Abstract
High angular resolution diffusion imaging (HARDI) demonstrates transient radial coherence of telencephalic white matter in the human fetus. Our objective was to define the neuroanatomic basis of this radial coherence through correlative HARDI- and postmortem tissue analyses. Applying immunomarkers to radial glial fibers (RGFs), axons, and blood vessels in 18 cases (19 gestational weeks to 3 postnatal years), we compared their developmental profiles to HARDI tractography in brains of comparable ages (n = 11). At midgestation, radial coherence corresponded with the presence of RGFs. At 30–31 weeks, the transition from HARDI-defined radial coherence to corticocortical coherence began simultaneously with the transformation of RGFs to astrocytes. By term, both radial coherence and RGFs had disappeared. White matter axons were radial, tangential, and oblique over the second half of gestation, whereas penetrating blood vessels were consistently radial. Thus, radial coherence in the fetal white matter likely reflects a composite of RGFs, penetrating blood vessels, and radial axons of which its transient expression most closely matches that of RGFs. This study provides baseline information for interpreting radial coherence in tractography studies of the preterm brain in the assessment of the encephalopathy of prematurity.
Keywords: axons, blood vessels, encephalopathy of prematurity, periventricular leukomalacia, radial glial fibers, vimentin
Introduction
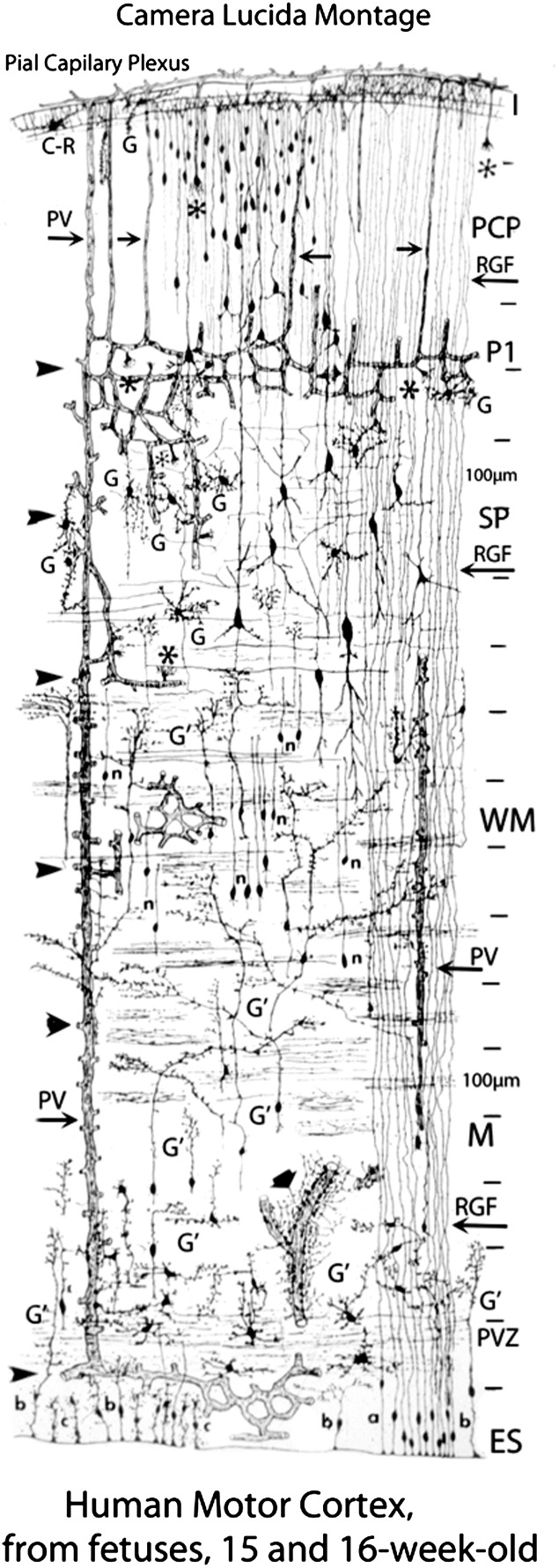
The hallmark of the developing human fetal telencephalon is its radial organization perpendicular to the pial surface. This organization is defined by the linear direction (like “rays/radia” from the sun) of the radial glial fibers (RGFs) as they shape the radial or vertical columns of the cerebral cortex critical for cognitive processing (Rakic 1988, 2006; Rakic et al. 2009; Marin-Padilla 2011). RGFs form the scaffolding for the early migration of neuroblasts from the germinal zones lining the ventricle of the dorsal telencephalon (pallium) to their final destination as pyramidal/glutamatergic neurons in the vertical columns of the overlying cortex (Rakic 1988, 2006; Rakic et al. 2009; Marin-Padilla 2011; Rubenstein 2011). They also provide guidelines for the late migration of interneurons from the ganglionic eminence, the origin of two-thirds of GABAergic neurons in the human cortex (Letinic and Rakic 2001). In addition, postmitotic, tightly packed migrating neuroblasts form linear chains along their length very early in gestation (Marin-Padilla 2011). In parallel to the RGFs is the well-recognized radial organization of developing (arterial and venous) vascularity of the telencephalon (Virgintino et al. 1998; Bertossi et al. 1999; Marin-Padilla 2011), as well as the segments of the axons directly entering and exiting the cortical plate, and the apical dendrites of the pyramidal neurons within the cortex itself (Hevner 2000; Kostovic and Judas 2002; Marin-Padilla 2011). This stunning radial organization of RGFs, neuroblastic chains, blood vessels, axons, and pyramidal apical dendrites in parallel is captured in the Golgi drawing of the human motor cortex and underlying white matter at 15 and 16 weeks by Marin-Padilla (Fig. 1) (Marin-Padilla 2011). His exquisite camera lucida drawing is reprinted here with his permission for reference because it guides the present study (Fig. 1).
Figure 1.
The overall radial organization of the human fetal telencephalon is illustrated for reference in the composite Golgi camera lucida drawing from fetuses at 15 and 16 gestational weeks in the motor cortex by Marin-Padilla (2011) (with permission). The complete telencephalic wall is illustrated from the pial surface to the ventricle. Abbreviations: ES, ependymal surface; G, glia; P1, first pyramidal cell stratum; PCP, pyramidal cell plate; PV, penetrating vessel; PVZ, paraventricular zone; n, ascending neuronal processes; SP, subplate; RGF, radial glial fiber; WM, white matter.
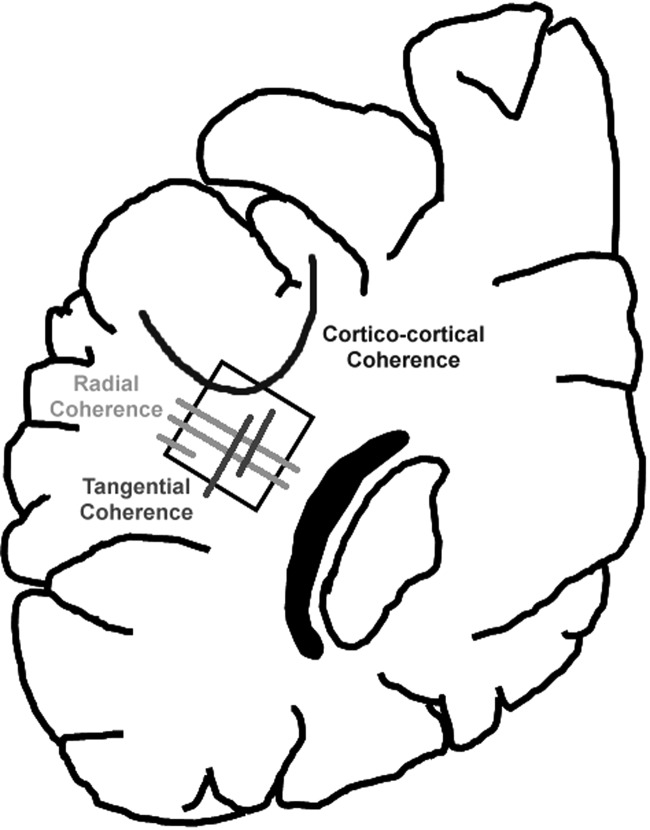
Advanced neuroimaging of the telencephalic wall of the human fetal brain with high angular resolution diffusion imaging (HARDI) (Tuch et al. 2003) likewise reveals a distinct pattern of radial coherence (Takahashi et al. 2012), yet its precise neuroanatomic substrate is uncertain. Radial coherence detected by HARDI is a measure of the directionality of tissue water diffusion that in turn reflects an overall linear direction of one or more anatomic structures. We use the term “radial coherence” and “tangential coherence” as the diffusion coherence (tractography pathways) radial and tangential to the brain surface, respectively, and the term “corticocortical coherence” as the diffusion coherence continuous between cortical regions. Without knowledge of the neuroanatomic substrate of the radial coherence defined by HARDI in the normal human preterm brain, the clinical interpretation of the diagnostic and prognostic significance of its potential disruption in the abnormal preterm brain, such as in the encephalopathy of prematurity (EOP) or periventricular leukomalacia (PVL) is impossible (Volpe 2001; Kinney and Volpe 2009; Kinney 2009; Volpe et al. 2011). Accordingly, the objective of the current study was to establish the anatomic correlates of the HARDI-detected radial coherence in the human fetal white matter with tractography-guided tissue analysis. In essence, we addressed the question: Does HARDI radial coherence reflect RGFs, radial chains of migrating neuroblasts, radial vasculature, and/or radial axonal fascicles? We tested the hypothesis that the neuroanatomic substrate of the radial coherence delineated by HARDI in the human fetal white matter correlates primarily with RGFs in tissue sections. We focused on the white matter as it is a preferential site of preterm brain injury, and baseline information concerning radial coherence by tractography analysis is of immediate relevance to the clinical care of premature infants.
Material and Methods
Design
We correlated the spatial orientation and distribution and temporal sequence of the RGFs in tissue sections with that of the HARDI-imaged radial coherence in a standardized region of the telencephalon, that is, the occipital lobe at the level of the atrium of the lateral ventricle. We chose this region because it is a site of predilection for PVL, the dominate white matter lesion in preterm infants (Banker and Larroche 1962; Kinney and Volpe 2009), and its overlying cortex is critical to cognitive visual processing that is commonly affected in long-term survivors with PVL (Fazzi et al. 2009). We analyzed the spatial and temporal profile of RGFs, axons, and blood vessels with antibodies to vimentin (key cytoskeletal marker of RGFs), SMI 312 (pan-axonal neurofilament marker), and CD31 (marker of vascular wall), respectively with immunocytochemical methods in alternate sections. Owing to technical issues, we were unable to perform immunocytochemistry for the antibodies in the same brains that we analyzed with HARDI. Nevertheless, we compared the immunocytochemical findings in the same standardized occipital region as that in the imaged brains, thereby providing developmental profiles for comparison between the 2 modalities.
HARDI Analysis
Dataset
Table 1 shows age brackets and number of specimens that were used for HARDI tractography. The ages of the preterm and full-term cases were expressed as postconceptional age (PCA) that equals gestational age and postnatal age. The infant and adult brains were analyzed as mature indices for comparison, given that RGFs are known not be present at these ages. The 2 preterm and 2 adult brains at autopsy were obtained from the Department of Pathology, Brigham and Women's Hospital, Boston, MA. All brains were imaged in toto for analysis of the occipital white matter at the level of the atrium (Fig. 2). The infant specimen was obtained from the Brain and Tissue Bank for Developmental Disorders supported by the Eunice Kennedy Shriver National Institute of Child Health and Development (http://medschool.umaryland.edu/BTBank/). This infant specimen consisted of a 2-cm-thick coronal slice of the occipital lobe at the level of the atrium of the lateral ventricle. Whole infant brains were not available from this resource or from our autopsy services. One of the normal adult brains was donated to Harvard Medical School, Boston, MA, and further clinical information, including the exact age and cause of death, was not available. The known primary causes of death were complications of prematurity (n = 2 at 20 and 21 gestational weeks); pneumonia at 3 postnatal months (n = 1); and leukemia in the adult (n = 1). All living patients had MRI studies that were clinically interpreted as showing no brain abnormalities. Indications for imaging included concern for hypoxic ischemic injury, apnea, and transient choreiform movements following an upper respiratory tract infection. None of the cases raised the clinical concerns of congenital malformations or genetic disorders. Standard autopsy examination of all brains undergoing postmortem HARDI revealed no gross abnormalities and no or only minimal microscopic abnormalities. All brains utilized for HARDI and tissue analysis were analyzed under institutional review board approved protocols.
Table 1.
Summary of HARDI and neuroanatomic observations in the white matter of the occipital lobe in 11 cases with neuroimaging and 18 cases with immunocytochemistry (total n = 29)
| Case # | Ex vivo/ in vivo | Age | HARDI radial coherence | RGFs | Vimentin-positive cell bodies | Dominant unidirectional axons | Multidirectionla axonsa | Penetrating radial blood vessels | Nuclear chains in interstitial white matter | |
|---|---|---|---|---|---|---|---|---|---|---|
| Midgestation | 1 | Ex vivo | 19 weeks | NA | + | − | − | + | + | − |
| 2 | Ex vivo | 20 weeks | NA | + | − | − | + | + | − | |
| 3 | Ex vivo | 20 weeks | + | NA | NA | NA | NA | NA | NA | |
| 4 | Ex vivo | 21 weeks | + | NA | NA | NA | NA | NA | NA | |
| Early preterm | 5 | Ex vivo | 24 weeks | NA | + | − | − | + | + | − |
| 6 | Ex vivo | 26 weeks | NA | + | − | − | + | + | − | |
| 7 | Ex vivo | 27 weeks | NA | + | − | − | + | + | − | |
| Mid Preterm | 8 | Ex vivo | 30 weeks | NA | + | + | − | + | + | − |
| 9 | In vivo | 30 weeks | +/− | NA | NA | NA | NA | NA | NA | |
| 10 | In vivo | 30 weeks | +/− | NA | NA | NA | NA | NA | NA | |
| Late preterm | 11 | Ex vivo | 34 weeks | NA | + | + | − | + | + | − |
| 12 | In vivo | 34 weeks | +/− | NA | NA | NA | NA | NA | NA | |
| 13 | Ex vivo | 36 weeks | NA | + | + | − | + | + | − | |
| 14 | Ex vivo | 36 weeks | NA | + | + | − | + | + | − | |
| Term | 15 | Ex vivo | 37 weeks | NA | + | + | − | + | + | − |
| 16 | Ex vivo | 38 weeks | NA | + | + | − | + | + | − | |
| 17 | Ex vivo | 40 weeks | NA | − | + | − | + | + | − | |
| 18 | Ex vivo | 40 weeks | NA | − | + | − | + | + | − | |
| 19 | In vivo | 40 weeks | − | NA | NA | NA | NA | NA | NA | |
| 20 | In vivo | 40 weeks | − | NA | NA | NA | NA | NA | NA | |
| 21 | Ex vivo | 41 weeks | NA | − | + | − | + | + | − | |
| Infancy | 22 | Ex vivo | 3 months | − | NA | NA | NA | NA | NA | NA |
| 23 | Ex vivo | 9 months | NA | − | − | + | − | + | − | |
| Toddler | 24 | In vivo | 2 years | − | NA | NA | NA | NA | NA | NA |
| 25 | Ex vivo | 2 years | NA | − | − | + | − | + | − | |
| 26 | Ex vivo | 3 years | NA | − | − | + | − | + | − | |
| 27 | Ex vivo | 3.5 years | NA | − | − | + | − | + | − | |
| Mature | 28 | Ex vivo | Adult | − | NA | NA | NA | NA | NA | NA |
| 29 | Ex vivo | Adult | − | NA | NA | NA | NA | NA | NA |
Note: aMultidirectional axons are defined as axons visualized with SMI 312 immunocytochemistry that are oriented in multiple directions, including radially, horizontally, and obliquely, with no predominate pattern. Abbreviations: weeks, postconceptional weeks; months, postnatal months; years, postnatal years; NA, not available.
Figure 2.
This diagram indicates the site in the central (interstitial) white matter in the occipital lobe that was analyzed by HARDI and tissue-based methods. This level is at the atrium of the lateral ventricle. The square in the interstitial white matter indicates the standardized level of the immunocytochemical analysis. The distal optic radiation is indicated in black for reference. Examples of radial coherence, tangential coherence, and corticocortical coherence are also shown.
Tissue preparation for HARDI
At the time of autopsy, the fetal and adult brains were fixed immediately in a 4% paraformaldehyde solution containing 1 mM gadolinium (Gd-DTPA) MRI contrast agent for at least 1 week to reduce the T1 relaxation time while ensuring sufficient T2-weighted signals (Buxton 2003). We soaked the cerebral hemispheric specimen of the 3-month-old case in 4% paraformaldehyde solution containing 1 mM Gd-DTPA. During image acquisition, the brains were placed in Fomblin solution (Ausimont, Thorofare, NJ) to minimize so-called susceptibility artifacts that may be caused by an applied magnetic field, typically near the interfaces of substances of different magnetic susceptibility (e.g., between the air, water, and brain tissue) (Shapiro et al. 2004).
HARDI procedures
The postmortem brain specimens were imaged on a 4.7 T Bruker Biospec MR system (specimens from 20 weeks to 3 postnatal months) and on a 3T Siemens MR system (adult specimens), A. A. Martinos Center, Massachusetts General Hospital, Boston, MA. Different scanner systems were used to accommodate the different brain sizes, and magnetic resonance (MR) coils that best fit each brain sample were used to ensure optimal imaging. For the specimens from 20 weeks to 3 postnatal months, the pulse sequence used for acquisition was a 3D diffusion-weighted spin-echo echo-planar imaging (SE-EPI) sequence, repetition time/echo time (TR/TE) 1000/40 (ms), with an imaging matrix of 80 × 112 × 96 pixels for the specimens at 20 and 21 gestational weeks and 70 × 100 × 80 pixels for the specimen at 3 postnatal months. Sixty diffusion-weighted measurements (with the strength of the diffusion weighting, b = 8000 s/mm2) and 1 nondiffusion-weighted measurement (no diffusion weighting or b = 0 s/mm2) were acquired with the duration of the diffusion gradients, δ = 12.0 ms, the time interval between the start of the 2 diffusion gradients, Δ = 24.2 ms. The total acquisition time was approximately 2 h for each imaging session.
For the adult specimen, diffusion data were acquired using a 3D diffusion-weighted steady-state free precession sequence (McNab et al. 2009; McNab and Miller 2008) with a 3DFT readout using following parameters: TR/TE 27.8/22.9 ms, δ = 18.0 ms, diffusion gradient amplitude = 3.2 G/cm, and matrix size 96 × 88 × 64 pixels. Forty-four diffusion-weighted measurements and four nondiffusion-weighted volumes (b = 0 s/mm2) were acquired. The total acquisition time was approximately 5 h and 30 min for each imaging session. The spatial resolution was 700 × 700 × 700 µm for the specimens at 20 and 21 gestational weeks; 1,000 × 660 × 706 μm for the specimen at 3 postnatal months; and 1 × 1 × 1 mm for the adult specimen. Higher spatial resolution was needed to reveal the smaller structures of the younger (smaller) brains in the preterm period compared to the adult brain. We determined the highest spatial resolution for each brain specimen with an acceptable signal-to-noise ratio of more than 130, and within a reasonable scan time.
The brains of living patients were imaged on a 3T Siemens MR system, Children's Hospital Boston, Boston, MA. The HARDI pulse sequence used for imaging live subjects was a diffusion-weighted SE-EPI sequence, TR/TE 8320/88 ms, with an imaging matrix of 128 × 128 × 64 pixels. The spatial resolution was 2 × 2 × 2 mm. Thirty diffusion-weighted measurements (b = 1000 seconds/mm2) and 5 nondiffusion-weighted measurements (b = 0 s/mm2) were acquired with δ = 40 ms, Δ = 68 ms. The total acquisition time was approximately 5 min.
Reconstructions
We reconstructed and analyzed the radial coherence in the occipital lobe in each case with the Diffusion Toolkit and TrackVis (http://trackvis.org). Orientation distribution function was calculated using an established method (Hess et al. 2006). We used a streamline algorithm with HARDI reconstruction for diffusion tractography (Mori et al. 1999), as in previous publications (Takahashi et al. 2010, 2011, 2012; Schmahmann et al. 2007; D'Arceuil et al. 2008). Trajectories were propagated by consistently pursuing the orientation vector of least curvature. We terminated tracking when the angle between 2 consecutive orientation vectors was greater than the given threshold (40°), or when the fibers extended outside of the brain surface. For the latter determination, we used mask (boundary) images of the brains created by MRIcro (www.sph.sc.edu/comd/rorden/mricro.html) to determine coherence in the brain itself and not in the surrounding immersion fluid. Of note, the mask image is a 3D binary image created from a mean diffusion image of each brain assessment with endpoints constrained to the brain tissue itself. This process is routinely used to remove skull images or susceptibility-related artifacts around the brain surface. We used brain mask volumes to terminate tractography structures instead of the fractional anisotropy (FA) threshold because progressive myelination and crossing fibers in the developing brain can result in low FA values that may potentially incorrectly terminate tractography tracing in the gray matter (Schmahmann et al. 2007; D'Arceuil et al. 2008; Wedeen et al. 2008; Vishwas et al. 2010; Takahashi et al. 2010, 2011, 2012).
For visualization purposes in Figures 4–7, we restricted the percentages of the number and the directionality of detected tractography connections by the demonstration of 75% of the connections that touched the coronal brain slice with a thickness of 10 voxels. Tractography pathways were then restricted to start and end within the plane (slice filter). The tract visualization was standardized using the same slice filter thickness (10 voxels). In the images in Figures 4 and 5, coherence patterns perpendicular to the brain slice, that is, coursing in the anterior–posterior plane, are thus excluded in the images as they do not “start and stop” in the coronal brain slice analyzed, but rather, pass through the slice. These anterior–posterior pathways are therefore not color-coded by illustrated in black in Figures 4 and 5, with an overlying asterisk for clarity. The color-coding of tractography connections was based on a standard red-green-blue (RGB) code that was applied to the vector between the end-points of each structure (green for right–left, red for dorsal–ventral, and blue for anterior–posterior, the latter in pathways that are confined to the coronal slice analyzed).
Figure 4.
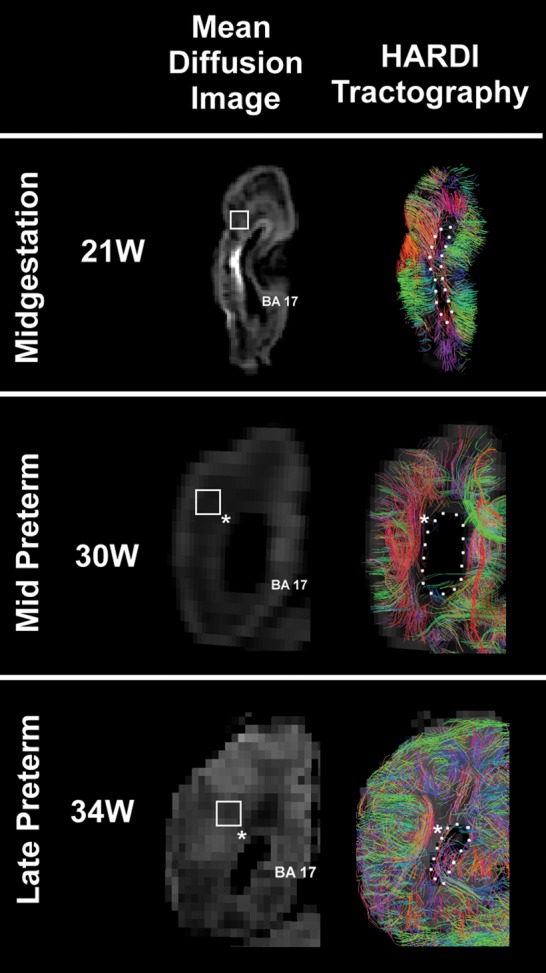
HARDI images (right) are presented with the comparable mean diffusion image (left) at midgestation and the mid and late preterm periods at the level of the atrium of the lateral ventricle in the occipital lobe. Only radial coherence that begins and ends in the occipital tissue slice is displayed. The asterisks indicate the location where tractography pathways run anterior to/from posterior through the tissue plane (also shown in black). The boundary of the lateral ventricle is highlighted with a dotted white line. The square represents the tissue sample in the central white matter illustrated in Figures 6 and 7, but rotated 90°. At 30 weeks, the black in region lateral to the atrium of the ventricle represents the site of the optic radiation (asterisk). The color-coding of tractography connections is based on a standard red-green-blue (RGB) code applied to the vector between the end-points of each structure (green for right–left, red for dorsal–ventral, and blue for anterior–posterior). Abbreviations: BA, Brodmann area; W, weeks. The 21-week images are from a postmortem specimen, and the 30-week and 34-week images are from living subjects.
Figure 5.
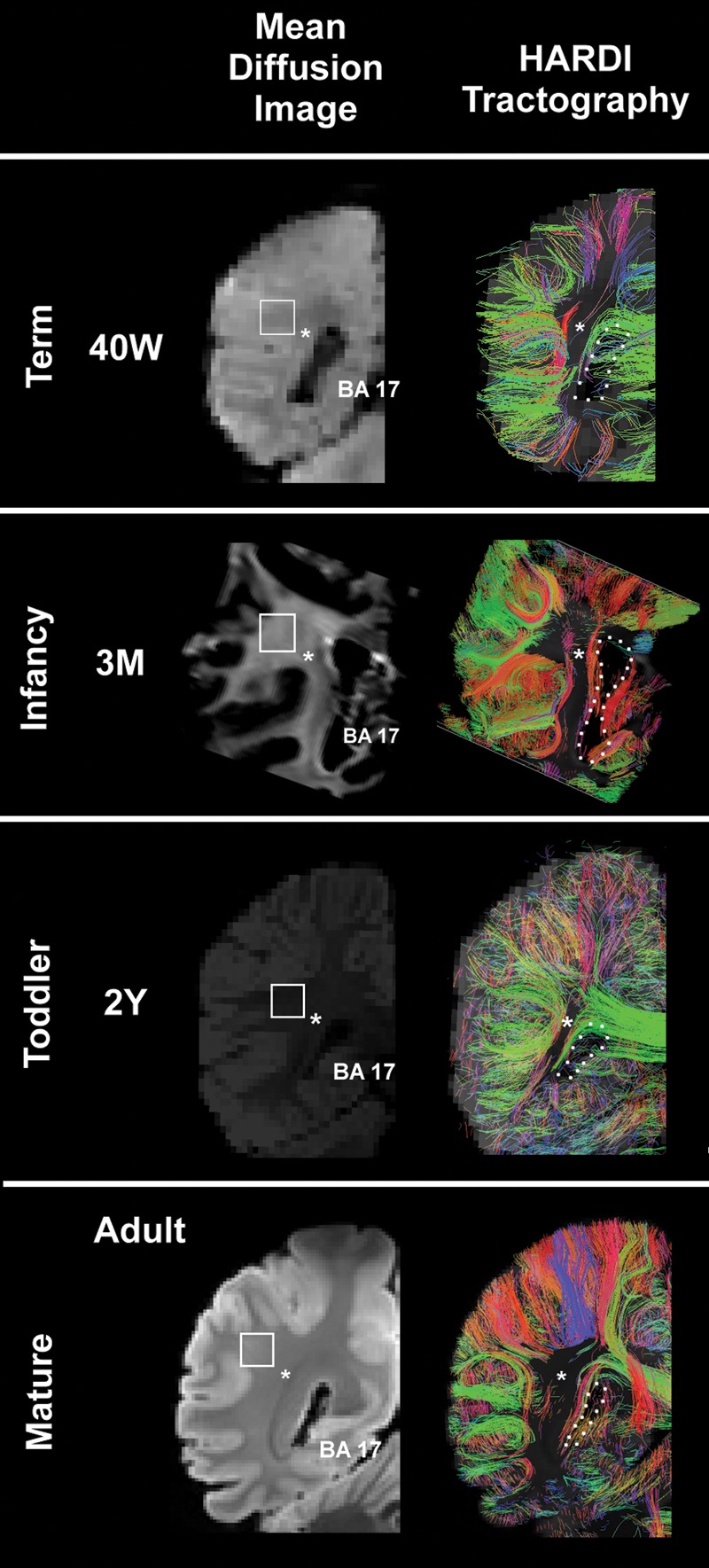
HARDI images (right) are presented with the comparable mean diffusion image (left) at term, infancy, toddler, and adult time points at the level of the atrium of the lateral ventricle of the occipital lobe. Only radial coherence that begins and ends in the occipital tissue slice is displayed. The asterisks indicate the location where tractography pathways run anterior to posterior through the tissue plane. The boundary of the lateral ventricle is shown with a dotted white line. The square represents the tissue sample in the central white matter illustrated in Figures 6 and 7, but rotated 90°. The color-coding of tractography connections is based on a standard red-green-blue (RGB) code applied to the vector between the end-points of each structure (green for right–left, red for dorsal–ventral, and blue for anterior–posterior). Abbreviations: BA, Brodmann area; M, postnatal months; W, weeks. The 3 postnatal month and adult images are from postmortem specimens, and the 40 gestational week and 2-year images are from living subjects.
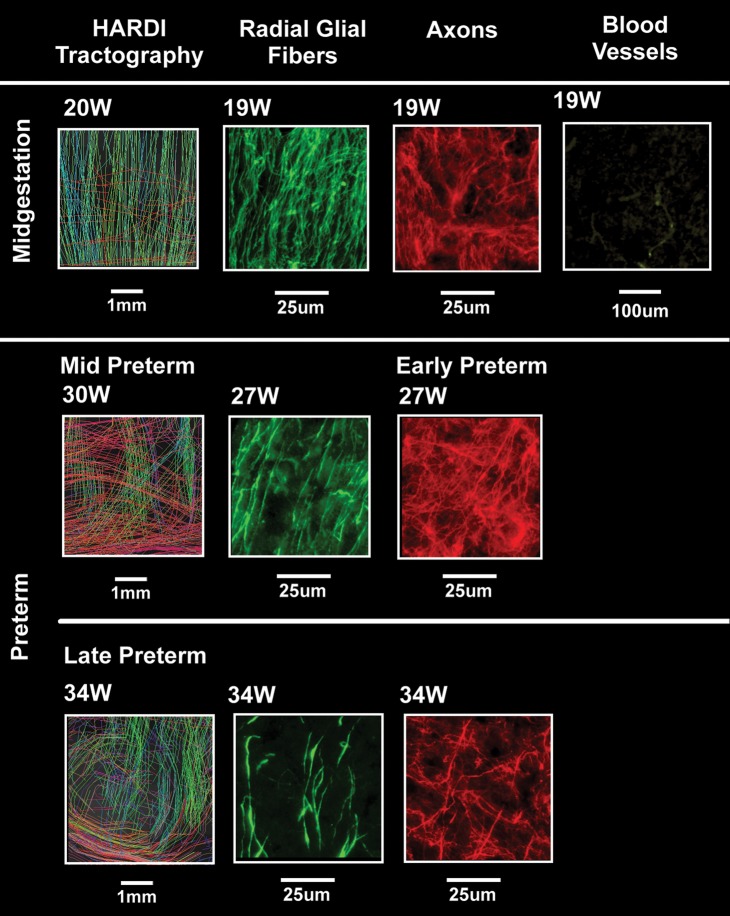
Figure 6.
Composite image of HARDI data (left) and tissue data (middle and right) at midgestation and in the preterm period at the central site of the occipital white matter indicated by squares in Figure 3 but rotated 90°. The tissue analysis was performed with immunocytochemical methods with antibodies to RGFs (vimentin), axons (SMI 312), and blood vessels (CD31. All images are oriented with the pial surface toward the top of the page. HARDI images were magnified images of white rectangles in Figure 4, using standard options of TrackVis software. The field of view of the HARDI images was approximately 5 mm.
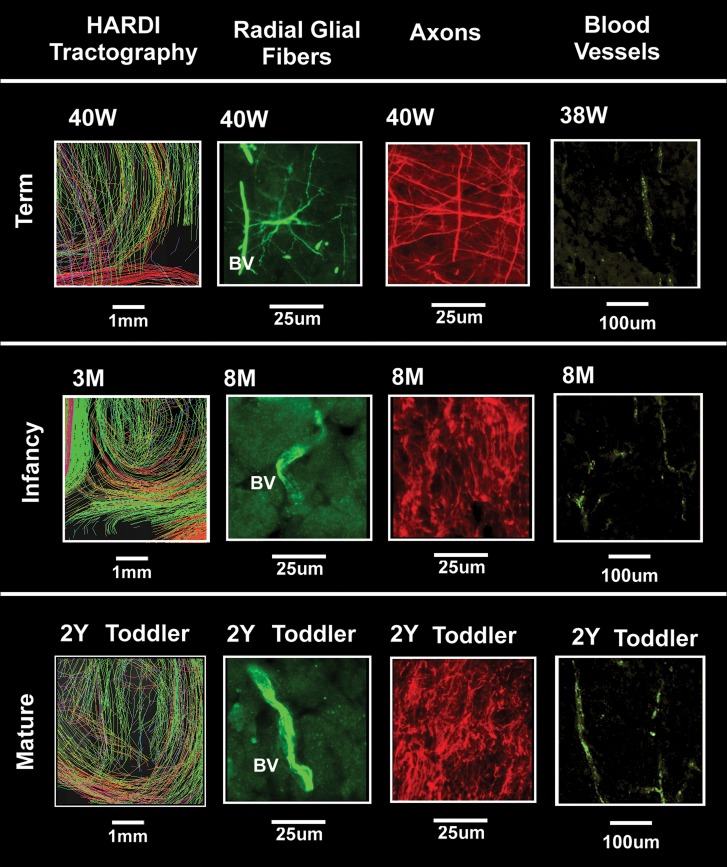
Figure 7.
Composite image of HARDI data (left) and tissue data (middle and right) in the term, infant, preschool, and adult time-period at the central site of the occipital white matter indicated by squares in Figure 5 but rotated 90°. The tissue analysis was performed with immunocytochemical methods with antibodies to RGFs (vimentin), axons (SMI 312), and blood vessels (CD31). All images are oriented with the pial surface toward the top of the page. The scales vary among the tissue preparations. HARDI images were magnified images of white rectangles in Figure 5 using a standard option of TrackVis software. The field of view of the HARDI images was approximately 5 mm.
Neuroanatomic Analysis
For the immunocytochemical studies, we used tissue samples from brains that were scanned for HARDI because the antibodies required processing in frozen tissues, and the HARDI-scanned brains were fixed in paraformaldehyde and then scanned in Fomblin oil.
Histological survey
We defined the overall histological features of the developing white matter at the standardized occipital site with the cresyl violet stain in 7 cases ranging from 20 gestational weeks to 8 postnatal months (Fig. 3). The PCAs of the cases examined with cresyl violet staining were 20 weeks (n = 3), 31 weeks (n = 1), 34 weeks (n = 1), 43 weeks (n = 1), and 72 weeks (n = 1). Tissues sections at 5 μm thickness were obtained from the formalin-fixed, paraffin-embedded blocks and stained with cresyl violet according to standard methods. Cases with EOP, including PVL, and known disorders of neuronal migration were excluded. There were no or minimal neuropathologic changes, for example, mild white matter gliosis, in the cases. Anatomic observations concerning radial organization in the telencephalic wall were made with the standard light microscope.
Figure 3.
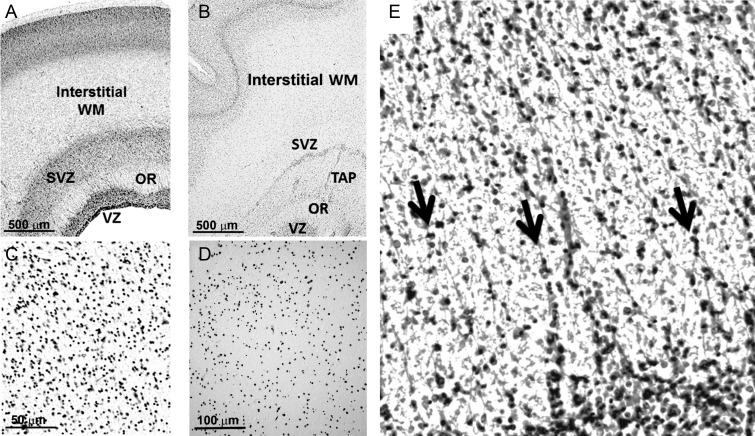
Neuroblastic nuclei do not form linear chains suggestive of migration along RGFs in the interstitial white matter at 20 gestational weeks (A and C) and 31 gestational weeks (B and D) in microscopic sections stained with cresyl violet. Low-power views of the occipital pole are shown at ×4 in A and B. High-power views of the occipital white matter are shown in C and D at ×40 magnification. (E) Radial nuclear chains (arrows) are prominent in the septae of the human optic radiation at midgestation. Cresyl violet, ×40. Abbreviations: OR, optic radiation; SVZ, subventricular zone; TAP, tapetum; V, ventricular zone; WM, interstitial white matter.
Immunocytochemical dataset
Eighteen human brain specimens were obtained for immunocytochemical analysis from the autopsy service of the Department of Pathology, Brigham and Women's Hospital, Boston, MA, as well as from the Maryland Developmental Brain and Tissue Bank. We analyzed occipital tissues from 9 preterm infants (19–36 weeks), 5 full-term newborns (37–41 weeks), 1 infant, and 3 toddlers (Table 1). The primary causes of death were prematurity (n = 8), congenital heart disease (n = 4), stillbirth (n = 3), renal dysplasia and Potter's sequence (n = 1), prenatal cocaine exposure (n = 1), and drowning (n = 1). Standard examination of the whole brain, including the occipital cortex, was performed in all samples. There were no or minimal abnormalities upon standard neuropathologic examination in all brains undergoing tissue analysis.
Single-label immunocytochemistry
For the single-label studies involving vimentin, SMI 312, and CD31, standardized tissue blocks from the occipital lobe (Fig. 2) were frozen immediately at autopsy on dry ice and stored at −80°C until tissue processing. The frozen occipital tissue samples were embedded in Tissue-Tek O.C.T compound (Sakura Finetek USA Inc.) and cut at 50 μm thickness with a Leica cryostat (Leica, Germany), and the tissue sections were postfixed in 4% paraformaldehyde. We identified radial RGFs with an antibody to vimentin (Brown et al. 2001) that is a cytoskeletal intermediate filament protein, a marker of neuroepithelial and mesenchymal origin, thereby labeling RGFs and vascular smooth muscle cells, respectively (Table 2). Vimentin is also considered a marker of immature astrocytes, as opposed to glial fibrillary acidic protein (GFAP) that labels mature astrocytes (Bertossi et al. 1999). We preformed preimmune absorption by incubating the antibody with vimentin peptide (Santa Cruz Biotechnology Inc., Santa Cruz, CA; SC-7557P) (1 μg peptide per 1 μg antibody) for 1 h at room temperature (RT) before immunostaining the tissue sections. We labeled axons with antibody to pan-axonal neurofilaments (SMI 312) (Haynes et al. 2005), which recognizes both high and medium molecular weight phosphorylated neurofilament (Table 2). The identification of blood vessels was facilitated by the application of an antibody to CD31, also known as platelet endothelial cell adhesion molecule-1, which is constitutively expressed by developing and adult endothelial cells and mediates cell–cell adhesion.
Table 2.
Summary of antibodies used for analysis of radial structures in the cerebral white matter of the developing human
| Antibodies | Host | Source/cat # | Immunogens | Dilution | Tissue labeling | References |
|---|---|---|---|---|---|---|
| Vimentin | Goat Polyclonal IgG | Santa cruz/Sc-7557 | Synthetic peptide to C-terminus of vimentin of human origin | 1:100 | Detection of vimentin In RGFS in humans and rodents | Chiu et al. (2000) |
| Pan-axonal Neurofilament SMI 312 | Mouse IgG1 cocktail | Covance/SMI312R | Not available | 1:1000 | Detection of phosphorylated neurofilament | Haynes et al. (2005) |
| CD31 | Rabbit polyclonal IgG | Abcam/Ab28364 | Synthetic peptide corresponding to C-terminus of mouse CD31 | 1:50 | Expressed on endothelial cells, as well as platelets and leukocytes | Lu et al. (2008) |
| MAP2 | Rabbit polyclonal IgG | Santa cruz/Sc-20172 | Amino acids 1–300 at the N-terminus of MAP2 of human origin | 1:200 | Reacts with MAP2 of humans and rodents | Andiman et al. (2010) |
| GFAP | Mouse monoclonal IgG | Covance/SMI-22 | Derived from the Bigner-Eng clones MAb1B4, MAb2E1, and MAb4A11 | 1:500 | Intermediate filaments in resting and reactive astrocytes | McLendon et al. (1986) |
The mounted tissue sections were washed twice with phosphate buffered saline (PBS, pH 7.4) with 0.3% Triton X-100 (PBST, PH 7.4) for 5 min. Nonspecific immunostaining was blocked by incubating sections in PBST containing 10% normal donkey serum for 1 h at RT. The primary antibody incubation was performed overnight at 4°C. After 2 washes (5 min each) in PBST, the sections were incubated in Alexa Fluor donkey anti-goat IgG 488 secondary antibody (for vimentin, 1:1000 in PBST, Invitrogen), Alexa Fluor donkey anti-mouse IgG 594 secondary antibody (for neurofilament, 1:1000 in PBST, Invitrogen), and Alexa Fluor donkey anti-rabbit IgG 488 secondary antibody for CD31 (1:1000 in PBST, Invitrogen) for 1 h at RT. Nuclei were stained with Hoechst 33258 (1:10 000, Sigma-Aldrich). Negative controls omitted the primary antibodies. Immunofluorescence was visualized with the Olympus BX51 microscope (Olympus America, Inc., Melville, NY) using FITC, TRITC, and 4′,6-diamidino-2-phenylindole filters. Images were captured with a Cool SNAP fx camera (Photometrics, Tuscan, AZ) and MCID Elite 6.0 software (Life Sciences, Piscataway, NJ). For single label analysis examining SMI 312 in the optic radiation, a block of tissue at the level of the optic radiation was fixed in 4% paraformaldehyde, cryopreserved in PBS containing 30% sucrose, frozen at −20°C, and cut at 50 μm on a cryostat. Free-floating fixed frozen sections were incubated overnight at 4°C with SMI 312 followed by the same staining procedure as above. Sections were mounted onto slides and visualized as above.
Double-label Immunocytochemistry
This technique was performed with the astrocytic marker to GFAP and the pan-neuronal (cytoplasmic) marker to microtubule-associated protein 2 (MAP2) to analyze the timing of the transformation of RGFs into astrocytes and/or neurons during fetal telencephalic development. Tissues were processed as above and the sections were then incubated with GFAP or MAP2 antibodies. Fluorescence was detected with Alexa Fluor donkey anti-mouse 594 and Alexa Fluor donkey anti-goat 488 secondary antibodies (1:1000; Invitrogen). Negative controls omitted the primary antibodies. The microscopic examination and photography of the immunostained tissue sections was identical to that for single-label immunocytochemistry.
Results
We compared the spatial distribution and developmental profile of the HARDI-defined radial coherence in the human fetal occipital lobe (Figs 2, 4 and 5) with histological findings in the white matter (Fig. 3), and immunocytochemical findings of white matter RGFs, axons, and blood vessels (Figs 6, 7; Table 2). At each developmental epoch, we first present below the HARDI analysis (Figs 4, 5), followed by the neuroanatomic analysis (Figs 6, 7). The combined observations in each case analyzed are summarized in Table 1 for reference.
Midgestation (19–21 Gestational weeks)
HARDI Analysis
At this age bracket, the cerebral cortex was agyric with only formation of the calcarine sulcus (Fig. 4). Radial coherence dominated the central white matter in the 2 brains analyzed at 20 weeks and 21 weeks (Fig. 4). Radial coherence was present from the pial surface, through the cerebral cortex, underlying white matter, and developing optic radiation to the ventricular wall (Fig. 4). The majority of fibers from the pial surface, however, terminated at the boundary of the distal optic radiation Fig. 8), with a minority extending through the optic radiation to the ventricular surface (Fig. 8). The optic radiation, running in the anterioposterior plane, was present but not visualized in the imaged coronal slab as the image was restricted to in-plane fibers (Fig. 4). There is no black region at the site of the optic radiations as radially coherent fibers that coexisted with the optic radiations remained after exclusion of the optic radiations. Short tangential fibers, indicative of corticocortical pathways, were rare in the white matter at this age.
Figure 8.
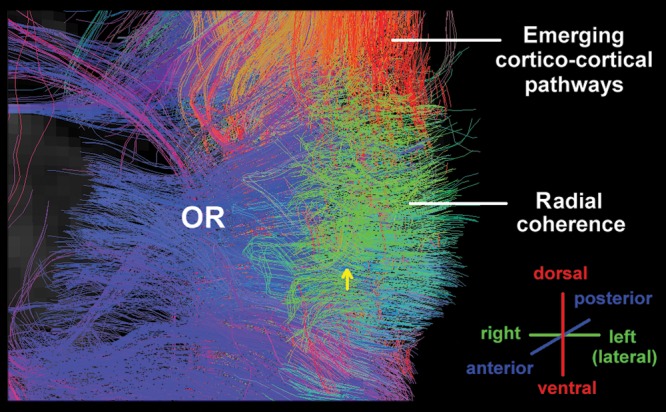
An oblique view of radial coherence with HARDI in the human occipital wall at the level of the atrium of the lateral ventricle at 21 postconceptional weeks. The orientation of the pathways is shown in the lower right corner. Abbreviations: OR, optic radiation.
Neuroanatomic Analysis
Examination of the telencephalic wall at midgestation was performed in 3 cases at 20 gestational weeks with tissues stained with cresyl violet from low (×4) to high (×40) magnification with the standard light microscope. Germinal matrix was prominent at the ventricular wall of the atrium, adjacent to the developing optic radiation and subventricular zone (Fig. 3). The optic radiation was well formed (Fig. 3), with bundles of axons in the anterioposterior plane divided by glial septae (Fig. 3E). Radial nuclear chains of variable length were noted only in the septae of the optic radiation (Fig. 3). They were not found in the subventricular zone or interstitial white matter (Fig. 3; Table 1). Rather, nuclei appeared scattered throughout the interstitial white matter, in contrast to the radial pattern of neuroblastic nuclei in the optic radiations and developing cerebral cortex (Fig. 3). We also analyzed the pattern of RGFs, axons, and blood vessels relative to each other with immunocytochemistry at the standardized central white matter site in a case at 19 weeks and one at 20 weeks at low and high magnifications under the microscope (Fig. 6) (Table 1). The RGFs were long, slender, densely packed processes with varicosities that were distributed in a radial pattern perpendicular to the pial surface and that immunostained positively with the vimentin antibody (Fig. 6), consistent with observations in other mammalian studies (Noctor et al. 2001; Campbell and Gotz 2002; Rakic 2006). It was not always possible to follow single RGFs the entire distance from the pial to the ventricular surface; rather, the processes entered and exited the section plane, with their radial path recognized over short (100 μm) distances. Axons were identified as long, thin processes that immunostained positively with the SMI 312 antibody (Fig. 6). In contrast to the uniformly radial pattern of the RGFs, we observed at low- and high-magnification segments of axons oriented in multiple directions, that is, radial, oblique, and tangential, in a seemingly random pattern and with extensive crossing (Fig. 6). This multidirectional pattern in the central white matter contrasted with the bundled pattern (i.e., with axons in one direction) in the adjacent developing optic radiation, such that the optic radiation served as an internal control for the capability to detect a unidirectional pattern of axons, if present, at this age (Fig. 9). The blood vessel walls, weakly labeled with the CD31 antibody, revealed a radial organization of the penetrating arteries in the telencephalic wall at this age (Fig. 6). Horizontal collateral branching was also identified with this antibody between the penetrating vessels in the deep central white matter (Fig. 6). Nuclear chains were not noted in the interstitial white matter in the 50-μm-thick sections for immunocytochemistry at this age bracket or all subsequent ages (data not shown).
Figure 9.
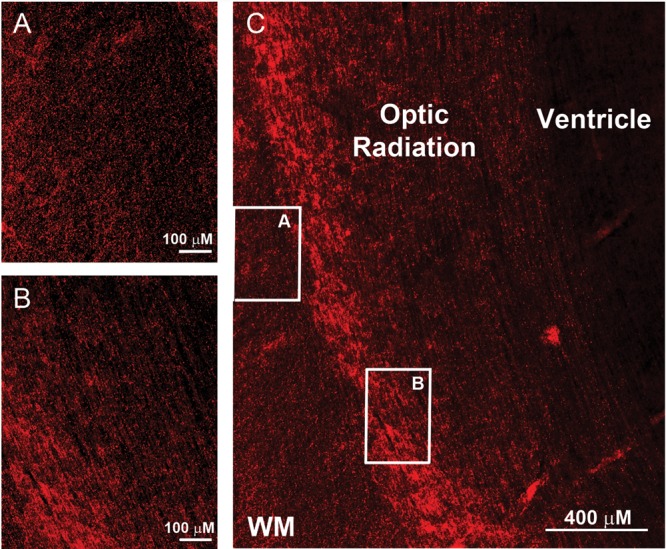
Two distinct axonal patterns are demonstrated by immunocytochemistry with the pan-axonal antibody SMI 312 at midgestation (C). The axons in the optic radiation are bundled and project in one direction (A), as illustrated in the horizontal plane (B). The axons in the central white matter at this age, however, are multidirectional with projections in the radial, horizontal, and oblique planes (A). Scale bar = 100 μm for panels A and B, 400 μm for panel C.
Early Preterm Period (24–29 weeks)
HARDI Analysis
Brains were not available for HARDI at this age bracket.
Tissue Morphology
We analyzed the distribution pattern of RGFs, axons, and blood vessels in 3 cases at 24, 26, and 27 weeks (Fig. 6). In this age bracket, the RGFs in the central white matter appeared less densely packed than at midgestation (Fig. 6), and the overall nuclear density in the central white matter appeared decreased relative to midgestation (data not shown).
Mid Preterm Period (30–33 Weeks)
HARDI analysis
Two fetal brain datasets at 30 weeks were available for HARDI analysis. In addition to the calcarine sulcus, the cingulate gyrus and parietooccipital sulcus were observed (Fig. 4). At this period, the dominant pattern of radial coherence began to disappear (Fig. 4; Table 2). In the white matter lateral to the ventricle, a region of tangential coherence in the anterior–posterior plane intercepted radial coherence (Fig. 4, asterisk) such that it was no longer continuous from the pial to ventricular surface. Of note, the radial coherence pattern extended contiguously into the pial surface, that is, through the cerebral cortex. The tangential pattern of coherence suggested longitudinal axonal fascicles characteristic of long association pathways, including the optic radiation just lateral to the ventricle (Fig. 3). In addition, we identified corticocortical coherence, indicating the emergence of short-range corticocortical association fibers, consistent in part with subcortical association (U) fibers, at this age (Fig. 4).
Tissue Morphology
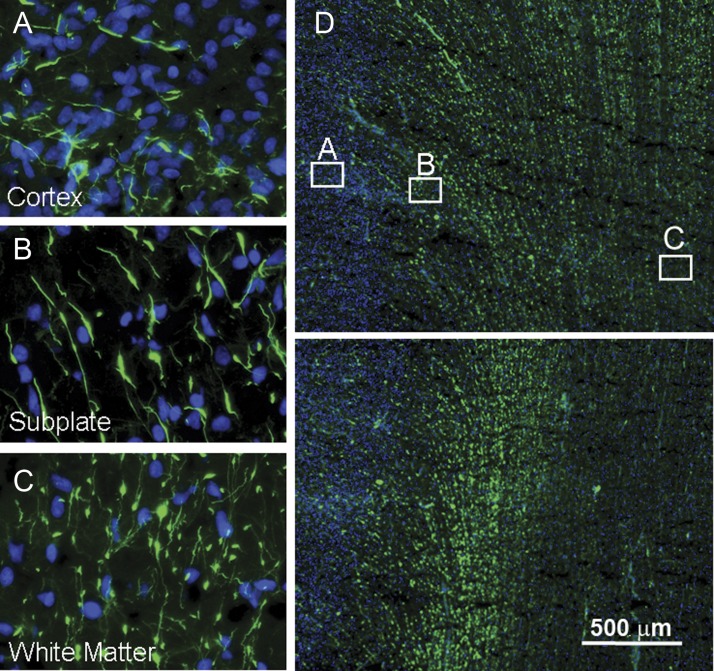
Examination of cresyl violet-stained sections of the telencephalic wall at 31 postconceptional weeks (n = 1) revealed no radial nuclear chains the periventricular or interstitial white matter (Fig. 3) (Table 1). Nuclear chains were also not prominent in the septae of the optic radiation at this age but rather, appeared singly dispersed (data not shown). In a single case available for immunocytochemical analysis in this age bracket (30 weeks), we first observed the presence of vimentin-positive cell bodies with multiple, delicate processes in the central white matter, suggesting the onset of the transformation of RGFs to astrocytes at this site (Fig. 6). Still, RGFs remained prominent, and axons traversed the white matter in multiple directions. The radial orientation of the penetrating blood vessels persisted.
Late Preterm Period (34–36 Weeks)
HARDI Analysis
One fetal brain dataset at 34 weeks was available for HARDI analysis (Fig. 4). The calcarine, cingulum, and parietooccipital sulci became deeper than the previous stage. Additional shallow sulci were also emerging. Thus, the pattern of radial coherence continued to be in transition. The site of the optic radiation in the anterioposterior direction was notable (Fig. 4, asterisk).
Tissue Morphology
Examination of cresyl violet-stained sections of the telencephalic wall at 34 postconceptional weeks (n = 1) revealed no nuclear radial chains in the white matter (data not shown). Immunocytochemical analysis was preformed in 1 case at 34 and 2 cases at 36 weeks. At this age bracket, changes in the RGFs were marked. At 34 weeks, the RGFs were visually decreased in number relative to that in younger epochs (Fig. 7), suggesting disintegration and/or transformation. The RGFs were thicker and shorter, and their varicosities were more prominent than at younger time-points (Fig. 7). Vimentin-positive cell bodies now populated the white matter (Fig. 7), as well as the overlying cerebral cortex (data not shown). The axons continued to demonstrate a multidirectional pattern (Fig. 6). The intensity of CD31 in the walls of the blood vessels appeared increased compared to younger ages, but the pattern of radial orientation of the penetrating vessels and short horizontal pattern of the collateral microvasculature persisted (data not shown).
Term Birth (37–42 weeks)
HARDI Analysis
All the gyral structure was present at this stage. The brains of 2 in vivo individuals at 40 postconceptional weeks were analyzed. At this age, radial coherence from the ventricular wall to the pial surface was no longer evident and was replaced by short in-plane corticocortical coherence (Fig. 5). In addition, we identified tangential coherence, consistent with the optic radiation and the superior and inferior longitudinal fasciculi running in an anterior–posterior direction through the coronal plane (Fig. 5).
Tissue morphology
We performed immunocytochemical studies in 5 cases at 37 weeks (n = 1), 38 weeks (n = 1), 40 weeks (n = 2), and 41 weeks (n = 1). In this age bracket, the long RGFs virtually all disappeared (Fig. 7), first in the cerebral cortex (Fig. 10), followed by the white matter (Fig. 10). At 38 weeks, RGFs were short and sparse in the cerebral cortex, whereas in the subplate region and white matter they were still relatively dense in comparison. There were, however, short and thick fibers with large varicosities in the subplate region, and thin fibers with small varicosities in the underlying white matter (Fig. 10). Owing to the loss of RGFs at this age, the radially oriented blood vessels immunostained with CD31 appeared especially in the tissue sections (Fig. 7).
Figure 10.
The presence of RGFs is differentially identified in the cerebral cortex (A), subplate region (B), and gyral white matter (C) in the occipital lobe term birth (38 weeks), suggesting that they disappear/transform first in the cerebral cortex followed by the white matter. At low magnification, RGFs are most prominent in the subplate region and superficial white matter, the latter shown at the crest (D) of the gyrus and within the gyrus (E). The RGFs are short and sparse in the cerebral cortex (A), short and thick with large varicosities in the subplate region (B), and thin and dense with small varicosities in the deep white matter (C).
In this age bracket, we performed double-label immunocytochemistry to determine if the RGFs transformed into astrocytes and/or neurons, both possibilities suggested previously in the human and animal literature (Gray and Sane 1992; De Azevedo et al. 2003). We found that small cells with multiple delicate processes (i.e., astrocytic morphology) expressed both GFAP and vimentin (Fig. 11), suggesting transformation of RGFs to astrocytes. We also identified a small population of GFAP-positive, vimentin-negative cells with the astrocyte morphology (Fig. 11), suggesting that they were mature and had lost the expression of this early astroglial marker.
Figure 11.
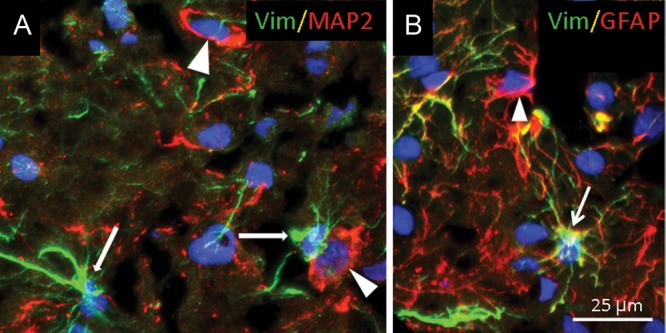
Vimentin-immunopositive cells transform into GFAP-expressing astrocytes (B) but not into neurons that express MAP2 in the central occipital white matter, as demonstrated with double-label immunocytochemistry in a term newborn. A. Vimentin-immunopositive cells (likely astrocytes) (thin arrows) do not colocalize with MAP2 in neurons (arrow heads). B. Vimentin is present in GFAP-positive astrocytes (arrow). There are, however, also GFAP-expressing astrocytes that do not express vimentin (arrow head).
We did not identify MAP2-immunopositive neurons that coexpressed vimentin in the white matter in which neurons at this site suggest subplate neurons and/or late migrating GABAergic neurons (Gang Xu et al. 2011). At this age range, the axons in the white matter continued to display multidirectional orientations (Fig. 7), and the large blood vessels, a prominent radial pattern (Fig. 7).
Infancy (1–12 Postnatal Months)
HARDI Analysis
In the infant postmortem specimen at 3 postnatal months, the short corticocortical tracts appeared denser than in the preceding age brackets (Fig. 5). Additional short-range corticocortical fibers were identified coursing in all directions, but only those in the plane of brain slide analyzed were visualized (see above). Tangential coherence persisted in the optic radiation and in the superior and inferior longitudinal fasciculi running in the anterior–posterior direction through the coronal plane (Fig. 5).
Tissue Morphology
In the infant brain analyzed at 8 postnatal months, there were no RGFs, as demonstrated by vimentin immunostaining (Fig. 7). At the age, there was a striking change in the axonal pattern compared to younger ages with the presence now of unidirectional axonal bundles in the central white matter (extrinsic to the distal optic radiation) (Fig. 7). These axonal bundles in the standardized block examined were likely contained with the superior longitudinal fasciculus. Radial (and nonradial) blood vessels stained positively for vimentin (Fig. 7), and the radial pattern of the penetrating vessels persisted.
Toddler Period (1–5 Postnatal Years)
HARDI Analysis
The brain of a living child at 2 years was analyzed. The tractography patterns were unchanged from term (data not shown).
Tissue Morphology
Immunocytochemical analysis was performed in this age bracket in 1 case each at 1 and 2 years. Throughout the white matter, there were no RGFs (Fig. 7). There were also no cells with astrocytic morphology that stained with both vimentin and GFAP antibodies, but rather, only with GFAP (Fig. 7). Vimentin immunolabeling was only identified in the radial and nonradial blood vessels. At this age bracket, the axons in the central white matter demonstrated unidirectional trajectories (Fig. 7), as in the infant case in the previous age bracket. The walls of the radial blood vessels strongly stained with the CD31 antibody (Fig. 7).
Adulthood
HARDI Analysis
In the 2 adult cases, no radial coherence was present in the white matter, in contrast to multiple corticocortical bundles that were oriented in multiple directions (Fig. 5). The tangential coherence of the optic radiation and the superior and inferior longitudinal fasciculi were unchanged from previous periods. The adult pattern of corticocortical connectivity that has been described in depth by others was observed in these 2 cases (Mori et al. 2005; Catani et al. 2008).
Tissue Morphology
Immunocytochemistry was not performed.
Discussion
Diffusion tractography in the study of the brains of preterm infants is deepening our understanding of neural development and injury over the second half of gestation. Nevertheless, its diagnostic value depends upon accurate correlations of imaging findings with structures defined in the human brain itself. The objective of the current study was to define the neuroanatomic basis of the radial coherence demonstrated in the telencephalic white matter of the preterm infant with HARDI by correlations with structures defined by postmortem morphologic analyses. We reasoned that the neuroanatomic substrate(s) for the radial coherence identified with HARDI should follow a similar time course in appearance and regression. We found that while radial coherence in the fetal white matter likely reflects a composite of RGFs, penetrating blood vessels, and radial axons, its transient expression most closely matches that of the RGFs. In the following discussion, we highlight the temporal correlations between radial coherence and radial structures, critical scaling issues, potential limitations of the study, and the clinical implication of our findings.
Radial Glial Fibers in the Human Preterm Brain
The adult human cortex is organized into vertical (radial) columns in which iterative neuronal modules extend radially from Layer VI to Layer II underneath the superficial Layer I (Rakic 1988, 2006; Rakic et al. 2009). Rakic has proposed that cohorts of cortical neurons originate over time from a single neuronal progenitor in the germative zone of the ventricular wall to form a so-called ontogenic column (Rakic 1988), and that a “proto-map” in the embryonic proliferative zones specifies the basic neuronal classes and their final localization in the different cortical areas (Rakic et al. 2009), with a single functional column likely formed by multiple ontogenic columns. The migration of the early arriving pyramidal neurons along the RGFs establishes the radial architecture of the cortex; thereafter, cortical development continues with the arrival of GABAergic interneurons (via tangential and radial migration), glial cells, and axonal afferents (Rakic 2006; Marin-Padilla, 2011; Xu et al. 2011). In the current study, we found that from 19 to 27 weeks, RGFs are present in the human white matter, suggesting that neuroblasts migrate along them over this time frame, well into the third trimester. The transition from HARDI-defined radial coherence to corticocortical coherence begins in the mid preterm period (30–31 weeks), at the same time that RGFs begin to transform into white matter astrocytes. From 30 weeks to term gestation (37–41 weeks), the RGFs progressively disappear and the white matter becomes populated with transformed astrocytes, as visualized by the coexpression of the RGF immunomarker, vimentin, and the astrocytic immunomarker, GFAP, in delicate small cells with the stellate morphology of astrocytes, as previously noted by others (Honig et al. 1996; De Azevedo et al. 2003). By term gestation, radial coherence and RGFs have both completely disappeared, thereby marking the end of radial migration of cortical neurons. From midgestation onward, we did not detect vertical chains of nuclei in the interstial white matter that would suggest neuroblasts migrating upward along RGFs, a histological finding reported earlier in cortical plate formation in the embryonic period (e.g., Marin-Padilla 2011).
Of major interest was our finding that the anterioposterior coherence of the distal optic radiation (at the level of the atrium of the lateral ventricle) was intermixed with radial coherence at midgestation, even though this structure was well formed at this age by histological examination, as supported by previous human studies (Gilles et al. 1976). Moreover, closer analysis with HARDI at midgestation revealed that the majority of the radial coherence terminated at the boundary of the optic radiation, with the appearance of a minority “passing through” to the ventricular surface. At play here, we believe, is the boundary, or “cordone”, between 2 different neural structures, that is, the axonal projections of the optic radiation in the anterioposterior plane, and the vertical trajectory of RGFs extending from the ventricular to pial surface. Such boundaries represent the barriers between 2 sets of structures with different origins, terminations, functions, and developmental timetables—at this site, between axons and RGFs. HARDI graphically illustrates this boundary that has not been previously appreciated. The molecular cues that guide the RGFs through the optic radiation to the pial surface without veering are unknown. We speculate that the dominant radial nuclear chains we detect in the optic radiation at midgestation reflect RGFs extending through this structure. The boundary or barrier between axons and RGFs at the optic radiation in the developing human telencephalon needs further investigation.
The onset of the retraction of RGFs around 30 weeks is similar to that defined by others in human studies also utilizing the vimentin antibody (Honig et al. 1996; De Azevedo et al. 2003; Ulfig and Briese 2004). A major strength of the current study, however, is the large sample size of brains spanning the last half of gestation and at term (n = 14), thereby allowing a more precise timetable of RGF development. Yet, in contrast to previous rodent and rare human studies of RGFs (Gray and Sane 1992; De Azevedo et al. 2003), we were unable to establish that RGFs transform into neurons utilizing double-label immunocytochemistry for coexpression of vimentin with the neuronal immunomarker, MAP2, a phenomenon that would suggest the RGF capability for neuronal and glial differentiation. It is possible, however, that MAP2 is not an optimal marker of the immature neuronal phenotype that characterizes the transition state. Additional studies with specific markers of neuronal precursors or progenitors are needed.
Axonal Development in the Human Preterm Brain
We investigated the possibility that axons entering and leaving the cerebral cortex account for radial coherence in the preterm telencephalic white matter with the application of the pan-axonal marker SMI 312 in tissue sections adjacent to those used for vimentin analysis. We found that single axonal processes are oriented in radial, tangential, and oblique directions in the interstitial white matter of the occipital lobe (lateral to the optic radiation) in the fetal period. Nevertheless, we cannot exclude the possibility that HARDI is biased toward the detection of the radially oriented axons that are visualized among the single tangential and oblique processes detected in tissue sections. The multidirectional trajectories of the single axons in the preterm white matter may reflect the ongoing growth of axons as they “search” for their final synaptic targets, with initial overgrowth followed by programmed regression. Indeed, the expression levels of the phosphoprotein growth associated protein-43 (GAP-43), a marker of axonal elongation, are remarkably high during the second half of human gestation in the parietooccipital white matter (Haynes et al. 2005), underscoring the relative immaturity of preterm axonal development. We found, however, an obvious unidirectional bundle in the interstitial white matter after infancy, well after radial coherence has disappeared. This bundle likely is the superior longitudinal fascicule emerges after term with diffusion tensor magnetic resonance imaging (Huang et al. 2006, 2009; Zhang et al. 2007).
Radial Vasculature in the Human Preterm Brain
We considered the possibility that the transient radial coherence in the fetal white matter reflects radially oriented blood vessels. Beginning around 8 gestational weeks, telencephalic blood vessels are known to form from leptomeningeal capillaries that perforate the marginal glia and external basal lamina of Layer I, and advance from the pial surface to the subventricular zone (Marin-Padilla 2011). The leading endothelial “sprouts” of adjacent perforators grow vertically and establish horizontal interconnections via a capillary anastomotic plexus that is well in place by 15 weeks (Marin-Padilla 2011). By 12 weeks, vimentin-positive cytoplasmic extensions of the RGFs contribute to the glial envelope that tightly surrounds the cortical vessels (Bertossi et al. 1999), and the RGFs provide a supportive guideline to the perforating vessels (Virgintino et al. 1998), as they do for the migrating neuroblasts from the subventricular zone. In the present study, the radial pattern of vascularity persisted from gestation through childhood, well beyond the transient appearance of radial coherence documented between 20 and 30 weeks. While the developmental profiles of the radial blood vessels and RGFs did not precisely match one another, the penetrating vessels nevertheless likely contribute to the transient radial coherence, albeit not necessarily as its determining factor.
Scaling between HARDI and Neuroanatomic Images
A major challenge in correlating HARDI and tissue findings is the difference in scale between MR images and histological sections that can be several orders of magnitude. Revitalized interest in complimentary MR diffusion techniques and tissue probes of detailed microstructure, including angular bipolar double-PFG techniques, is mounting (Shemesh and Cohen 2011). Notably, the structures in tissue sections are at the same order of magnitude as the diffusion distance in slices of 50 μm thickness with field dimensions of 100–200 μm a side; the average voxel size with MR imaging, on the other hand, is 0.4–2.0 mm a side. Therefore, the orientation of a structure that appears “random” in a thin (5 μm) tissue section may appear nonrandom or coherent when viewed in thick (50 μm) and multiple sections. To account for this possibility, we examined tissue sections in thick sections (50 μm) and multiple sections, as well as at low magnification (×10). We emphasize that the so-called “fibers” visualized by tractography are indeed not specific anatomic structures and their sizes are defined post hoc mainly for visualization purposes. That is, there is not a direct relationship between axonal or RGF diameter and “fiber” diameter.
Potential Limitations of the Study
A potential limitation of this study is that the HARDI and tissue findings may not entirely reflect normality as the studies were performed in living patients with suspected or diagnosed brain disorders, and in autopsy patients who died in extremis. This approach to human pediatric brain analysis, however, is unavoidable as rarely, if ever, do completely “normal” premature and term infants undergo neuroimaging studies, or, in the case of the autopsy, die without complex hospital courses (Kinney 2009). We address this concern by analyzing only HARDI in living patients who, despite neurological signs, had unremarkable conventional MR imaging clinically, and by studying brains at autopsy in which there were no or only minimal pathologic changes upon a comprehensive neuropathologic survey.
A second potential limitation of the study is the combined analysis of in vivo and ex vivo brains, considered a necessity to reach a reasonable sample size for interpretation. We found that HARDI radial coherence disappeared around 30 weeks; yet, this finding needs to be considered in the context that the brains prior to 30 weeks were ex vivo, and after 30 weeks, in vivo, raising the possibility that radial coherence after 30 weeks was not detected due to suboptimal imaging parameters for detection with in vivo HARDI. We considered 4 factors that may account for the possible inferiority of in vivo compared to ex vivo imaging. First, motion could obscure underlying structures in the brains imaged in vivo. Nevertheless, motion would presumably affect all fiber tracts simultaneously; given the high quality of the tracts identified in the in vivo imaged brains of our dataset, motion is unlikely a factor. Second, fewer diffusion gradient directions in the in vivo compared to ex vivo imaging primarily impacts the quality of the tracts (length and number). However, fewer directions do not change the primary tissue coherence detected. Higher numbers of diffusion directions help tract coherence over longer ranges and help resolve crossing fibers but the primary direction of the majority of the tracts is unchanged. Thus since the detected white matter organization reflected major axonal pathways, radial coherence was no longer the dominant tissue organization. In addition, absence of radial coherence in the white matter was not a selective inability to detect radial diffusion, as we were able to detect radial coherence in the cortex.
The third factor for consideration is the difference in spatial resolution in the in vivo imaging (2 mm) versus ex vivo (0.4–0.7 mm) imaging. Despite this difference in resolution, in all cases (both in vivo or ex vivo), there are multiple voxels (5–7 voxels) that span the cerebral mantel which would allow for detection of radial coherence. Finally, the fixation process itself could alter tissue structure. Many prior studies, however, confirm that the tissue coherence is preserved with tissue fixation (e.g. Schmahmann et al. 2007; Huang et al. 2009). We conclude that the absence of radial coherence after 30 weeks is not an “artifact” of the different parameters of in vivo versus ex vivo imaging, but rather, a definite biological change, a conclusion supported by the immunocytochemical data that demonstrates the transformation of RGFs to astrocytes after 30 weeks.
Relevance of Study to the Clinical Care of Preterm Infants
Our findings are most germane to diagnostic neuroimaging in preterm brains at risk for EOP. The major white matter component of this disorder is PVL, which is characterized by periventricular necrotic foci and diffuse gliosis and microglial activation in the surrounding white matter (Kinney and Volpe 2009; Kinney 2009; Volpe et al. 2011). The possibility of RGF damage has not yet been specifically addressed in PVL, either by neuroimaging or postmortem tissue analysis. The establishment of injury to RGFs in preterm infants with diffusion tractography, utilizing the baseline information provided here, will provide critical insight into mechanisms underlying cognitive impairments in long-term survivors of prematurity.
Notes
The authors thank the Eunice Kennedy Shriver NICHD Brain and Tissue Bank for Developmental Disorders (NO1-HD-9-0011) for the provision of developmental cerebral cortical tissue for HARDI (brain slice at 3 postnatal months) and tissue analysis. This work was supported by the National Institute of Neurological Diseases and Stroke (PO1-NS38475) (HCK, JJV), Cerebral Palsy International Research Foundation (RLH), and Eunice Shriver Kennedy National Institute of Child Health and Development (R21HD069001) (ET), (P30-HD018655) (Children's Hospital Developmental Disabilities Research Center). This research was carried out in part at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41RR14075, a P41 Regional Resource supported by the Biomedical Technology Program of the National Center for Research Resources (NCRR), National Institutes of Health. This work also involved the use of instrumentation supported by the NCRR Shared Instrumentation Grant Program (1S10RR023401, 1S10RR019307, and 1S10RR023043) and High-End Instrumentation Grant Program (S10RR016811). The content of the manuscript is the view of the authors and not that the official view of the Eunice Shriver Kennedy National Institute of Child Health and Development. Conflict of Interest: None declared.
References
- Andiman SE, Haynes RL, Trachtenberg FL, Billiards SS, Folkerth RD, Volpe JJ, Kinney HC. The cerebral cortex overlying periventricular leukomalacia: analysis of pyramidal neurons. Brain Pathol. 2010;20:803–814. doi: 10.1111/j.1750-3639.2010.00380.x. doi:10.1111/j.1750-3639.2010.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker BQ, Larroche JC. Periventricular leukomalacia of infancy. A form of neonatal anoxic encephalopathy. Arch Neurol. 1962;7:386–410. doi: 10.1001/archneur.1962.04210050022004. doi:10.1001/archneur.1962.04210050022004. [DOI] [PubMed] [Google Scholar]
- Bertossi M, Virgintino D, Errede M, Roncali L. Immunohistochemical and ultrastructural characterization of cortical plate microvasculature in the human fetus telencephalon. Microvasc Res. 1999;58:49–61. doi: 10.1006/mvre.1999.2154. doi:10.1006/mvre.1999.2154. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Hallam JA, Colucci-Guyon E, Shaw S. Rigidity of Circulating Lymphocytes Is Primarily Conferred by Vimentin Intermediate Filaments. J Immunol. 2001;166:6640–6646. doi: 10.4049/jimmunol.166.11.6640. [DOI] [PubMed] [Google Scholar]
- Buxton R. Introduction to functional magnetic resonance imaging. Principles and techniques. Cambridge, UK: Cambridge University Press; 2003. pp. 181–183. [Google Scholar]
- Campbell K, Gotz M. Radial glia: multi-purpose cells for vertebrate brain development. Trends Neurosci. 2002;25:235–238. doi: 10.1016/s0166-2236(02)02156-2. doi:10.1016/S0166-2236(02)02156-2. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. doi:10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Chiu IM, Touhalisky K, Liu Y, Yates A, Frostholm A. Tumorigenesis in transgenic mice in which the SV40 T antigen is driven by the brain-specific FGF1 promoter. Oncogene. 2000;19:6229–6239. doi: 10.1038/sj.onc.1204021. doi:10.1038/sj.onc.1204021. [DOI] [PubMed] [Google Scholar]
- D'Arceuil H, Liu C, Levitt P, Thompson B, Kosofsky B, de Crespigny A. Three dimensional high-resolution diffusion tensor imaging and tractography of the developing rabbit brain. Dev Neurosci. 2008;30:262–275. doi: 10.1159/000110503. doi:10.1159/000110503. [DOI] [PubMed] [Google Scholar]
- De Azevedo LC, Fallet C, Moura-Neto V, Daumas-Duport C, Hedin-Pereira C, Lent R. Cortical radial glial cells in human fetuses: depth-corrected transformation into astrocytes. J Neurobiol. 2003;55:288–298. doi: 10.1002/neu.10205. doi:10.1002/neu.10205. [DOI] [PubMed] [Google Scholar]
- Fazzi E, Bova S, Giovenzana A, Signorini S, Uggetti C, Bianchi P. Cognitive visual dysfunctions in preterm children with periventricular leukomalacia. Dev Med Child Neurol. 2009;51:974–981. doi: 10.1111/j.1469-8749.2009.03272.x. doi:10.1111/j.1469-8749.2009.03272.x. [DOI] [PubMed] [Google Scholar]
- Gray GE, Sane JR. Lineage of radial glia in the chicken optic tectum. Development. 1992;114:271–283. doi: 10.1242/dev.114.1.271. [DOI] [PubMed] [Google Scholar]
- Gilles FH, Dooling E, Fulchicro A. Sequence of myelination in the human fetus. Tran Am Neurol Assoc. 1976;101:244–246. [PubMed] [Google Scholar]
- Haynes RL, Borenstein NS, DeSilva TM, Folkerth RD, Liu LG, Volpe JJ, Kinney HC. Axonal development in the cerebral white matter of the human fetus and infant. J Comp Neurol. 2005;484:156–167. doi: 10.1002/cne.20453. doi:10.1002/cne.20453. [DOI] [PubMed] [Google Scholar]
- Hess CP, Mukherjee P, Han ET, Xu D, Vigneron DB. Q-ball reconstruction of multimodal fiber orientations using the spherical harmonic basis. Magn Reson Med. 2006;56:104–117. doi: 10.1002/mrm.20931. doi:10.1002/mrm.20931. [DOI] [PubMed] [Google Scholar]
- Hevner RF. Development of connections in the human visual system during fetal mid-gestation: a DiI-tracing study. J Neuropathol Exp Neurol. 2000;59:385–392. doi: 10.1093/jnen/59.5.385. [DOI] [PubMed] [Google Scholar]
- Honig LS, Herrmann K, Shatz CJ. Developmental changes revealed by immunohistochemical markers in human cerebral cortex. Cereb Cortex. 1996;6:794–806. doi: 10.1093/cercor/6.6.794. doi:10.1093/cercor/6.6.794. [DOI] [PubMed] [Google Scholar]
- Huang H, Xue R, Zhang J, Ren T, Richards L, Yarowsky P, Miller MIs, Mori S. Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. J Neurosci. 2009;29:4263–4273. doi: 10.1523/JNEUROSCI.2769-08.2009. doi:10.1523/JNEUROSCI.2769-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Zhang J, Wakana S, Zhang W, Ren T, Richards L, Yarowsky P, Donohue P, Graham E, van Zjil PCM, Mori S. White and gray matter development in human fetal, newborn and pediatric brains. NeuroImage. 2006;33:27–38. doi: 10.1016/j.neuroimage.2006.06.009. doi:10.1016/j.neuroimage.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Kinney HC. The encephalopathy of prematurity: one pediatric neuropathologist's perspective. Semin Pediatr Neurol. 2009;16:179–190. doi: 10.1016/j.spen.2009.09.003. doi:10.1016/j.spen.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Volpe JJ. Perinatal Panencephalopathy in the Premature Infant: Is It due to Hypoxia-Ischemia? In: Haddad GG, Ping Yu S, editors. Perinatal Brain Hypoxia and Ischemia. Contemporary Clinical Neuroscience. New York, NY: Humana Press; 2009. pp. 153–185. [Google Scholar]
- Kostovic I, Judas M. Correlation between the sequential ingrowth of afferents and transient patterns of cortical lamination in preterm infants. Anat Rec. 2002;267:1–6. doi: 10.1002/ar.10069. doi:10.1002/ar.10069. [DOI] [PubMed] [Google Scholar]
- Letinic K, Rakic P. Telencephalic origin of human thalamic GABAergic neurons. Nat Neurosci. 2001;4:931–936. doi: 10.1038/nn0901-931. doi:10.1038/nn0901-931. [DOI] [PubMed] [Google Scholar]
- Lu P, Li L, Kuno K, Wu Y, Baba T, Li YY, Zhang X, Mukaida N. Protective roles of the fractalkine/CX3CL1-CX3CR1 interactions in alkali-induced corneal neovascularization through enhanced antiangiogenic factor expression. J Immunol. 2008;180:4283–4291. doi: 10.4049/jimmunol.180.6.4283. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. The Human Brain: Prenatal Development and Structure. Heidelberg Germany: Springer; 2011. pp. 1–145. [Google Scholar]
- McLendon RE, Burger PC, Pegram CN, Eng LF, Bigner DD. The immunohistochemical application of three anti-GFAP monoclonal antibodies to formalin-fixed, paraffin-embedded, normal and neoplastic brain tissues. J Neuropathol Exp Neurol. 1986;45:692–703. doi: 10.1097/00005072-198611000-00007. doi:10.1097/00005072-198611000-00007. [DOI] [PubMed] [Google Scholar]
- McNab JA, Jbabdi S, Deoni SC, Douaud G, Behrens TE, Miller KL. High resolution diffusion-weighted imaging in fixed human brain using diffusion-weighted steady state free precession. Neuroimage. 2009;46(3):775–785. doi: 10.1016/j.neuroimage.2009.01.008. doi:10.1016/j.neuroimage.2009.01.008. [DOI] [PubMed] [Google Scholar]
- McNab JA, Miller KL. Sensitivity of diffusion weighted steady state free precession to anisotropic diffusion. Magn Reson Med. 2008;60(2):405–413. doi: 10.1002/mrm.21668. doi:10.1002/mrm.21668. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. doi:10.1002/1531-8249(199902)45:2<265::AID-ANA21>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Mori S, Van Zijl PCM, Wakana S. MRI Atlas of Human White Matter. Amsterdam The Netherlands: Elsevier Science & Technology; 2005. [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. doi:10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Rakic P. A century of progress in corticoneurogenesis: from silver impregnation to genetic engineering. Cereb Cortex. 2006;(Suppl 1):i3–i17. doi: 10.1093/cercor/bhk036. doi:10.1093/cercor/bhk036. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. doi:10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rakic P, Ayoub AE, Breunig JJ, Dominguez MH. Decision by division: making cortical maps. Trends Neurosci. 2009;32:291–301. doi: 10.1016/j.tins.2009.01.007. doi:10.1016/j.tins.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL. Annual research review: development of the cerebral cortex: implications for neurodevelopmental disorders. J Child Psychol Psychiatry. 2011;52:339–355. doi: 10.1111/j.1469-7610.2010.02307.x. doi:10.1111/j.1469-7610.2010.02307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, de Crespigny AJ, Wedeen VJ. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. doi:10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Shapiro EM, Skrtic S, Sharer K, Hill JM, Dunbar CE, Koretsky AP. MRI detection of single particles for cellular imaging. Proc Natl Acad Sci USA. 2004;101:10901–10906. doi: 10.1073/pnas.0403918101. doi:10.1073/pnas.0403918101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemesh N, Cohen Y. Microscopic and compartment shape anisotropies in gray and white matter revealed by angular bipolar double-PFG MR. Magn Reson Med. 2011;65:1216–12127. doi: 10.1002/mrm.22738. doi:10.1002/mrm.22738. [DOI] [PubMed] [Google Scholar]
- Steindler DA. Glial boundaries in the developing nervous system. Annu Rev Neurosci. 1993;16:445–470. doi: 10.1146/annurev.ne.16.030193.002305. doi:10.1146/annurev.ne.16.030193.002305. [DOI] [PubMed] [Google Scholar]
- Takahashi E, Dai G, Rosen GD, Wang R, Ohki K, Folkerth RD, Galaburda A, Wedeen VJ, Grant PE. Developing neocortex organization and connectivity in cats revealed by direct correlation of diffusion tractography and histology. Cereb Cortex. 2011;21:200–211. doi: 10.1093/cercor/bhq084. doi:10.1093/cercor/bhq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi E, Dai G, Wang R, Ohki K, Rosen GD, Galaburda A, Grant PE, Wedeen VJ. Development of cerebral fiber pathways in cats revealed by diffusion spectrum imaging. Neuroimage. 2010;49:1231–1240. doi: 10.1016/j.neuroimage.2009.09.002. doi:10.1016/j.neuroimage.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi E, Folkerth RD, Galaburda A, Grant PE. Emerging cerebrain connectivity in the human fetal brain: an MR tractography study. Cereb Cortex. 2012;22:455–464. doi: 10.1093/cercor/bhr126. doi:10.1093/cercor/bhr126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuch DS, Reese TG, Wiegell MR, Wedeen VJ. Diffusion MRI of complex neural architecture. Neuron. 2003;40:885–895. doi: 10.1016/s0896-6273(03)00758-x. doi:10.1016/S0896-6273(03)00758-X. [DOI] [PubMed] [Google Scholar]
- Ulfig N, Briese M. Evidence for the presence of the sphingosine-1-phosphate receptor Edg-8 in human radial glial fibers. Acta Histochem. 2004;106:373–378. doi: 10.1016/j.acthis.2004.08.002. doi:10.1016/j.acthis.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Virgintino D, Maiorano E, Errede M, Vimercati A, Greco P, Selvaggi L, Roncali L, Bertossi M. Astroglia-microvessel relationship in the developing human telencephalon. Int J Dev Biol. 1998;42:1165–1168. [PubMed] [Google Scholar]
- Vishwas M, Chitnis T, Pienaar R, Healy BC, Grant PE. Tract based analysis of callosal, projection and association pathways in pediatric patients with multiple sclerosis: a preliminary study. Am J Neuroradiol. 2010;31:121–128. doi: 10.3174/ajnr.A1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553–562. doi: 10.1203/00006450-200111000-00003. doi:10.1016/j.ijdevneu.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Volpe JJ, Kinney HC, Jensen FE, Rosenberg PA. The developing oligodendrocyte: key cellular target of brain injury in the premature infant. Int J Dev Neurosci. 2011;29:423–440. doi: 10.1016/j.ijdevneu.2011.02.012. doi:10.1016/j.ijdevneu.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WY, Dai G, Pandya DN, Hagmann P, D'Arceuil H, de Crespigny AJ. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. NeuroImage. 2008;41:1267–1277. doi: 10.1016/j.neuroimage.2008.03.036. doi:10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Xu G, Broadbelt KG, Haynes RL, Folkerth RD, Borenstein NS, Belliveau RA, Trachtenberg FS, Volpe JJ, Kinney HC. Late development of the GABAergic system in the human cerebral cortex. J Neuropathol Exp Neurol. 2011;70:841–858. doi: 10.1097/NEN.0b013e31822f471c. doi:10.1097/NEN.0b013e31822f471c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Evans A, Hermoye L, Lee SK, Wakana S, Zhang W, Donohue P, Miller MI, Huang H, Wang X, van Zijl PC, Mori S. Evidence of slow maturation of the superior longitudinal fasciculus in early childhood by diffusion tensor imaging. Neuroimage. 2007;38:239–247. doi: 10.1016/j.neuroimage.2007.07.033. doi:10.1016/j.neuroimage.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]