpura, resulting in a fatal intracranial hemorrhage, and has also been associated with triggering the potentially serious complication of macrophage activation syndrome in other autoimmune diseases [3,55].
CMV & the immunocompromised host
Immunosuppressant medications are frequently prescribed for treatment of cSLE disease manifestations, contributing to dysregulation of the immune system. Therefore, the susceptibility for both primary and secondary CMV infection is greatest in cSLE patients on immunosuppressants [49]. In the case of anti-CMV IgM positivity, there is an increased likelihood of progression from CMV infection to CMV disease if immunosuppressive therapies such as corticosteroids or cyclophosphamide are prescribed [22,56].
Corticosteroids can impair lymphocyte proliferation, inhibit T-cell function and block the production of inflammatory cytokines [53]. Therefore, in the face of the lymphopenia of SLE, primary infection or reactivation of latent CMV infection is more likely in patients on corticosteroids. Lastly, corticosteroids can impair other immunologic cells and alter inflammatory responses in a dose-dependent manner [57,58]. The CMV infection risk is further increased when other immunosuppressant agents such as cyclophosphamide, azathioprine or mycophenolate mofetil, are given concomitantly with corticosteroids [39]. The most commonly used biologic response modifiers in cSLE alter B-cell numbers and/or function. The risk of CMV following anti-B-cell therapies is unknown, although one study reported reactivation of CMV infection in 20% of patients during anti-CD20 therapy [25,59].
Treatment of CMV in cSLE
Standard treatment for CMV infection in the cSLE patient varies by the clinical presentation (i.e., CMV infection vs CMV disease); however, some general principles apply to treating both primary CMV infection and reactivation of latent CMV disease. Treatment of CMV infection in a patient with active SLE, as for any immunocompromised patient, requires balancing the degree of immunosuppression to control the SLE disease while minimizing its use to control the replication of CMV and prevent the progression to CMV disease. Specific treatment for CMV can be toxic, and can limit use of immunosuppressive treatments that are prescribed for SLE [21,60]. Consultation with an infectious disease specialist is recommended when considering or initiating treatment of CMV in patients with cSLE. See Table 1 for a summary of treatment guidelines.
Table 1.
Treatment guidelines for cytomegalovirus infection in childhood-onset systemic lupus erythematosus
| Category of infection | Antiviral therapies | Adjunctive management |
|---|---|---|
| CMV infection: primary or secondary | Consider pre-emptive treatment based on viral DNA copy number and clinical situation (no definitive threshold for treatment based on DNA copy number) | Follow viral load Consider modifying immunosuppression if feasible |
| CMV syndrome | Treat: iv. gancyclovir first line; foscarnet and others if intolerant | Monitor immunosuppressants Consider CMV IVIg (Cytogam®) |
| CMV disease | Treat: iv. gancyclovir first line; foscarnet and others if intolerant | Consider modifying immunosuppression if feasible Consider CMV IVIg (Cytogam) |
CMV: Cytomegalovirus; iv.: Intravenous; IVIg: Intravenous immunoglobin.
Gancyclovir is the first-line drug of choice, whereby it interferes with viral DNA replication to inhibit DNA polymerase [15,21]. It was the first drug used to treat CMV retinitis, and has potentially serious side effects including neutropenia, anemia, phlebitis and gastrointestinal disturbances [31,61]. Foscarnet is a first-line choice for those individuals who cannot tolerate gancyclovir due to side effects such as the significant neutropenia. Although it causes less myelosuppression, foscarnet runs the risk of nephrotoxicity, electrolyte abnormalities, and neurotoxicity [26]. Its mechanism of action is similar to gancyclovir as it also inhibits the CMV DNA polymerase.
CMV Intravenous Immune Globulin (Cytogam®) is given as prophylaxis for CMV infection in the case of organ transplant of a CMV-positive organ into a CMV-negative recipient. In the setting of SLE and cSLE it may be given as part of treatment of organ-specific CMV involvement (i.e., kidney and liver), and CMV pneumonitis [34,62].
Leflunomide is a drug used by rheumatologists for rheumatoid arthritis, in addition to juvenile arthritis and even in cSLE patients with resistant arthritis. In vitro it is an effective anti-CMV drug, and has recently been used to treat CMV refractory to gancyclovir [31,32,62,63]. Leflunomide’s mechanism of action targets viral capsid assembly, and due to its different mechanism of action, it offers less potential for the cross-resistance that develops following prolonged antiviral therapy in refractory CMV disease. Side effects include increased liver enzymes, peripheral neuropathy, nausea and diarrhea. Due to its long half-life, it can be discontinued earlier than the complete resolution of symptoms to prevent more permanent (but infrequent) sequelae such as peripheral neuropathy [22,64]. Cidofovir is an additional second-line therapy for CMV that is a nucleotide analog that requires phosphorylation in order to inhibit DNA synthesis. The metabolites of cidofovir have very long half-lives, so one advantage is that the drug can be administered once weekly. The most frequent side effect of cidofovir is is nephrotoxicity, but it can also lead to neutropenia and metabolic acidosis. Other experimental agents including maribavir [65] are currently being evaluated for the treatment of resistant CMV syndrome and CMV disease.
Early treatment with antiviral therapy should be initiated at the onset of symptoms, in order to optimize survival and decrease the risk of adverse outcomes [36,53,60]. In the transplant population, early treatment after CMV detection is associated with faster resolution of antigenemia and decreased morbidity and mortality [11,19,66]. Moreover, selective treatment of infection (pp65 antigenemia or high DNA viral load) may prevent progression to CMV disease [13,19]. As a general recommendation, treatment of CMV in the immunocompromised individual should continue until quantitative viral studies are negative, and for a minimum of 2 weeks, with longer treatments reserved for primary infections and high viral load at baseline [26].
Unfortunately, CMV infection in the SLE patient may result in death, perhaps more frequently than with other viruses [53,67]. Poor prognostic markers for mortality from CMV infection in the context of SLE include cytopenia (especially lymphopenia), prior cyclophosphamide therapy, multisystem involvement and delayed initiation of antiviral therapy [45,53,68].
Surveillance
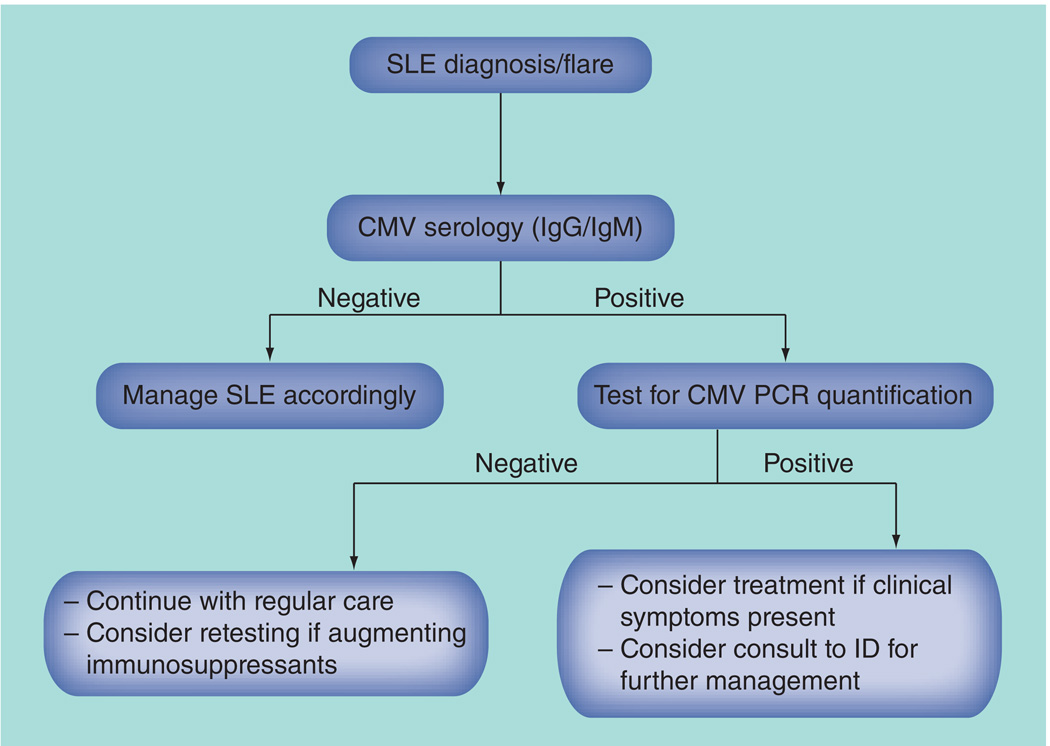
Although effective treatments exist, the goal in a patient with cSLE is early detection of CMV infection, and where possible, prevention of the progression to CMV disease [69]. Routine and frequent screening in the absence of symptoms is neither warranted nor cost effective; however, screening in the setting of a disease flare, or at disease onset requires consideration, particularly prior to initiating immunosuppressive therapies [70]. At the Hospital for Sick Children, we have adopted the following surveillance plan in our large ethnically diverse cSLE clinic (Figure 2): check CMV IgG and CMV IgM serology at the time of cSLE diagnosis; if CMV IgM antibodies are present, run quantitative CMV PCR to determine false positivity, and to differentiate between latent and active infection; when indicated, selectively check CMV PCR when augmenting immunosuppressive therapies; treat the CMV infection if the patient has significant symptoms including colitis, nephritis, pneumonitis and pancreatitis, or requires significant ongoing or escalating immunosuppression; balance immunosuppressants against CMV infection and treatment, depending on SLE disease activity; and recheck quantitative PCR at regular intervals until the infection has cleared.
Figure 2. Surveillance algorithm for cytomegalovirus in childhood-onset systemic lupus erythematosus.
CMV: Cytomegalovirus; ID: Infectious disease; SLE: Systemic lupus erythematosus.
Future perspective
Given the paucity of literature on CMV and cSLE, an expansion of studies beyond case reports is required to provide greater knowledge of the impact of CMV, specifically defining accurate clinical and laboratory markers that differentiate CMV disease versus active cSLE. In addition, large studies evaluating the utility of CMV screening at onset of cSLE with respect to cSLE treatment would help to determine the utility of this testing and resources associated with this endeavour. Lastly, prospective epidemiologic study of healthy children that elucidate the timeline of developing CMV antibodies and susceptible (SLE-associated) autoantibodies would help shed light on the conundrum of ‘which came first – SLE or CMV’? This type of study might only be possible by leveraging large population-based sera banks, but could give us insight into how the virus is ultimately implicated in the pathogenesis of cSLE.
At present, the field of CMV research is focused on vaccine development [26,71]; should vaccination be successful, evaluation of trends in cSLE and autoantibody prevalence would be interesting. Further studies establishing the relationships between CMV PCR viral load, histopathology and clinical outcomes will help better guide treatment and regulation of immunosuppression in patients with cSLE.
Supplementary Material
Executive summary.
Epidemiology, transmission & manifestations of cytomegalovirus in the immunocompetent host
-
▪
Cytomegalovirus (CMV) is a β-herpesvirus that is widespread in the population (60–100%) and is most often horizontally transmitted.
-
▪
Manifestations vary from asymptomatic or flu-like illnesses to the possibility of fatal infection in the immunocompromised host.
-
▪
CMV infection elicits both a T- and B-cell response from the immune system, mainly towards pp65 antigen on CMV, with persistence in circulating T cells to maintain latency of infection.
Detection of CMV
-
▪
Several methods exist for the detection of CMV infection, with molecular testing and PCR superior for quantifying viral burden and response to therapy.
-
▪
Although organ biopsy and immunostaining are the gold standard for diagnosis, this invasive testing should be used with discretion.
CMV in systemic lupus erythematosus: specific autoantigens
-
▪
CMV infection has been linked to anti-RNP, anti-Sm, anti-Ro and anti-La autoantibodies, all of which are also frequent in patients with childhood-onset systemic lupus erythematosus (cSLE).
-
▪
Cross-activation with anti-CMV pp65 antibodies has been shown to induce autoimmunity in susceptible individuals.
CMV in SLE: clinical manifestations
-
▪
It remains unclear whether CMV is a trigger for the onset of SLE, or whether CMV reactivation is triggered by the immunocompromised host.
-
▪
CMV in SLE can present as CMV syndrome or CMV disease with features including pancreatitis, colitis or pneumonitis.
-
▪
Persistent fever despite prednisone use in SLE should increase suspicion of an underlying infectious etiology such as CMV.
CMV in SLE: treatment
-
▪
First-line therapy is gancyclovir, with alternatives including foscarnet, cidofovir and leflunomide available depending on medication tolerance.
-
▪
Concurrent modulation of immunosuppressive therapy must be considered in the setting of CMV infection.
Surveillance & conclusion
-
▪
Mortality has been linked to lymphopenia, pancytopenia, delay in antiviral therapy and prior cyclophosphamide therapy.
-
▪
Screening of CMV serology of newly diagnosed patients with SLE is recommended. If screen is positive, perform molecular quantitative testing for determination of viral DNA copies.
-
▪
Always consider treatment versus modulation of immunosuppressant therapy as clinically indicated. Consultation with infectious disease specialists may be warranted as there are no consensus guidelines on the CMV viral load required to treat disease.
-
▪
Further prospective and observational studies are required to examine the associations between CMV and cSLE.
Acknowledgments
D Levy acknowledges the support of the NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant K23-AR053202).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Kamphuis S, Silverman ED. Prevalence and burden of pediatric-onset systemic lupus erythematosus. Nat. Rev. Rheumatol. 2010;6(9):538–546. doi: 10.1038/nrrheum.2010.121. [DOI] [PubMed] [Google Scholar]
- 2. Ljungman P, Griffiths P, Paya C. Definitions of Cytomegalovirus infection and disease in transplant recipients. Clin. Infect. Dis. 2002;34(8):1094–1097. doi: 10.1086/339329.▪▪ Article outlining classification and definitions of Cytomegalovirus infection and disease applicable to immunocompromised individuals.
- 3.White DW, Suzanne Beard R, Barton ES. Immune modulation during latent herpes virus infection. Immunol. Rev. 2012;245(1):189–208. doi: 10.1111/j.1600-065X.2011.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cannon MJ, Schmid DS. Review of Cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 2010;20:202–213. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- 5.Staras SAS, Flanders WD, Dollard SC, Pass RF, McGowan JE, Cannon MJ. Cytomegalovirus seroprevalence and childhood sources of infection: a population-based study among pre-adolescents in the United States. J. Clin. Virol. 2008;43(3):266–271. doi: 10.1016/j.jcv.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the National Health and Nutrition Examination Surveys, 1988–2004. Clin. Infect. Dis. 2010;50(11):1439–1447. doi: 10.1086/652438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pickering LK. Red Book: 2012 Report of the Committee of Infectious Diseases. 29th Edition. Elk Grove Village, IL, USA: American Academy of Pediatrics; 2012. Cytomegalovirus infection; pp. 300–305. [Google Scholar]
- 8.Hamprecht K, Maschmann J, Vochem M, Dietz K, Speer CP, Jahn G. Epidemiology of transmission of Cytomegalovirus from mother to preterm infant by breastfeeding. Lancet. 2001;357(9255):513–518. doi: 10.1016/S0140-6736(00)04043-5. [DOI] [PubMed] [Google Scholar]
- 9.Pergam SA, Xie H, Sandhu R, et al. Efficiency and risk factors for CMV transmission in seronegative hematopoietic stem cell recipients. Biol. Blood Marrow Transplant. 2012;18(9):1391–1400. doi: 10.1016/j.bbmt.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rafailidis PI, Mourtzoukou EG, Varbobitis IC. Severe Cytomegalovirus infection in apparently immunocompetent patients: a systematic review. Virol J. 2008;5:47. doi: 10.1186/1743-422X-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boeckh M. Late Cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2002;101(2):407–414. doi: 10.1182/blood-2002-03-0993. [DOI] [PubMed] [Google Scholar]
- 12.Bego MG, St Jeor S. Human Cytomegalovirus infection of cells of hematopoietic origin: HCMV-induced immunosuppression, immune evasion, and latency. Exp. Hematol. 2006;34(5):555–570. doi: 10.1016/j.exphem.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto D, Matsushima A, Nagao M, Takakura S, Ichiyama S. Risk factors associated with elevated blood Cytomegalovirus pp65 antigen levels in patients with autoimmune diseases. Mod. Rheumatol. doi. 2012 doi: 10.1007/s10165-012-0651-8. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Gérard L, Leport C, Flandre P, et al. Cytomegalovirus (CMV) viremia and the CD4+ lymphocyte count as predictors of CMV disease in patients infected with human immunodeficiency virus. Clin. Infect. Dis. 1997;24(5):836–840. doi: 10.1093/clinids/24.5.836. [DOI] [PubMed] [Google Scholar]
- 15.Pérez-Mercado AE, Vilá-Pérez S. Cytomegalovirus as a trigger for systemic lupus erythematosus. J. Clin. Rheumatol. 2010;16(7):335–337. doi: 10.1097/RHU.0b013e3181f4cf52. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths PD. Principles and Practice of Clinical Virology. Chichester, UK: John Wiley & Sons Ltd; 2009. Cytomegalovirus; pp. 161–197. [Google Scholar]
- 17.Sylwester AW. Broadly targeted human Cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 2005;202(5):673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddehase MJ. Antigens and immunoevasins: opponents in Cytomegalovirus immune surveillance. Nat. Rev. Immunol. 2002;2(11):831–844. doi: 10.1038/nri932. [DOI] [PubMed] [Google Scholar]
- 19. Hsieh AH, Jhou YJ, Liang CT, Chang M, Wang SL. Fragment of tegument protein pp65 of human Cytomegalovirus induces autoantibodies in BALB/c mice. Arthritis Res. Ther. 2011;13(5):R162. doi: 10.1186/ar3481.▪ Investigating the relationship between Cytomegalovirus protein pp65 and autoimmunity, highlighting differences between patients with systemic lupus erythematosus and healthy controls.
- 20. Razonable RR, Paya CV, Smith TF. Role of the laboratory in diagnosis and management of Cytomegalovirus infection in hematopoietic stem cell and solid-organ transplant recipients. J. Clin. Microbiol. 2002;40(3):746–752. doi: 10.1128/JCM.40.3.746-752.2002.▪ Outlining various diagnostic tests of Cytomegalovirus in the immunocompromised host, highlighting the solid-organ transplant population.
- 21. Eisenstein EM, Wolf DG. Cytomegalovirus infection in pediatric rheumatic diseases: a review. Pediatr. Rheumatol. Online J. 2010;8:17. doi: 10.1186/1546-0096-8-17.▪ Comprehensive review outlining mechanisms of Cytomegalovirus disease, and manifestations in several autoimmune conditions, including childhood-onset systemic lupus erythematosus.
- 22.Palafox Sanchez CA, Satoh M, Chan EKL, et al. Reduced IgG antismall nuclear ribonucleoprotein autoantibody production in systemic lupus erythematosus patients with positive IgM anti Cytomegalovirus antibodies. Arthritis Res. Ther. 2009;11(1):R27. doi: 10.1186/ar2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cannavan FP, Costallat LT, Bertolo MB, Rossi CL, Costa SC. False positive IgM antibody tests for human Cytomegalovirus (HCMV) in patients with SLE. Lupus. 1998;7(1):61–62. doi: 10.1191/096120398678919741. [DOI] [PubMed] [Google Scholar]
- 24.Humar A, Mazzulli T, Moussa G, et al. Clinical utility of Cytomegalovirus (CMV) serology testing in high-risk CMV D+/R-transplant recipients. Am. J. Transplant. 2005;5(5):1065–1070. doi: 10.1111/j.1600-6143.2005.00797.x. [DOI] [PubMed] [Google Scholar]
- 25.Burbelo PD, Issa AT, Ching KH, et al. Highly quantitative serological detection of antiCytomegalovirus (CMV) antibodies. Virol. J. 2009;6(1):45. doi: 10.1186/1743-422X-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kotton CN, Kumar D, Caliendo AM, et al. International consensus guidelines on the management of Cytomegalovirus in solid organ transplantation. Transplantation. 2010;89(7):779–795. doi: 10.1097/TP.0b013e3181cee42f.▪ International Consensus Guidelines on diagnosis and management of Cytomegalovirus in patients post-transplant applicable to any immunosuppressed patient. The updated version for 2013 is in press
- 27.Emery VC, Sabin CA, Cope AV, Gor D, Hassan-Walker AF, Griffiths PD. Application of viral-load kinetics to identify patients who develop Cytomegalovirus disease after transplantation. Lancet. 2000;355(9220):2032–2036. doi: 10.1016/S0140-6736(00)02350-3. [DOI] [PubMed] [Google Scholar]
- 28.Gerna G, Baldanti F, Torsellini M, et al. Evaluation of Cytomegalovirus DNAemia versus pp65-antigenaemia cutoff for guiding preemptive therapy in transplant recipients: a randomized study. Antivir. Ther.(Lond.) 2007;12(1):63–72. [PubMed] [Google Scholar]
- 29.Badley AD, Patel R, Portela DF, et al. Prognostic significance and risk factors of untreated Cytomegalovirus viremia in liver transplant recipients. J. Infect. Dis. 1996;173(2):446–449. doi: 10.1093/infdis/173.2.446. [DOI] [PubMed] [Google Scholar]
- 30.Chemaly RF, Yen-Lieberman B, Castilla EA, et al. Correlation between viral loads of Cytomegalovirus in blood and bronchoalveolar lavage specimens from lung transplant recipients determined by histology and immunohistochemistry. J. Clin. Microbiol. 2004;42(5):2168–2172. doi: 10.1128/JCM.42.5.2168-2172.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Newkirk MM, van Venrooij WJ, Marshall GS. Autoimmune response to U1 small nuclear ribonucleoprotein (U1 snRNP) associated with Cytomegalovirus infection. Arthritis Res. 2001;3(4):253–258. doi: 10.1186/ar310.▪ Analysis of healthy individuals and patients with systemic lupus erythematosus describing the association between the autoimmune responses to Cytomegalovirus infection and autoantibodies.
- 32. Berkun Y, Zandman-Goddard G, Barzilai O, et al. Infectious antibodies in systemic lupus erythematosus patients. Lupus. 2009;18(13):1129–1135. doi: 10.1177/0961203309345729.▪ Case–control study evaluating the prevalence of Cytomegalovirus and other viruses in patients with systemic lupus erythematosus, speculating on the triggering effects of Cytomegalovirus for systemic lupus erythematosus.
- 33.Rozenblyum EV, Levy DM, Harvey E, Hebert D, Silverman ED. Cytomegalovirus in pediatric systemic lupus erythematosus: screening and therapeutic implications. Arthritis Rheum. 2011;63(Suppl. 10):2045. [Google Scholar]
- 34.Baboonian C, Venables PJ, Booth J, Williams DG, Roffe LM, Maini RN. Virus infection induces redistribution and membrane localization of the nuclear antigen La (SS-B): a possible mechanism for autoimmunity. Clin. Exp. Immunol. 1989;78(3):454–459. [PMC free article] [PubMed] [Google Scholar]
- 35.Haaheim LR, Halse AK, Kvakestad R, Stern B, Normann O, Jonsson R. Serum antibodies from patients with primary Sjögren’s syndrome and systemic lupus erythematosus recognize multiple epitopes on the La (SS-B) autoantigen resembling viral protein sequences. Scand. J. Immunol. 1996;43(1):115–121. doi: 10.1046/j.1365-3083.1996.d01-2.x. [DOI] [PubMed] [Google Scholar]
- 36.Getts MT, Miller SD. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: triggering of autoimmune diseases by infections. Clin. Exp. Immunol. 2010;160(1):15–21. doi: 10.1111/j.1365-2249.2010.04132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soderberg-Naucler C. Fragment of tegument protein pp65 of human Cytomegalovirus induces autoantibodies in BALB/c mice. Arthritis Res. Ther. 2012;14(1):101. doi: 10.1186/ar4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perdan-Pirkmajer K, Koren-Kranjc M, Tomsic M. A successfully treated pancreatitis caused by a CMV infection in a lupus patient. Lupus. 2011;20(10):1104–1105. doi: 10.1177/0961203311398514. [DOI] [PubMed] [Google Scholar]
- 39.Strasser C, Wolf EM, Kornprat P, Hermann J, Münch A, Langner C. Opportunistic Cytomegalovirus infection causing colonic perforation in a patient with systemic lupus erythematosus. Lupus. 2012;21(4):449–451. doi: 10.1177/0961203311425529. [DOI] [PubMed] [Google Scholar]
- 40.Azuma N, Hashimoto N, Yasumitsu A, et al. CMV infection presenting as a cavitary lung lesion in a patient with systemic lupus erythematosus receiving immunosuppressive therapy. Intern. Med. 2009;48(24):2145–2149. doi: 10.2169/internalmedicine.48.2495. [DOI] [PubMed] [Google Scholar]
- 41.Cunha BA, Gouzhva O, Nausheen S. Severe Cytomegalovirus (CMV) community-acquired pneumonia (CAP) precipitating a systemic lupus erythematosus (SLE) flare. Lupus. 2009;38(3):249–252. doi: 10.1016/j.hrtlng.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Sankar J, Lodha R, Sankar MJ, Mitra DK, Kumar R, Kabra SK. Unusual lymphocytosis with systemic lupus erythematosus. Indian J. Pediatr. 2008;75(7):748–750. doi: 10.1007/s12098-008-0142-2. [DOI] [PubMed] [Google Scholar]
- 43.Nawata M, Seta N, Yamada M, Sekigawa I, Lida N, Hashimoto H. Possible triggering effect of Cytomegalovirus infection on systemic lupus erythematosus. Scand. J. Rehabil. Med. 2001;30(6):360–362. doi: 10.1080/030097401317148570. [DOI] [PubMed] [Google Scholar]
- 44.Akagi S, Ichikawa H, Suzuki J, Makino H. Systemic lupus erythematosus associated with Cytomegalovirus infection. Scand. J. Rehabil. Med. 2004;33(1):58–59. doi: 10.1080/03009740410005331. [DOI] [PubMed] [Google Scholar]
- 45. Ramos-Casals M, Cuadrado MJ, Alba P, et al. Acute viral infections in patients with systemic lupus erythematosus. Medicine. 20 08;87(6):311–318. doi: 10.1097/MD.0b013e31818ec711.▪ Case series and literature review outlining associations of Cytomegalovirus at diagnosis and flare in systemic lupus erythematosus patients, examining ethnicity, clinical symptoms and fatal outcomes.
- 46. Hrycek A, Kusmierz D, Mazurek U, Wilczok T. Human Cytomegalovirus in patients with systemic lupus erythematosus. Autoimmunity. 2005;38(7):487–491. doi: 10.1080/08916930500285667.▪ Case–control study of Cytomegalovirus prevalence in systemic lupus erythematosus versus general population, highlighting consideration of Cytomegalovirus during systemic lupus erythematosus treatment.
- 47.Barzilai O, Sherer Y, Ram M, Izhaky D, Anaya J-M, Shoenfeld Y. Epstein–Barr virus and Cytomegalovirus in autoimmune diseases: are they truly notorious? A preliminary report. Ann. NY Acad. Sci. 2007;1108:567–577. doi: 10.1196/annals.1422.059. [DOI] [PubMed] [Google Scholar]
- 48.Zandman-Goddard G, Shoenfeld Y. Infections and SLE. Autoimmunity. 2005;38(7):473–485. doi: 10.1080/08916930500285352. [DOI] [PubMed] [Google Scholar]
- 49.Cuchacovich R, Gedalia A. Pathophysiology and clinical spectrum of infections in systemic lupus erythematosus. Rheum. Dis. Clin. North Am. 2009;35(1):75–93. doi: 10.1016/j.rdc.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Franssila R, Hedman K. Infection and musculoskeletal conditions: Viral causes of arthritis. Best Pract. Res. Clin. Rheumatol. 2006;20(6):1139–1157. doi: 10.1016/j.berh.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 51.Labarca JA, Rabaggliati RM, Radrigan FJ, et al. Antiphospholipid syndrome associated with Cytomegalovirus infection: case report and review. Clin. Infect. Dis. 1997;24(2):197–200. doi: 10.1093/clinids/24.2.197. [DOI] [PubMed] [Google Scholar]
- 52.Usta Guc B, Cengiz N, Yildirim SV, Uslu Y. Cytomegalovirus infection in a patient with atypical Kawasaki disease. Rheumatol. Int. 2008;28(4):387–389. doi: 10.1007/s00296-007-0440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Takizawa Y, Inokuma S, Tanaka Y, et al. Clinical characteristics of Cytomegalovirus infection in rheumatic diseases: multicentre survey in a large patient population. Rheumatology. 2008;47(9):1373–1378. doi: 10.1093/rheumatology/ken231.▪ Observational study examining Cytomegalovirus infection in rheumatic diseases commenting on risk factors for fatalities in this cohort.
- 54.Chang M, Pan MR, Chen DY, Lan JL. Human Cytomegalovirus pp65 lower matrix protein: a humoral immunogen for systemic lupus erythematosus patients and autoantibody accelerator for NZB/W F1 mice. Clin. Exp. Immunol. 2006;143(1):167–179. doi: 10.1111/j.1365-2249.2005.02974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murakami H, Takahashi S, Kawakubo Y, Kinukawa N, Funaki S, Harada K. Adolescent with Henoch–Schönlein purpura glomerulonephritis and intracranial hemorrhage possibly secondary to the reactivation of latent CMV. Pediatr. Int. 2008;50(1):112–115. doi: 10.1111/j.1442-200X.2007.02531.x. [DOI] [PubMed] [Google Scholar]
- 56.Yoda Y, Hanaoka R, Ide H, et al. Clinical evaluation of patients with inflammatory connective tissue diseases complicated by Cytomegalovirus antigenemia. Mod. Rheumatol. 2006;16(3):137–142. doi: 10.1007/s10165-006-0470-x. [DOI] [PubMed] [Google Scholar]
- 57.Sfriso P, Ghirardello A, Botsios C, et al. Infections and autoimmunity: the multifaceted relationship. J. Leukoc. Biol. 2010;87(3):385–395. doi: 10.1189/jlb.0709517. [DOI] [PubMed] [Google Scholar]
- 58.Doria A, Zampieri S, Sarzi-Puttini P. Exploring the complex relationships between infections and autoimmunity. Autoimmun. Rev. 2008;8(2):89–91. doi: 10.1016/j.autrev.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 59.Kelesidis T, Daikos G, Boumpas D, Tsiodras S. Does rituximab increase the incidence of infectious complications? A narrative review. Int. J. Infect. Dis. 2011;15(1):e2–e16. doi: 10.1016/j.ijid.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oliveira MJ, Mouta J, Rodriguez A, Henriques C, Grilo A, Riso N. Cytomegalovirus infection in systemic lupus erythematosus. Lupus. 2011;20(4):388–389. [Google Scholar]
- 61.McGregor A, Choi KY. Cytomegalovirus antivirals and development of improved animal models. Exp. Opin. Drug Metab. Toxicol. 2011;7(10):1245–1265. doi: 10.1517/17425255.2011.613824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Avery RK, Mossad SB, Poggio E, et al. Utility of leflunomide in the treatment of complex Cytomegalovirus syndromes. Transplantation. 2010;90(4):419–426. doi: 10.1097/TP.0b013e3181e94106. [DOI] [PubMed] [Google Scholar]
- 63.Shahira E, Thomas B, Talwar M, Salazar M, et al. Leflunomide: A novel therapeutic agent for ganciclovir-resistant Cytomegalovirus in kidney transplant recipients. Am. J. Kidney Dis. 2011;57(4):A87. [Google Scholar]
- 64.Avery RK. Management of late, recurrent, and resistant Cytomegalovirus in transplant patients. Transplant. Revs. 2007;21(2):65–76. [Google Scholar]
- 65.Avery RK, Marty FM, Strasfeld L, et al. Oral maribavir for treatment of refractory or resistant Cytomegalovirus infections in transplant recipients. Transpl. Infect. Dis. 2010;12(6):489–496. doi: 10.1111/j.1399-3062.2010.00550.x. [DOI] [PubMed] [Google Scholar]
- 66.Boeckh M, Boivin G. Quantitation of Cytomegalovirus: methodologic aspects and clinical applications. Clin. Microbiol. Rev. 1998;11(3):533–554. doi: 10.1128/cmr.11.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoon KH, Fong KY, Tambyah PA. Fatal Cytomegalovirus infection in two patients with systemic lupus erythematosus undergoing intensive immunosuppressive therapy: Role for Cytomegalovirus vigilance and prophylaxis? J. Clin. Rheumatol. 2002;8(4):217–222. doi: 10.1097/00124743-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 68.Goncalves A, Guedes C, Martins C, et al. Risk factors for opportunistic infections in a cohort of systemic lupus erythematosus. Lupus. 2011;20:389–390. [Google Scholar]
- 69.Barber C, Gold WL, Fortin PR. Infections in the lupus patient: perspectives on prevention. Curr. Opin. Rheumatol. 2011;23(4):358–365. doi: 10.1097/BOR.0b013e3283476cd8. [DOI] [PubMed] [Google Scholar]
- 70.Bendiksen S, Van Ghelue M, Rekvig OP, Gutteberg T, Haga H-J, Moens U. A longitudinal study of human Cytomegalovirus serology and viruria fails to detect active viral infection in 20 systemic lupus erythematosus patients. Lupus. 2000;9(2):120–126. doi: 10.1191/096120300678828118. [DOI] [PubMed] [Google Scholar]
- 71.Gandhi MK, Khanna R. Human Cytomegalovirus: clinical aspects, immune regulation and emerging treatments. Lancet Infect. Dis. 2004;4:725–738. doi: 10.1016/S1473-3099(04)01202-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.