Abstract
Mutations of the fragile X mental retardation 1 (FMR1) gene are the genetic cause of fragile X syndrome (FXS). The presence of significant socioemotional problems has been well documented in FXS although the brain basis of those deficits remains unspecified. Here, we investigated amygdala dysfunction and its relation to socioemotional deficits and FMR1 gene expression in children and adolescents on the FX spectrum (i.e., individuals whose trinucleotide CGG repeat expansion from 55 to over 200 places them somewhere within the fragile X diagnostic range from premutation to full mutation). Participants performed an fMRI task in which they viewed fearful, happy, and scrambled faces. Neuroimaging results demonstrated that FX participants revealed significantly attenuated amygdala activation in Fearful > Scrambled and Fearful > Happy contrasts compared with their neurotypical counterparts, while showing no differences in amygdala volume. Furthermore, we found significant relationships between FMR1 gene expression, anxiety/social dysfunction scores, and reduced amygdala activation in the FX group. In conclusion, we report novel evidence regarding a dosage response of the FMR1 gene on fear-specific functions of the amygdala, which is associated with socioemotional deficits in FXS.
Keywords: amygdala, emotion, FMR1 gene, fragile X syndrome, functional brain imaging
Introduction
Fragile X syndrome (FXS) is the most common inherited form of intellectual disability, resulting from a trinucleotide repeat expansion (full mutation > 200 CGG repeats) in the 5′-untranslated region of the fragile X mental retardation 1 (FMR1) gene located at Xq27.3. This expansion results in hypermethylation of the FMR1 gene promoter and the subsequent reduction or absence of the gene's protein product (FMRP), ultimately causing cognitive and behavioral impairments characteristic of the syndrome (Fu et al. 1991; Tassone et al. 1999). For example, a lack of FMRP production is associated with abnormal brain development and function in affected people and animal knockout (KO) models of the disorder (Barnea-Goraly et al. 2003; Hessl et al. 2004; Bagni and Greenough 2005; Lightbody and Reiss 2009). The severity of brain dysfunction and resulting cognitive and behavioral impairment varies across people with FXS and may partly be related to amount of FMRP production (Keysor and Mazzocco 2002; Reiss and Dant 2003).
Along with intellectual disability, the presence of significant problems of hyper arousal, social anxiety, withdrawal, social deficits, and gaze aversion have been well documented in FXS (Bregman et al. 1988; Cohen et al. 1988; Cohen et al. 1991; Hagerman et al. 1991; Hessl et al. 2001; Hessl et al. 2005; Reiss and Freund 1992). However, research on the brain basis of these behaviors in individuals with FXS has been limited, especially in children and adolescents for whom these deficits can be particularly impactful. There have been a few studies focusing on the pronounced gaze aversion characteristic of young children with FXS, suggesting that enhanced and sustained amygdala activity in response to direct gaze may underlie the gaze aversion observed in children with FXS (Garrett et al. 2004; Watson et al. 2008). No prior studies have investigated possible linkages between neural mechanisms of emotional problems and FMR1 genetic factors in children and adolescents on the FX spectrum, although a recent study reported a significant relationship between FMR1 gene expressions and frontostriatal activation to other cognitive functions, such as executive control, in adolescents with FXS (Hoeft et al. 2007). Two recent studies from our group have shown dosage response of FMR1 gene expression on emotional dysfunctions in adults with the fragile X (FX) premutation (55–200 CGG repeats), where we found significant relationships between amygdala activity to emotional stimuli and abnormal elevation of FMR1 mRNA (Hessl et al. 2007) and reduced FMRP (Hessl et al. 2011) in adult men with the FX premutation.
The aforementioned studies suggest atypical amygdala activity in response to gaze and emotional processing in individuals on the FX spectrum; namely, sustained amygdala activity to direct gaze in children with FXS and reduced amygdala activity to emotional facial expression in FX premutation carriers. In order to evaluate the role of the amygdala in FX, however, it is necessary to understand the functional role of the amygdala in typical development. The amygdala is a key region of the brain known to play a crucial role in fear processing (Davis 1992; Adolphs et al. 1995). Both human and animal studies have demonstrated amygdala involvement in the acquisition, storage, and expression of conditioned fear learning (Phelps and LeDoux 2005; LeDoux 2007). Moreover, recent studies of the amygdala have shown that this structure is involved in several aspects of social cognition, such as recognizing emotion in faces, judging the trustworthiness of a person, and generating a sense of personal space (Adolphs 2010). In addition to brain imaging studies, lesion studies support the role of the amygdala in social cognition and fear processing as well. For instance, patients with amygdala lesions have demonstrated deficits in fear conditioning (Bechara et al. 1995) and the perception of fear in both facial expressions (Adolphs et al. 1994) and voices (Scott et al. 1997). Furthermore, amygdala lesions in humans early in life produce impairments in identifying emotional facial expressions, particularly those depicting fear (Adolphs et al. 1994).
Although the amygdala has been recognized as a core brain region for social cognition and fear processing, findings on the structural and functional features of the amygdala in FXS have been somewhat inconsistent. For example, some researchers found reduced amygdala volumes in children with FXS (Gothelf et al. 2008; Hazlett et al. 2009), whereas other researchers found no significant differences in amygdala volumes between children with FXS and matched controls (Kates et al. 1997; Hoeft et al. 2008). In functional imaging research, some studies revealed amplified and sustained amygdala activation in young children with FXS associated with gaze aversion (Garrett et al. 2004; Watson et al. 2008), while abnormal amygdala activity related to gaze processing was not evident in adults and adolescents with FXS (Dalton et al. 2008). With regards to emotion processing, Hagan et al. (2008) tested adults with FXS on a facial-emotion judgment test using happy, sad, and neutral faces. Although the researchers reported significantly reduced anterior cingulate cortex (ACC) activity for emotional faces in the FXS group compared with the control group, no significant group differences in amygdala activation were reported. Using a face-encoding task with fearful faces, Holsen et al. (2008) also did not find differences in amygdala activation between adolescents and young adults with FXS and their matched controls. However, studies on men with the FX premutation revealed significant reduction in amygdala activation in response to the emotional expression (i.e., “fearful > scrambled faces” and “emotion faces (fear and anger) > shapes”) compared with their neurotypical counterparts (Hessl et al. 2007, 2011).
One of the possible reasons for such inconsistent findings on amygdala function in FXS may be the wide range of experimental designs and the diversity among the patient groups tested. That is, some studies focused on gaze aversion in individuals with FXS (e.g., Garrett et al. 2004), whereas others investigated face or emotion processing in general (e.g., Hagan et al. 2008). Furthermore, previous studies only recruited one specific group of participants on the FX spectrum, such as girls with FXS (Garrett et al. 2004), boys with FXS (Watson et al. 2008), women with FXS (Hagan et al. 2008), or men with the FX premutation (Hessl et al. 2007, 2011). By contrast, in the present study, we recruited a range of FX participants including female children and adolescents with the FX full mutation and male and female children and adolescents with mosaicism (i.e., a “FX spectrum” group). In the case of mosaicism, the FMR1 gene is not fully methylated and some FMRP may be produced. Likewise, in the case of full mutation females, FMRP may be produced depending on the percentage of cells that have the normal X chromosome as the active X chromosome (Abrams et al. 1994). Taking advantage of having a group of FX participants who are all producing some amount of FMRP, in the current study, we have focused our questions on the fear-related function of the amygdala in FX spectrum samples and its relationship to both the FMR1 gene and socioemotional deficits.
In the present study, we hypothesized that children and adolescents on the FX spectrum would show reduced amygdala activity in response to fear-related events (i.e., Fearful > Happy and Fearful > Scrambled contrasts) compared with their neurotypical counterparts. Also, we hypothesized that fear-related amygdala functioning in our FX spectrum group would be negatively correlated with their socioemotional deficits. Finally, we expected to find, in the FX group, a dosage response of FMR1 gene expression such that amygdala activation for processing fear-related stimuli would be related to the degree to which the FMR1 gene is behaving normally.
Materials and Methods
Participants
Eighteen children and adolescents on the FX spectrum (FX group; mean age: 14.0 years, SD = 2.97; Female: 12) and 20 neurotypical age-matched controls (NT group; mean age: 13.7 years, SD = 3.24; Female: 10) with normal or corrected-to-normal vision participated in the experiment. The FX group consisted of girls with full mutation and boys and girls with mosaicism: 9 girls with full mutation, 3 girls with methylation mosaicism (i.e., all cells containing a full mutation, but with varying methylation patterns), 5 boys with methylation mosaicism, and 1 boy with size mosaicism (i.e., some cells containing a full mutation and some containing a premutation). Allele status was confirmed for all participants by FMR1 DNA testing. Two participants in the FX group (i.e., 2 girls with full mutation) and 4 participants in the NT group (i.e., 3 girls and 1 boy) were excluded from further analyses due to excessive movement (>3 mm) in the MRI scanner. Group demographic statistics and FMR1 data are shown in Table 1. Participants in the FX group were recruited and clinically evaluated at the UC Davis MIND Institute, and control participants were recruited through letters to families, fliers, and word of mouth in Northern California. The Institutional Review Board at the University of California, Davis, approved the experimental protocol. Prior to the experiment, each participant's caregiver provided informed written consent, and all were compensated with $25 for our fMRI experiment, which lasted for ∼1 h. Screening for MR safety was also completed on the day of scanning to ensure eligibility for MRI. Ten FX participants in our sample were taking medications (7 were on stimulants, 2 were on antidepressants, and 2 were taking medications for ADHD symptoms). Participants received a battery of neuropsychological tests (detailed below) to assess intellectual ability and social and emotional deficits.
Table 1.
Group demographic and FMR1 data
| NT |
FX |
||||||
|---|---|---|---|---|---|---|---|
| N | Mean (SD) | Range | N | Mean (SD) | Range | t (P) | |
| Age | 16 | 14 (3.2) | 8–17 | 16 | 14 (3.0) | 9–18 | |
| CGG repeat size | 9 | 28.2 (4.1) | 16–32 | 16 | N/A | 222–1367 | N/A |
| AR | None | N/A | N/A | 10 | 0.58 (0.20) | 0.17–0.83 | N/A |
| FMR1 % unmethylation | None | N/A | N/A | 6 | 0.51 (0.29) | 0.14–0.84 | N/A |
| FSIQ | 10 | 111.8 (17.7) | 88–137 | 16 | 80.3 (17.2) | 53–117 | −4.5 (<0.001) |
| ASD (ADOS) | None | None | 16 | 5* | N/A | N/A | |
| SRS total t-score | 15 | 40.3 (5.5) | 34–51 | 15 | 61.5 (15.7) | 39–92 | 4.99 (<0.001) |
| ADAMS | 15 | 0.6 (1.2) | 0–4 | 12 | 6.8 (4.3) | 1–14 | 4.82 (<0.001) |
| General anxiety | |||||||
| ADAMS | 15 | 0.5 (1.2) | 0–4 | 12 | 7.2 (5.5) | 1–18 | 4.08 (0.002) |
| Social avoidance | |||||||
| ADAMS | 15 | 0.5 (1.4) | 0–5 | 12 | 3.3 (4.3) | 0–13 | 2.17 (0.05) |
| Depression | |||||||
| ADAMS Manic/hyperactive | 15 | 0.6 (1.0) | 0–3 | 12 | 3.3 (2.9) | 0–9 | 3.06 (0.009) |
| ADAMS Obsessive/compulsive | 15 | 0.1 (0.4) | 0–1 | 12 | 1.1 (2.1) | 0–6 | 1.58 (0.142) |
| Anxiety disorders based on K-SADS | None | None | N/A | 14 | 10* | N/A | N/A |
IQ measures (WASI, WISC-IV, WAIS-III, Stanford–Binet Intelligence Scales); Social Responsiveness scale (SRS) t-scores criteria: severe (≥76), mild to moderate (60 ≤ x ≤ 75), normal (59≤).
*indicates number of people who had each disorder at the time of the study.
Neuropsychological Assessments
Cognitive ability was assessed for each participant based on Full-Scale IQ (FSIQ) using 1 of 4 scales: the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler 1999), Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV; Wechsler 2004), Wechsler Adult Intelligence Scale, Third Edition (WAIS-III; Wechsler 1997), or the Stanford–Binet Intelligence Scales, Fifth Edition (Thorndike et al. 1986). Social dysfunction was measured based on caregiver-report scores from the Social Responsiveness Scale (SRS; (Constantino and Todd 2005). In addition, each participant's anxiety level was assessed by caregivers using the Anxiety Depression and Mood Scale (ADAMS; Esbensen et al. 2003), which consisted of 5 subscale scores: General Anxiety, Social Avoidance, Depression, Manic/Hyperactive, and Obsessive/Compulsive Behavior. For the FX group only, autism phenotype features were also assessed with the Autism Diagnostic Observation Schedule Module 3 or 4 depending on the participant's expressive language level (ADOS; Lord et al. 1989). Finally, to diagnose anxiety disorders in FX participants, the Kiddie-Schedule for Affective Disorders and Schizophrenia (K-SADS; Puig-Antich and Chambers 1978) was administered by trained clinicians. All neuropsychological assessments were administered at the UC Davis MIND institute. Some of the participants could not complete the assessments on the day of the study but agreed to fill out the questionnaires and return them via mail. Nonetheless, we could not obtain full compliance for data that was not collected during the participant's visit. Table 1 presents the scores from the neuropsychological tests as well as the number of participants who completed each of the neuropsychological assessments. All of the statistical tests including the between-group analyses and correlation analyses on demographic, neuropsychological, brain volumes, and eye tracking data, and the regression analyses of brain activity and FMR1 gene expression/behavioral data were conducted using IBM SPSS statistics 20 (http://www.ibm.com/software/analytics/spss/products/statistics).
Molecular Genetic Measures
CGG Repeat Size
Seven of NT controls opted out of the blood draw portion of the study and thus their molecular standing could not be verified. However, their performance on all standardized measures confirmed their diagnosis of typical development. For the remaining 9 NT participants and all of the 16 FX participants, genomic DNA was isolated from peripheral blood lymphocytes using standard methods (Puregene Kit; Gentra Inc). CGG sizing and methylation status were assessed by Southern Blot and polymerase chain reaction (PCR) analyses as detailed in Tassone et al. (2008). Analysis and calculation of the repeat size for both Southern Blot and PCR analysis were carried out using an Alpha Innotech FluorChem 8800 Image Detection System (Tassone et al. 2008).
FMR1 % Unmethylation and Activation Ratio
Activation ratio (AR) was calculated for female participants on the FX group, which represents the percentage of cells that have the normal allele on the active X chromosome (Abrams et al. 1994). AR was measured as described in Tassone et al. 1999. It has been demonstrated that a number of cognitive functions (e.g., as measured by performance IQ) are positively correlated with AR (Abrams et al. 1994; Tassone et al. 1999) in females with the full mutation. Furthermore, researchers have found a strong and positive correlation between AR and FMRP levels in a large sample of females with FXS (Tassone et al. 1999; Loesch et al. 2004; Godler et al. 2010). For males, FMR1% methylation was calculated using Southern Blot analysis (Tassone et al. 2008). As in the case of AR, a strong positive relationship has been found between FMRP levels and FMR1% unmethylation in mosaic males as the cells with an unmethylated FMR1 gene are able to produce FMRP though with lower efficiency (Feng et al. 1995; Tassone et al. 1999; Kenneson et al. 2001; Primerano et al. 2002; de Vries et al. 2003). The reverse values of the % methylation were used for further analyses (i.e., 1 − [FMR1% methylation]: FMR1% unmethylation) as an analog of the AR for male participants in the FX group. Because both AR and FMR1% unmethylation indicate the % of cells carrying an unmethylated allele (i.e., transcriptionally active allele), higher values for these 2 variables represent the degree of functional activity of the FMR1 gene.
Imaging Methods and Analyses
Brain Image Acquisition
All imaging data were acquired on a Siemens 3 Tesla Trio scanner with Echospeed gradients and a Siemens 8-channel whole head coil located at UC Davis Imaging Research Center. Visual stimuli were projected onto a screen and viewed on an MR compatible mirror mounted above the participant's head. Head motion was restricted with a pillow and foam inserts. For each participant, an anatomical scan was acquired using a high-resolution T1-weighted MPRAGE sequence (TR = 2170 ms, TE = 4.82 ms, flip angle = 7°, FOV = 256 × 256 mm, 192 slices, 1.0 mm slice thickness) for the purpose of manual segmentation and to aid in localization, coregistration, and normalization of functional data. After the anatomical scan, 2 functional runs were acquired for each participant. During the functional runs, imaging was performed using a T2*-weighted gradient echo planar pulse sequence (TR = 2000 ms, TE = 13 ms, flip angle = 84°). Each brain volume was composed of 38 transverse slices (FOV = 220 × 220, matrix = 64 × 64, 3.4 × 3.4 × 3.4-mm resolution) aligned to the AC–PC line, collected interleaved, inferior to superior. For all functional runs, data from the first 2 volumes were discarded to allow for stabilization of magnetic fields.
Image Processing and Statistical Analyses
Imaging data were preprocessed using SPM 5 (Wellcome Department of Cognitive Neurology, London) run within Matlab (Matlab Mathwork, Inc., Natick, MA). For preprocessing, data were slice-time corrected for acquisition order (referenced to the first slice), realigned, and unwarped to correct for motion across runs. Next, the images were spatially normalized (with trilinear interpolation and preserving the intensities of the original images) to the SPM EPI template corresponding to the MNI (Montreal Neurological Institute) defined standardized brain space, and then spatially smoothed with a Gaussian kernel of 5-mm FWHM. Motion estimates were not included as covariates in the preprocessing steps for the fMRI data analysis because all participants who were included in the analyses moved less than 3 mm in x, y, or z planes. The time series were high pass filtered at 128 s.
Statistical analyses were performed using the general linear model for event-related designs in SPM 5. For each participant, a whole-brain voxelwise analysis was conducted in which individual events were modeled as a canonical hemodynamic response. Each event type was first modeled for each participant using a fixed effects analysis. Then, the resulting least squares parameter estimates of the height of the modeled hemodynamic response for each condition were entered into random effects analyses. In order to determine the presence of significant clusters of activation, the joint expected probability distribution method (Poline et al. 1997) was used yielding a clusterwise significance level of P < 0.05, corrected for multiple comparisons. Group statistical maps were generated using a 1-sample t-test (height threshold z > 2.33, P < 0.01, extent threshold P < 0.05, at least 220 consecutive voxels). Group contrast maps were generated using a 2-sample t-test (height threshold z > 1.96, P < 0.05, extent threshold P < 0.05, at least 1200 consecutive voxels).
Amygdala Volume
Amygdala volumes were calculated via operator-guided manual segmentation using Mayo Biomedical Imaging Resource Analyze version 9.0 and 10.0. The tracing protocol was developed from anatomical exploration of postmortem human brains using histological sections of tissue cut and oriented perpendicularly to the axis of the hippocampus. For a more thorough description of the tracing protocol, see Schumann et al. 2004. The reliability of the tracer had been previously verified using a 10 case set, with reliability values of 99.1% for the left amygdala and 99.1% for the right amygdala. Once segmented, amygdala volumes were corrected using each participant's total cerebral volume (TCV).
Total Cerebral Volume
In order to calculate TCV, each case was manually edited using Mayo Biomedical Imaging Resource Analyze version 9.0 and 10.0. TCV was calculated with the exclusion of nonbrain structures, and the brainstem, the cerebellum, and the ventricles were identified and excluded using a Gaussian cluster multispectral thresholding tool.
fMRI Face Processing Task
The stimulus and task design were selected based on previous studies of the amygdala response to emotional facial expression (Thomas et al. 2001; Hessl et al. 2007) and modified to examine “fear-specific” activation of amygdala in an event-related design (Fig. 1). To test fear-specific amygdala function, rather than emotion-related amygdala function in general, we added happy expressions as control stimuli in addition to fearful expressions and directly compared brain activity elicited by the 2 salient but different emotion expressions. Specifically, participants saw fearful, happy, or scrambled versions of a calm face in each trial (selected from the NimStim Emotional Face Stimuli database; Tottenham et al. 2009). Hair and ears were stripped from each image to remove any nonfacial features. Each stimulus was presented for 1500 ms followed by jittered interstimulus interval (ISI) of 500, 2500, or 4500 ms, containing a central fixation circle. Each run consisted of 120 trials (40 trials per each face type) and each stimulus was presented twice during the functional run. Participants were instructed to look carefully at each picture during the functional runs. Because participants in the current study included children and adolescents with the FX full mutation as well as those with FX mosaicism, we chose a passive viewing paradigm with no overt response to reduce demands of the task to as low as possible (i.e., to prevent excluding some participants with the full mutation due to task difficulty). The passive viewing task with a rapid presentation of emotion face stimuli has been successfully used in our previous studies to activate bilateral amygdala (e.g., Hessl et al. 2007). Each participant completed 2 functional runs of this task and each run lasted for 8 min and 10 s.
Figure 1.
Trial sequence in the face-processing task. Participants saw a fearful, happy, or scrambled version of a calm face in each trial, and no overt response was required. Each stimulus was presented for 1500 ms followed by jittered interstimulus interval (ISI) of 500, 2500, or 4500 ms, containing a central fixation circle. The images were converted to grayscale here for presentation purpose.
Eye Tracking
Eye tracking data were collected concurrently with the fMRI face processing task using a long-range infrared eye tracking system (Applied Science Laboratories, Bedford, MA) and analyzed using Eyenal 6.0 (Eyetracker, North Sydney, Australia). An area of interests (AOI) was defined as a rectangle covering the whole face stimulus region for each face stimulus (Fearful, Happy, and Scrambled). Additionally, based on previous studies on the gaze patterns in FXS (Dalton et al. 2005; Dalton et al. 2008; Holsen et al. 2008), 2 AOIs were defined as rectangles covering the eye and mouth regions, separately, for the 2 emotion expression stimuli (Fearful and Happy). The gaze time to each AOI on each trial was defined as the amount of total time spent in milliseconds (minimum 50 ms) in which participant's gaze was fixated on each AOI. For each participant, the total gaze time to the whole face, the eye, and the mouth region was calculated as the average of gaze time within each of the 3 predefined regions for each face per run, then averaged across both runs.
Results
Neuropsychological and Molecular Data
Independent samples t-tests were conducted in order to test differences between the NT and FX groups in their demographic and neuropsychological test results. Table 1 shows descriptive and inferential statistics on participants' FMR1 genetic, demographic, and neuropsychological test scores. As expected, the FX group showed significantly lower FSIQ than the NT group (mean differences (MD) = −31.49, t(24) = −4.50, P < 0.001). Furthermore, the FX group revealed significantly higher scores on the SRS than the NT group (MD = 40.67, t(17.05) = 4.91, P < 0.001), indicating social deficits in FX participants in our study. Also, analyses on ADAMS scores indicated emotional dysfunction for FX participants compared with NT: 4 of the 5 subscales showed significantly higher scores for FX than NT, namely: ADAMS General Anxiety—MD = 6.15, t(12.35) = 4.824, P < 0.001; Social Avoidance—MD = 6.63, t(11.90) = 4.08, P = 0.002; Depression—MD = 2.8, t(12.75) = 2.17, P = 0.05; and Manic/Hyperactive—MD = 2.65, t(13.10) = 3.06, P = 0.009. Five FX participants (2 full mutation females, 1 mosaic female, and 2 mosaic males) had ADOS scores beyond the autism spectrum cutoff (7).
To examine relationship between scores from neuropsychological assessments and FMR1 gene expression (i.e., AR/% unmethylation) in the FX group, correlation analyses were conducted. There was a marginally significant negative correlation between FMR1 gene expression and scores from the SRS (r = −0.51, P = 0.052), suggesting the greater the degree to which the FMR1 gene is behaving normally, the smaller the deficit in social responsiveness. Among the ADAMS subscales, the social avoidance scale showed significant negative correlation with FMR1 gene expression (r = −0.74, P = 0.006). Finally, there was a positive but nonsignificant correlation between FSIQ and FMR1 gene expression (r = 0.48, P = 0.07) in the FX group.
Eye Tracking Results
Eye tracking data were collected and analyzed for 7/16 FX and 12/16 NT participants. The data for the remaining participants were not collected due to mechanical malfunctions or difficulties with data collection (e.g., problems with corneal reflection or calibration). First, a 3 (stimulus type—Fearful, Happy, Scrambled; within-subject factor) × 2 (group—NT, FX; between-subject factor) mixed ANOVA for the gaze time on the whole face AOI was run. The results showed a main effect of stimulus type (F(2,34) = 6.07, P = 0.006), suggesting that participants looked at emotional face stimuli (i.e., fearful and happy faces) significantly longer than scrambled face stimuli. Neither the main effect of group (F(1,17) = 1.86, P = 0.191) nor the interaction between stimulus type and group (F(2,34) = 0.489, P = 0.617) were significant, suggesting that gaze time to different stimulus types was not affected by FX diagnostic status. Next, to examine whether the gaze time to each AOI (i.e., eye and mouth) in each emotional face (i.e., fearful and happy) was different between the NT and FX groups, an additional 2 (stimulus type—fearful, happy; within-subject factor) × 2 (AOI—eye, mouth; within-subject factor) × 2 (group—NT, FX; between-subject factor) mixed ANOVA was conducted. Only the main effect of AOI was significant (F(1,17) = 6.83, P = 0.018) indicating that participants looked at the eye regions significantly longer than the mouth region regardless of stimulus type or their diagnostic status. The main effect of group (F(1,17) = 0.301, P = 0.591), 2-way interactions between AOI and group (F(1,17) = 1.20, P = 0.288), between the stimulus type and group (F(1,17) = 1.66 P = 0.215), and between AOI and the stimulus type (F(1,17) = 2.43, P = 0.137) as well as a 3-way interaction among AOI, the stimulus type, and the group (F(1,17) = 0.008, P = 0.930) were all nonsignificant. Finally, a set of independent samples t-tests confirmed that the gaze time to each AOI was not significantly different between the NT and the FX groups (Table 2).
Table 2.
Statistics for gaze time for each AOIs and the differences between NT and FX groups
| Contrast | Whole face (ms) |
Eye (ms) |
Mouth (ms) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NT | FX | MD | t (P) | NT | FX | MD | t (P) | NT | FX | MD | t (P) | |
| Fearful (F) | 815 | 639 | 176 | 1.3 (0.22) | 289 | 240 | 48 | 0.55 (0.59) | 117 | 140 | −23 | −0.76 (0.45) |
| Happy (H) | 852 | 630 | 222 | 1.5 (0.14) | 279 | 206 | 73 | 0.82 (0.43) | 125 | 138 | −13 | −0.03 (0.98) |
| Scrambled (S) | 746 | 566 | 179 | 1.2 (0.26) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| F-H | −38 | 9 | −47 | −0.80 (0.43) | 10 | 34 | −25 | −0.85 (0.41) | −13 | 9 | −22 | −1.3 (0.22) |
| F-S | 69 | 73 | −4 | −0.09 (0.93) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
Eye and Mouth AOIs were defined for “Fearful” and “Happy” faces only because “Scrambled” face does not have discrete areas of eye and mouth. “t” and “P” values are from independent samples t-tests between the NT and the FX group.
Total Cerebral and Amygdala Volumes
Descriptive statistics for TCV and amygdala volumes are shown in Table 3. Amygdala volumes for each participant were corrected for their TCV volume. Independent samples t-tests revealed no significant differences between FX and NT groups in TCV (MD = −54 053, t(30) = −1.12, P= 0.270), right amygdala (MD = 0.007, t(30) = 0.88, P= 0.384), nor left amygdala (MD = 0.007, t(30) = 0.97, P= 0.340). Moreover, none of the brain volumes (TCV, left, or right amygdala) showed significant correlation with AR/% unmethylation scores in the FX group (all P > 0.05). Finally, in both groups, none of the correlations between social and emotional deficit scores (SRS and ADAMS) and brain volumes (TCV, left, and right amygdala) were significant (all P > 0.05).
Table 3.
Descriptive statistics for total cerebral volume (TCV) and amygdala volumes
| NT | FX | |
|---|---|---|
| TCV (mm3) | 1 207 299 (113 634) | 1 153 246 (155 378) |
| L Amygdala (mm3) | 1849 (282) | 1841 (263) |
| R Amygdala (mm3) | 1991 (279) | 1981 (324) |
| L Amygdala after correction (%) | 0.15 (0.02) | 0.16 (0.02) |
| R Amygdala after correction (%) | 0.17 (0.02) | 0.17 (0.02) |
Numbers in parentheses indicate standard deviation.
Functional Imaging Results
Within-Group Analyses
WFU Pick Atlas Tool Version 2.4 (Lancaster et al. 1997; 2000; Maldjian et al. 2003) and Talairach Client (Version 1.1; Research Imaging Center, University of Texas Health Science Center, San Antonio, TX) were used to determine the anatomical loci of observed activation in all of the functional imaging results. Table 4 lists brain regions showing significant activation in the NT group for each contrast of interest. As hypothesized, the NT group showed significant bilateral amygdala activation when viewing fearful facial expressions compared with scrambled faces (Fig. 2A). They also showed robust activation in brain regions associated with face processing, social cognition, and emotional processing: namely, the right fusiform gyrus, right insula, left anterior cingulate (AC), left putamen, and bilateral orbitofrontal gyrus. When viewing happy faces compared with scrambled faces, however, the NT group did not show significant activation in the amygdala although they showed significant activation in the bilateral orbitofrontal gyrus and right fusiform gyrus. The NT group also revealed robust activation in the bilateral amygdala (Fig. 2A), bilateral AC, right orbitofrontal gyrus, bilateral insula, and bilateral fusiform gyrus when viewing fearful faces compared with happy faces. There was no suprathreshold cluster found in the NT group for happy faces compared with fearful faces. In contrast to the NT group, participants in the FX group showed no suprathreshold clusters in any of the 4 contrasts of interest.
Table 4.
Brain regions showing significant activation in the NT group
| Contrast | Brain regions | No. of voxels in cluster | Peak coordinates | t Value | Corrected P value of cluster |
|---|---|---|---|---|---|
| Fear > Scrambled | R orbitofrontal gyrus/IFG | 5342 | 28 22 −20 | 7.93 | <0.001 |
| R medial frontal gyrus | 8 52 4 | 6.82 | |||
| L anterior cingulate | −6 16 −10 | 5.56 | |||
| L orbitofrontal gyrus/IFG | −32 32 −6 | 5.34 | |||
| R parahippocampal gyrus | 22 8 −26 | 4.92 | |||
| R IFG | 44 26 −8 | 4.85 | |||
| L rectus/IFG | −14 28 −16 | 4.78 | |||
| R amygdala | 28 −2 −24 | 4.61 | |||
| R insula | 38 8 −4 | 3.37 | |||
| R fusiform gyrus | 1239 | 42 −44 −18 | 6.28 | <0.001 | |
| R inferior temporal gyrus | 48 −68 −6 | 5.95 | |||
| R inferior occipital gyrus | 48 −82 −8 | 5.23 | |||
| R middle occipital gyrus | 42 −74 0 | 5.18 | |||
| R middle temporal gyrus | 40 −58 −2 | 4.81 | |||
| R Cerebellum | 38 −64 −22 | 3.41 | |||
| L orbitofrontal gyrus/IFG | 437 | −24 18 −14 | 6.03 | 0.002 | |
| L amygdala | −24 2 −26 | 5.78 | |||
| L parahippocampal gyrus | −28 6 −22 | 4.35 | |||
| L Putamen | −20 12 2 | 4 | |||
| L caudate | −14 12 10 | 3.68 | |||
| L fusiform gyrus | 275 | −42 −42 −24 | 5.71 | 0.039 | |
| Fear > Happy | L anterior cingulate | 6933 | −10 36 30 | 8.74 | <0.001 |
| R orbitofrontal gyrus | 36 22 −8 | 6.64 | |||
| L putamen | −26 4 8 | 6.56 | |||
| L medial frontal gyrus | −10 34 38 | 6.22 | |||
| R superior frontal gyrus | 16 44 32 | 5.88 | |||
| R insula/IFG | 34 14 −20 | 5.76 | |||
| L superior frontal gyrus | −20 44 34 | 5.75 | |||
| R superior frontal gyrus | 18 44 40 | 5.62 | |||
| L insula | −34 18 −10 | 5.44 | |||
| L superior temporal gyrus | −34 6 −22 | 5.28 | |||
| R amygdala | 22 2 −20 | 5.14 | |||
| R anterior cingulate | 12 28 20 | 5.13 | |||
| L insula/IFG | −30 12 −20 | 5.13 | |||
| L amygdala | −20 2 −20 | 4.12 | |||
| L inferior occipital gyrus | 1084 | −40 −78 −8 | 7.71 | <0.001 | |
| L fusiform gyrus | −42 −40 −24 | 6.11 | |||
| L middle temporal gyrus | −52 −32 −2 | 4.89 | |||
| L middle occipital gyrus | −34 −92 −6 | 4.18 | |||
| L middle temportal gyrus | −52 −24 −14 | 3.38 | |||
| L cerebellum | −14 −42 −24 | 2.69 | |||
| R fusiform gyrus | 250 | 40 −64 −18 | 3.84 | 0.036 | |
| R cerebellum | 32 −64 −16 | 3.79 | |||
| Happy > Scrambled | R medial frontal gyrus | 386 | 4 45 2 | 4.43 | 0.001 |
| R rectal gyrus | 2 40 −24 | 4.3 | |||
| L superior frontal gyrus | −12 56 −2 | 3.74 | |||
| L medial frontal gyrus | −10 50 −10 | 3.5 | |||
| R orbitofrontal gyrus | 20 40 −20 | 3.47 | |||
| L rectal gyrus | −8 32 −24 | 3.46 | |||
| R anterior cingulate | 6 32 −10 | 3.29 | |||
| L orbitofrontal gyrus | −48 42 −8 | 2.77 |
All clusters significant at P <0.05 corrected.
Figure 2.
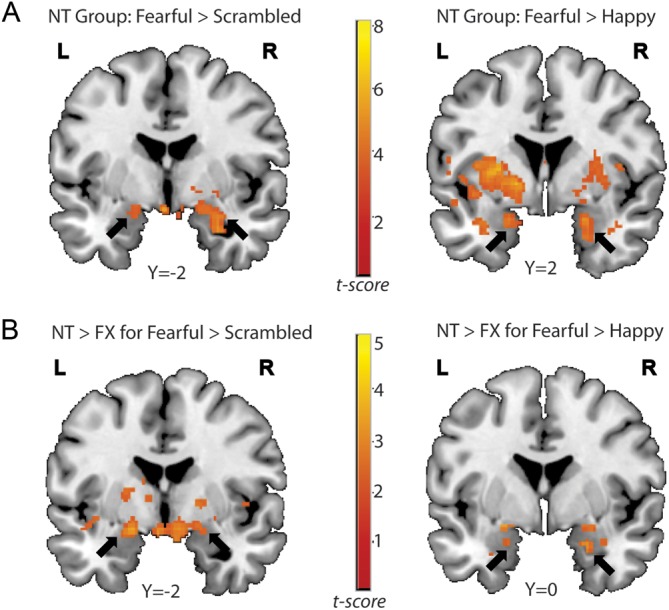
(A) Bilateral amygdala activation in the NT group. Bilateral amygdala regions were more active in response to fearful faces than to scrambled (left) or to happy faces (right) in the NT group. (B) Results from the between-group analyses. The NT group showed significantly greater activation in the bilateral amygdala than the FX group both in the Fearful > Scrambled contrast (left) and in the Fearful > Happy contrast (right).
Between-Group Analyses
As predicted from the within-group analyses, the control group showed significantly greater activation than the FX group in several brain areas associated with emotion and social cognition when viewing fearful faces compared with other types of stimuli (Table 5). Specifically, the NT group revealed greater activity in bilateral amygdala (Fig. 2B), bilateral AC, and bilateral insula compared with the FX group in the contrast of Fearful > Scrambled faces. Also, for the Fearful > Happy contrast, NT participants activated bilateral amygdala (Fig. 2B), left fusiform, bilateral AC, and bilateral insula significantly greater than the FX group. Unlike the response to fearful expressions, the NT group did not show greater activation than the FX group in any of the brain areas related to emotion processing when viewing happy faces compared with other types of stimuli. Instead, the NT group revealed greater activation than the FX group in nonemotion-related areas, such as right precuneus, superior/middle occipital gyrus, and right superior temporal gyrus (STG) in the Happy > Scrambled contrast. Finally, no suprathreshold clusters survived for the Happy>Fearful contrast for the NT>FX. As expected from the within-group analyses, the FX group did not show any suprathreshold clusters in any of the contrasts of interest when compared with the control group.
Table 5.
Brain regions showing greater activation in the NT group than the FX group
| Contrast | Brain regions | No. of voxels in cluster | Peak coordinates | t Value | Corrected P value of cluster |
|---|---|---|---|---|---|
| Fear > Scrambled | R parahippocampal gyrus | 2235 | 16 −10 −12 | 3.47 | 0.002 |
| R putamen | 18 6 −12 | 3.3 | |||
| L amygdala | −18 −2 −12 | 3.22 | |||
| L parahippocampal gyrus | −16 −12 −12 | 3.15 | |||
| R caudate | 12 14 10 | 3.12 | |||
| L caudate | −12 14 10 | 2.93 | |||
| L insula | −40 2 −6 | 2.55 | |||
| R insula | 36 8 8 | 2.31 | |||
| R amygdala | 20 −2 −12 | 2.18 | |||
| L anterior cingulate | 1935 | −24 42 16 | 3.27 | 0.006 | |
| L inferior frontal gyrus | −50 26 8 | 3.11 | |||
| L superior temporal gyrus | −46 16 −14 | 3.05 | |||
| R medial frontal gyrus | 12 52 6 | 2.96 | |||
| R anterior cingulate | 8 42 6 | 2.89 | |||
| R middle frontal gyrus | 32 48 30 | 2.6 | |||
| L superior frontal gyrus | −26 54 18 | 2.57 | |||
| L medial frontal gyrus | −8 58 16 | 2.52 | |||
| Fear > Happy | L fusiform gyrus | 8472 | −38 −40 −24 | 4.7 | <0.001 |
| R caudate | 6 18 14 | 3.83 | |||
| R anterior cingulate | 12 30 18 | 3.75 | |||
| L superior temporal gyrus | −50 6 6 | 3.72 | |||
| R superior temporal gyrus | 58 6 2 | 3.67 | |||
| R insula | 46 8 4 | 3.66 | |||
| L Putamen | −24 4 10 | 3.6 | |||
| R Putamen | 18 8 −8 | 3.41 | |||
| L anterior cingulate | 0 6 22 | 3.34 | |||
| R amygdala | 24 2 −24 | 3.1 | |||
| L insula | −36 6 0 | 3.08 | |||
| L amygdala | −20 2 −20 | 2.29 | |||
| Happy > Scrambled | R precuneus | 2702 | 28 −64 30 | 3.3 | <0.001 |
| R middle occipital gyrus | 32 −84 10 | 3.18 | |||
| R superior temporal gyrus | 64 −54 10 | 2.74 | |||
| R middle temporal gyrus | 46 −64 12 | 2.68 |
All clusters significant at P <0.05 corrected.
Relationships Between FMR1 Gene Expression, Neuropsychological Scores, and Brain Activation
To examine the relationships between FMR1 gene expression, severity of socioanxiety deficits, and fear-related brain activity in the FX group, a series of multiple regression analyses were conducted using AR/% unmethylation in the FX group, and scores from the SRS and the ADAMS general anxiety in both the NT and FX groups. We report regions that corresponded to areas found to be significant in our between-group analyses, which also are associated with emotional processing (i.e., bilateral amygdala, bilateral AC, and bilateral insula). The regions of interest (ROIs) for the bilateral amygdala, AC, and insula were anatomically defined using the WFU Pick Atlas Tool Version 2.4 (Lancaster et al. 1997, 2000; Maldjian et al. 2003), and peak voxels in those ROIs were determined in each contrast of interest (i.e., Fearful > Happy and Fearful > Scrambled) using multiple regression analysis module in SPM5. To estimate how much variance was accounted for by each variable of interest, parameter estimators (β coefficients) were extracted from each ROI using a 4-mm sphere centered on the coordinate of maximum activation in each region and regression analyses were conducted with the variables of interest. To rule out the possible effect of FSIQ on neural activity, FSIQ was used as a nuisance covariate in the multiple regression analyses.
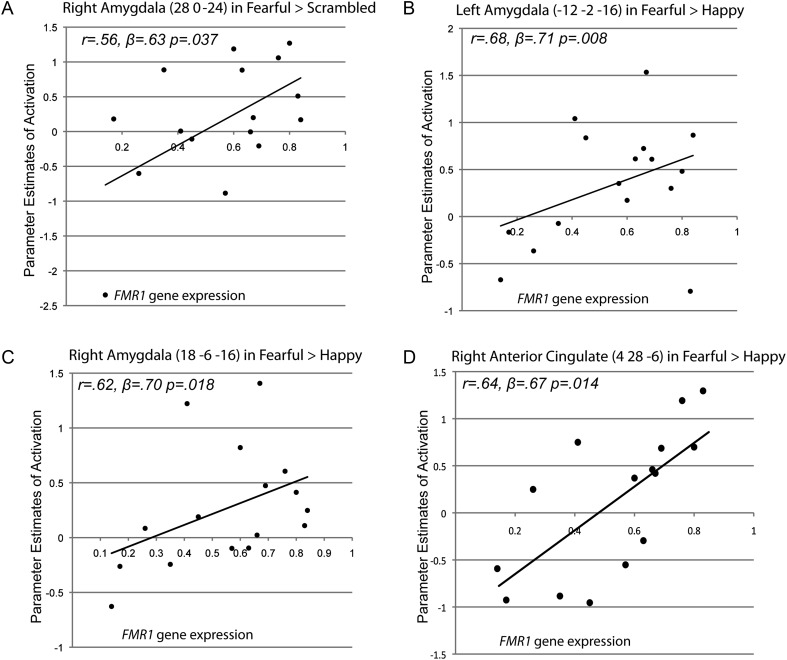
The results from the regression analyses supported our hypothesis about a dosage response of the FMR1 gene on fear-related emotion processing of the amygdala. Specifically, we found a significant positive correlation between AR/% unmethylation and right amygdala activation in the FX group in the Fearful > Scrambled contrast (r = 0.56, β = 0.63, P= 0.037; Fig. 3A), after regressing out the effect of FSIQ. Furthermore, after accounting for variance associated with FSIQ, AR/% unmethylation significantly predicted left and right amygdala activation in the Fearful > Happy contrast in the FX group (r = 0.68, β = 0.71, P= 0.008, and r = 0.62, β = 0.70, P= 0.018, respectively; Fig. 3B and C). No other contrasts showed significant relationships between the FMR1 gene expression and amygdala activation, suggesting that the dose response of FMR1 gene on amygdala activation is specific to the fear-related function of the amygdala. In addition to the amygdala, AR/% unmethylation significantly predicted the right AC activation in the Fearful > Happy contrast in the FX group after regressing out the effect of FSIQ (r = 0.64, β = 0.67, P= 0.014, Fig. 3D). All of the effects remained significant even when the effect of FSIQ was not controlled out.
Figure 3.
(A) A scatter plot showing a positive correlation between FMR1 gene expression and right amygdala activation in the FX group in the Fearful > Scrambled contrast. (B) Scatter plots showing a positive correlation between FMR1 gene expression and activation in the left amygdala in the FX group in the Fearful > Happy contrast. (C) Scatter plots showing a positive correlation between FMR1 gene expression and activation in the right amygdala in the FX group in the Fearful > Happy contrast. (D) Scatter plots showing a positive correlation between FMR1 gene expression and activation in the right anterior cingulate in the FX group in the Fearful > Happy contrast.
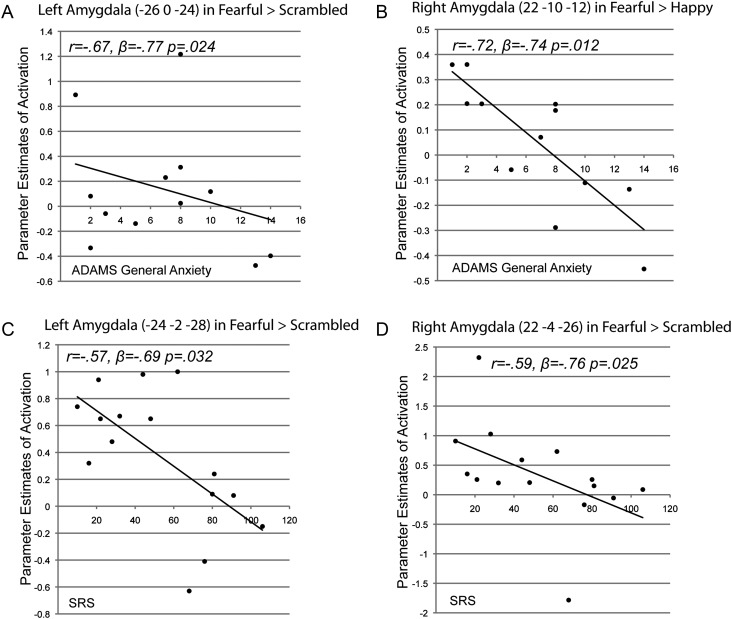
Next, to examine relationship between social and anxiety deficits and fear-related brain activation, a set of regression analyses were conducted using scores from the SRS and the ADAMS general anxiety scale in both NT and FX groups. The analytic procedures were identical to the regression analyses using AR/% unmethylation scores, described above. The results from the regression analyses indicate that the score from the general anxiety scale was a significant predictor for the fear-related amygdala activation in the FX group. That is, a significant negative correlation was found between ADAMS general anxiety scores and left amygdala activation in the Fearful > Scrambled contrast (r = −0.67, β = −0.77, P= 0.024; Fig. 4A) after removing the effect of FSIQ. Furthermore, there was a strong negative correlation between ADAMS general anxiety scores and the right amygdala activation in the Fearful > Happy contrast (r = −0.72, β = −0.74, P= 0.012; Fig. 4B), after accounting for variance associated with FSIQ. Measurement of social deficit also showed significant relationships with the fear-related amygdala activation in the FX group. After regressing out FSIQ, SRS scores were negatively correlated with left and right amygdala activation (r = −0.57, β = −0.69, P= 0.032 and r = −0.59, β = −0.76, P= 0.025, respectively; Fig. 4C and D) in the Fearful > Scrambled contrast, indicating a negative relationship between the severity of social deficits and fear-related amygdala activation. Such relationships between amygdala activation and socioemotional deficit scores were not found in the NT group probably due to a lack of variation in the deficit scores in the control group. Finally, no other significant correlations were found in other brain regions, such as AC and insula, with social and anxiety deficit scores in either group.
Figure 4.
(A) A scatter plot showing a negative correlation between ADAMS general anxiety scores and left amygdala activation in the FX group in the Fearful > Scrambled contrast. (B) A scatter plot showing a negative correlation between ADAMS general anxiety scores and right amygdala activation in the FX group in the Fearful > Happy contrast. (C) A scatter plot showing a negative correlation between SRS scores and right amygdala activation in the FX group in the Fearful > Scrambled contrast. (D) A scatter plot showing a negative correlation between SRS scores and left amygdala activation in the FX group in the Fearful > Scrambled contrast.
Relationship Between Gaze Time to the Eye Region and Brain Activation
Finally, we tested the relationship between the gaze time to the eye region and neural activity in brain regions associated with socioemotional processing (i.e., amygdala, insula, and AC) in the Fearful > Happy contrast in both NT and FX groups. Because our main contrast of interest for the brain activity was the “Fearful > Happy” contrast, corresponding contrast values for the gaze time to the eye regions (i.e., gaze time differences to the eye regions in Fearful > Happy) was used as a regressor and FSIQ was used as a nuisance variable. The analytic procedures were identical to the regression analyses described above. The results from the multiple regression analyses showed no significant relationship between brain activity in any of the ROIs and the gaze time differences to the eye regions in the Fearful > Happy contrast in either the NT or the FX group, indicating that the gaze time differences to the eye regions in the Fearful versus the Happy face conditions did not account for greater neural activity in brain areas associated with the socioemotional processing in the Fearful face condition than the Happy face condition found in our samples, particularly in the NT group. However, the results should be considered with caution given that the eye tracking dataset was incomplete.
Discussion
In the current study, we investigated fear-related amygdala function in girls with the FX full mutation and boys and girls with FX mosaicism using a passive viewing task with emotional face stimuli. Standardized clinical assessment indicated that our FX group had significant socioemotional problems, as well as significantly greater social responsiveness symptoms of autism spectrum disorder, compared with controls. Consistent with previous findings in FXS, the results from volumetric analyses showed no significant differences in TCV between the NT and FX groups (Cohen et al. 2011; Gothelf et al. 2008). In accordance with previous studies (Kates et al. 1997; Hoeft et al. 2008), the size of bilateral amygdala volumes were also strikingly similar between the NT and FX groups in the current study although there has been evidence that young children with FXS shows reduced amygdala volumes compared with their typically developing counterparts (Gothelf et al. 2008; Hazlett et al. 2009). Despite the comparable amygdala size between the NT and FX groups, the results from the functional imaging data in the present study demonstrate abnormal amygdala activation in the FX group when viewing fearful expressions compared with other stimuli. Specifically, compared with the control group, FX participants showed reduced amygdala activity in response to fearful compared with happy or scrambled face stimuli, along with attenuated activation in other brain regions associated with emotion processing and social cognition, namely, insula and ACC. Importantly, the attenuated amygdala activation in the FX group was fear specific because the reduced amygdala activity in the FX group was not observed when participants were viewing happy compared with other types of expressions. That is, unlike the healthy controls, FX participants did not show differentiated amygdala activation specific to the fearful expression compared with the other types of emotion or facial stimuli.
Results from the eye tracking analyses suggest that the different amygdala activation patterns between the groups for fearful compared with other types of facial expressions was not due to different patterns of gaze time to each stimulus type between the 2 groups. Although there was a tendency that the NT group showed longer gaze time to all types of face stimuli than the FX group (F(1,17) = 1.86, P = 0.191), the interaction between the type of stimulus and group did not even approach significance (F(2,34) = 0.489, P = 0.617), indicating that the overall gaze time patterns toward different face stimulus was not significantly different between-groups. Moreover, separate group comparison analyses on the gaze time to discrete areas of face (i.e., the eye and the mouth regions) showed no significant differences between groups on either region for either emotional face stimuli (i.e., fearful and happy). Overall, eye tracking results thus suggest that the increased amygdala activation for the fearful stimulus compared with the other types of stimulus (i.e., happy and scrambled faces) found only in the NT group was not due to the fact that NT participants looked at fearful expression or the eye region in the fearful expression significantly longer than the other types of stimulus compared with the FX group. Results from regression analyses with the gaze time differences to the eye region between the fearful and happy faces and brain activity in the Fearful > Happy contrast further support such interpretation as no relationship was found between fear-specific amygdala activity and the gaze time differences to the eye region in the Fearful > Happy contrast in either groups.
In the present study, we also found significant relationships between FMR1 gene expression, socioemotional deficits, and fear-related amygdala function in children and adolescents on the FX spectrum. In particular, we found that aberrant amygdala function in response to fearful expressions was strongly associated with AR/% unmethylation after controlling for FSIQ, indicating a dose response of FMR1 gene on fear-related amygdala function. In addition, consistent with a previous finding on positive relationship between FMRP levels and AC activation to emotional faces in females with FXS (Hagan et al. 2008), we found a significant positive correlation between right AC activity and AR/% unmethylation in the FX group, suggesting that aberrant activation of the AC may contribute to higher anxiety in individuals on the FX spectrum. Importantly, however, a significant relationship with participant's anxiety and social deficit levels in the FX group was only found with amygdala activity in response to fearful expressions, after accounting for variance associated with FSIQ. Our findings thus indicate significant relationships between abnormal fear-related amygdala functions, FMR1 gene expression, and socioemotional problems in children and adolescents on the FX spectrum.
Recently, our group tested differences in amygdala function for emotional processing in adult males with an FMR1 premutation allele (55–200 CGG repeats). In 2 separate studies, reduced amygdala activation in response to emotional facial expressions was observed in males with the FX premutation compared with matched control participants (Hessl et al. 2007, 2011). Furthermore, aberrant amygdala activation was found to be strongly associated with abnormal elevation of FMR1 mRNA (Hessl et al. 2007) and reduced FMRP (Hessl et al. 2011) in the premutation group. Although these 2 studies are closely related to the current study, there are several key differences between these previous studies and the present study.
First, unlike the previous studies, here we focused on fear-related amygdala functions in FX by comparing different emotional face stimuli. That is, we used both fearful and happy expressions and directly compared amygdala activation elicited by each emotional stimulus. Previous studies on amygdala function in healthy individuals have demonstrated that the amygdala is consistently engaged in response to fearful expression, possibly reflecting its role for detecting dangers in the environment (Adolphs et al. 1994; Morris et al. 1996; Canli et al. 2002). In particular, Canli et al. (2002) found consistent bilateral amygdala activation to fearful faces across participants, whereas the amygdala was variably engaged to happy faces across participants as a function of extroversion. Moreover, using PET, Morris et al. (1996) found that the neural response in the amygdala was significantly greater to fearful as opposed to happy expressions. Recent human and animal studies also support the idea of fear-specific function of the amygdala, by demonstrating amygdala involvement in the acquisition, storage, and expression of conditioned fear learning (Phelps and LeDoux 2005; LeDoux 2007). Based on such findings, and based on the higher anxiety levels seen in children and adolescents with FXS, we focused in the current study on fear-related amygdala function and its relation to FMR1 gene expression and to behavioral characteristics in our FX group. Consistent with previous reports on the amygdala, our imaging results showed robust amygdala activity specific to fearful expressions in healthy controls, and such activity was significantly reduced in the FX group compared with their age-matched controls, indicating abnormal amygdala functions responding to the fear-related stimuli in children and adolescents on the FX spectrum.
Second, unlike previous studies of young adult males with premutation, the current study tested girls with FX full mutation and boys and girls with FX mosaicism, who reveal significantly higher levels of anxiety and greater social dysfunction compared with controls and the majority of premutation carriers. Taking advantage of having FX participants on a continuous spectrum in terms of their genetic profiles, we conducted a regression analysis using the patients’ gene expression and amygdala activation and found a strong dosage response of FMR1 gene on fear-related amygdala function. That is, the greater the extent to which the FMR1 gene is behaving normally in a FX participant, the greater the amygdala activation triggered by fearful expressions. To our knowledge, this is the first study that demonstrates fear-related amygdala dysfunction and its relationship to the FMR1 molecular factors in children and adolescents on the FX spectrum. Furthermore, we also found a negative relationship between social and anxiety deficits in FX participants and their fear-related amygdala activation, indicating a significant relationship between amygdala dysfunction and participants' socioemotional functioning. Together, results from regression analyses suggest that FMR1 gene expression may be a common factor influencing both amygdala dysfunctions and neuropsychiatric symptoms observed in the FX group.
Counter to the current findings on a negative relationship between social and anxiety deficits and fear-specific amygdala activation in the FX group, prior fMRI research in FXS demonstrated a positive relationship between amygdala activation to emotional faces and social deficit scores (i.e., scores for autism characteristics) in individuals with FXS (Dalton et al. 2008). Moreover, several studies on anxiety disorders (e.g., post-traumatic stress disorder, panic disorder, and social anxiety disorder) have found hyperactivity of amygdala in the patient group relative to controls (Etkin and Wager 2007; Shah et al. 2009). Given the increased amygdala activation observed in studies with anxiety disorders and given the previous finding on a positive relationship between social deficit scores and amygdala activity in FXS, the finding of decreased amygdala activation observed in the current sample may seem surprising. However, it is important to note that the positive relationship between the social deficit scores and amygdala activity in the previous study (i.e., Dalton et al. 2008) ceased to be significant after accounting for the effect of FSIQ. Moreover, some studies with anxiety disorder or specific phobia have in fact failed to find amygdala hyperactivity in the patient group (Wright et al. 2003) and others have even found amygdala hypoactivity to fear-related stimuli in the patient group (Pillay et al. 2006). Interestingly, a recent twin study examining risk factors for anxiety disorder and depression indicated both hyper- and hypoactivity in amygdala as a risk factor for anxiety disorder (Wolfensberger et al. 2008). Specifically, the authors demonstrated that amygdala hypoactivity was related to genetic factors of anxiety disorder, whereas amygdala hyperactivity was related to environmental factors of the disorder. Thus, our findings of amygdala hypoactivity in FX participants, who have higher anxiety and whose disorder is defined by their genetic profile, are indeed in accordance with such prior findings. In addition, it is important to note that our task was specifically designed to test fear-related functions of the amygdala in FXS. That is, we found attenuated amygdala activity in the FX group not for the emotional stimuli in general but for the contrasts of Fearful > Scrambled and Fearful > Happy faces. These results suggest that children and adolescents on the FX spectrum exhibit amygdala dysfunction specifically related to its role of being differentially activated by fear-related stimuli to prepare for potential dangers (Canli et al. 2002; LeDoux 2007).
Earlier studies using the FMR1-knockout (KO) mouse, a widely used animal model for FXS, may also be of relevance to the findings of reduced fear-related amygdala activity in the FX group in the current study. For instance, amygdala-dependent fear learning has been shown to be impaired in FMR1-KO mice (Paradee et al. 1999). One of the forms of synaptic plasticity that underlies classical fear conditioning is long-term potentiation (LTP) mediated by metabotropic receptors (mGluR-LTP) at thalamic inputs to the lateral amygdala (LA) (Rodrigues et al. 2002). Interestingly, recent electrophysiological analysis using whole-cell recordings in brain slices found this form of mGluR-LTP to be deficient in LA principal neurons in FMR1-KO mice (Suvrathan et al. 2010). Biochemical analysis suggested that the impaired LTP was due to enhanced internalization of the AMPAR subunit, GluR1. In addition to these postsynaptic deficits, electrophysiological analysis demonstrated a lower probability of pre-synaptic transmitter release at thalamic inputs to LA principal neurons in KO mice (Suvrathan et al. 2010). Taken together, these electrophysiological and biochemical results point to both pre- and postsynaptic deficits underlying impaired amygdala synaptic plasticity (Suvrathan and Chattarji 2011) that may contribute to impaired fear learning in KO mice (Paradee et al. 1999). Such deficits in transmission and plasticity at excitatory synapses in the LA seen in the KO mice may help explain the significant reduction in fear-specific activation in the amygdala seen in FX individuals in our study.
In the present study, we also observed a somewhat paradoxical inverse relationship between fear-specific amygdala activation and anxiety scores in the FX individuals. Although a recent study showed a positive correlation between amygdala activation to emotional faces and social deficit scores in FXS without accounting for the effect of FSIQ (Dalton et al. 2008), our findings are in fact in accordance with other recent findings on negative relationship between neural activity and socioemotional deficit measures in individuals on the FX spectrum, such as studies on adults with FX premutation (Hessl et al. 2007, 2011) and those on adolescents and young adults with FXS (Holsen et al. 2008). Furthermore, recent findings on deficient inhibitory transmission in the amygdala of FMR1-KO mice may also be of significance in this context. According to a recent report (Olmos-Serrano et al. 2010), most interneurons in the basolateral amygdala express FMRP. In addition to a decrease in inhibitory synapse numbers, this study also found a robust reduction in both phasic and tonic inhibition (Olmos-Serrano et al. 2010). Together, these changes led to a lowering of inhibitory tone that in turn enhanced neuronal excitability. Similar to excitatory transmission (Suvrathan and Chattarji 2011; Suvrathan et al. 2010), presynaptic defects also contribute to the reduction in inhibitory transmission (Olmos-Serrano et al. 2010). Such reduction in inhibitory GABAergic tone, a major regulator of the neural circuitry in the amygdala (Ehrlich et al. 2009), is consistent with enhanced anxiety seen in both FXS humans and FMR1-KO mice (Spencer et al. 2005). Thus, disruption of the balance in excitatory and inhibitory transmission in the amygdala could give rise to divergent manifestations of emotional symptoms related to fear and anxiety wherein lower inhibitory tone could be anxiogenic, whereas impaired plasticity at excitatory synapses could specifically impair appropriate encoding of fear-related information.
Despite the novel findings on amygdala dysfunctions in children and adolescents on the FX spectrum, there are some limitations to the current study. First, it is important to note that the FMR1 molecular genetic measures were ascertained from blood samples, and therefore may not necessarily reflect what would have been found in brain tissue. Second, although the absence of interaction between the group, the stimulus type, and AOIs in the eye tracking data suggest that the reduced fear-specific amygdala activation in the FX group cannot be explained by selectively decreased visual fixation on fearful face stimuli or on the eye region in the FX group compared with the NT group, it must be noted that the incomplete eye tracking data from the participants (12 NT and 7 FX), weakens the power of these analyses. Third, we used combined molecular data (i.e., AR and FMR1% unmethylation) as a covariate of interest for our analyses with FMR1 gene expression. Even though the 2 variables are closely related to a person's FMRP production, mosaic males’ FMRP production might be less efficient due to the absence of a normal allele in their X chromosome. For this reason, we conducted an additional analysis using only data from the female FX participants in our study, and found the same pattern of regression results as reported in our study although the effect was weaker than the original results. That is, analysis of female FX participants’ data, alone, revealed a significantly positive correlation between their AR and bilateral amygdala activity in the Fearful > Happy contrast, suggesting that the dose response of the FMR1 gene on the fear-specific function of amygdala was not driven merely by the male mosaic participants. Finally, although it is reasonable to assume that our molecular variables are closely related to FMRP level in the FX group, we cannot definitively conclude that amygdala dysfunction in the FX group is exclusively due to reduced FMRP level without direct measurements of FMRP in our sample. Also, some individuals with FMR1 mosaicism demonstrate elevated FMR1 mRNA, which has been associated with amygdala dysfunction and psychiatric symptoms in our prior studies of men with the FX premutation. Future research with direct FMRP and mRNA measurements in a larger sample will allow us to test whether reduced FMRP is indeed a primary factor for the fear-related amygdala dysfunction in children and adolescents on the FX spectrum.
In conclusion, this study demonstrates novel findings on amygdala dysfunction associated with fear-related processing in children and adolescents on the FX spectrum. Our results indicate that children and adolescents with FX experience a failure to modulate amygdala response to different emotional faces, which in turn results in reduced fear-specific amygdala activation. Also, our findings with FMR1 gene expression suggest a dosage response of the FMR1 gene on fear-specific function of the amygdala in FXS. Finally, the correlation between anxiety and social dysfunction scores and fear-specific amygdala activation suggests that the higher level of anxiety and social deficits in individuals with FX are associated with atypical amygdala activation. Future research utilizing FMRP measures as well as a broader range of FX participants (e.g., adults on the FX spectrum) may be able to elucidate whether the reduced FMRP level is the critical factor for fear-related amygdala dysfunctions in individuals with FXS.
Funding
This work was supported by the National Institutes of Health Grant (R21 MH080025 to S.M.R.).
Notes
Conflict of Interest: None declared.
References
- Abrams MT, Reiss AL, Freund LS, Baumgardner TL, Chase GA, Denckla MB. Molecular-neurobehavioral associations in females with the fragile X full mutation. Am J Med Genet. 1994;51:317–327. doi: 10.1002/ajmg.1320510407. [DOI] [PubMed] [Google Scholar]
- Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. J Neurosci. 1995;15:5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio AR. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci. 2005;6:376–387. doi: 10.1038/nrn1667. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Eliez S, Hedeus M, Menon V, White CD, Moseley M, Reiss AL. White matter tract alterations in fragile X syndrome: preliminary evidence from diffusion tensor imaging. Am J Med Genet B Neuropsychiatr Genet. 2003;118B:81–88. doi: 10.1002/ajmg.b.10035. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Bregman JD, Leckman JF, Ort SI. Fragile X syndrome: genetic predisposition to psychopathology. J Autism Dev Disord. 1988;18:343–354. doi: 10.1007/BF02212191. [DOI] [PubMed] [Google Scholar]
- Canli T, Sivers H, Whitfield SL, Gotlib IH, Gabrieli JD. Amygdala response to happy faces as a function of extraversion. Science. 2002;296:2191. doi: 10.1126/science.1068749. [DOI] [PubMed] [Google Scholar]
- Cohen IL, Fisch GS, Sudhalter V, Wolf-Schein EG, Hanson D, Hagerman R, Jenkins EC, Brown WT. Social gaze, social avoidance, and repetitive behavior in fragile X males: a controlled study. Am J Ment Retard. 1988;92:436–446. [PubMed] [Google Scholar]
- Cohen IL, Sudhalter V, Pfadt A, Jenkins EC, Brown WT, Vietze PM. Why are autism and the fragile-X syndrome associated? Conceptual and methodological issues. Am J Hum Genet. 1991;48:195–202. [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Nichols T, Brignone L, Hall SS, Reiss AL. Insular volume reduction in fragile X syndrome. Int J Dev Neurosci. 2011;29:489–494. doi: 10.1016/j.ijdevneu.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Intergenerational transmission of subthreshold autistic traits in the general population. Biol Psychiatry. 2005;57:655–660. doi: 10.1016/j.biopsych.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Holsen L, Abbeduto L, Davidson RJ. Brain function and gaze fixation during facial-emotion processing in fragile X and autism. Autism Res. 2008;1:231–239. doi: 10.1002/aur.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, Alexander AL, Davidson RJ. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- de Vries BB, Severijnen LA, Jacobs A, Olmer R, Halley DJ, Oostra BA, Willemsen R. FMRP expression studies in blood and hair roots in a fragile X family with methylation mosaics. J Med Genet. 2003;40:535–539. doi: 10.1136/jmg.40.7.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Esbensen AJ, Rojahn J, Aman MG, Ruedrich S. Reliability and validity of an assessment instrument for anxiety, depression, and mood among individuals with mental retardation. J Autism Dev Disord. 2003;33:617–629. doi: 10.1023/b:jadd.0000005999.27178.55. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiat. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Zhang F, Lokey LK, Chastain JL, Lakkis L, Eberhart D, Warren ST. Translational suppression by trinucleotide repeat expansion at FMR1. Science. 1995;268:731–734. doi: 10.1126/science.7732383. [DOI] [PubMed] [Google Scholar]
- Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, Verkerk AJ, Holden JJ, Fenwick RG, Jr, Warren ST, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67:1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Garrett AS, Menon V, MacKenzie K, Reiss AL. Here's looking at you, kid: neural systems underlying face and gaze processing in fragile X syndrome. Arch Gen Psychiatry. 2004;61:281–288. doi: 10.1001/archpsyc.61.3.281. [DOI] [PubMed] [Google Scholar]
- Godler DE, Tassone F, Loesch DZ, Taylor AK, Gehling F, Hagerman RJ, Burgess T, Ganesamoorthy D, Hennerich D, Gordon L, et al. Methylation of novel markers of fragile X alleles is inversely correlated with FMRP expression and FMR1 activation ratio. Hum Mol Genet. 2010;19:1618–1632. doi: 10.1093/hmg/ddq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Furfaro JA, Hoeft F, Eckert MA, Hall SS, O'Hara R, Erba WH, Ringel J, Hayashi KM, Patnaik S, et al. Neuroanatomy of fragile X syndrome is associated with aberrant behavior and the fragile X mental retardation protein (FMRP) Ann Neurol. 2008;63:40–51. doi: 10.1002/ana.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan CC, Hoeft F, Mackey A, Mobbs D, Reiss AL. Aberrant neural function during emotion attribution in female subjects with fragile X syndrome. J Am Acad Child Adolesc Psychiatry. 2008;47:1443–1454. doi: 10.1097/CHI.0b013e3181886e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Amiri K, Cronister A. Fragile X checklist. Am J Med Genet. 1991;38:283–287. doi: 10.1002/ajmg.1320380223. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Lightbody AA, Gerig G, Macfall JR, Ross AK, Provenzale J, Martin A, Reiss AL, Piven J. Teasing apart the heterogeneity of autism: same behavior, different brains in toddlers with fragile X syndrome and autism. J Neurodev Disord. 2009;1:81–90. doi: 10.1007/s11689-009-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Dyer-Friedman J, Glaser B, Wisbeck J, Barajas RG, Taylor A, Reiss AL. The influence of environmental and genetic factors on behavior problems and autistic symptoms in boys and girls with fragile X syndrome. Pediatrics. 2001;108:E88. doi: 10.1542/peds.108.5.e88. [DOI] [PubMed] [Google Scholar]
- Hessl D, Rivera SM, Koldewyn K, Cordeiro L, Adams J, Tassone F, Hagerman PJ, Hagerman RJ. Amygdala dysfunction in men with the fragile X premutation. Brain. 2007;130:404–416. doi: 10.1093/brain/awl338. [DOI] [PubMed] [Google Scholar]
- Hessl D, Rivera SM, Reiss AL. The neuroanatomy and neuroendocrinology of fragile X syndrome. Ment Retard Dev Disabil Res Rev. 2004;10:17–24. doi: 10.1002/mrdd.20004. [DOI] [PubMed] [Google Scholar]
- Hessl D, Tassone F, Loesch DZ, Berry-Kravis E, Leehey MA, Gane LW, Barbato I, Rice C, Gould E, Hall DA, et al. Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premutation. Am J Med Genet B Neuropsychiatr Genet. 2005;139B:115–121. doi: 10.1002/ajmg.b.30241. [DOI] [PubMed] [Google Scholar]
- Hessl D, Wang JM, Schneider A, Koldewyn K, Le L, Iwahashi C, Cheung K, Tassone F, Hagerman PJ, Rivera SM. Decreased fragile x mental retardation protein expression underlies amygdala dysfunction in carriers of the fragile x premutation. Biol Psychiat. 2011;70:859–865. doi: 10.1016/j.biopsych.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, Parthasarathy S, Watson CL, Hall SS, Reiss AL. Fronto-striatal dysfunction and potential compensatory mechanisms in male adolescents with fragile X syndrome. Hum Brain Mapp. 2007;28:543–554. doi: 10.1002/hbm.20406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Lightbody AA, Hazlett HC, Patnaik S, Piven J, Reiss AL. Morphometric spatial patterns differentiating boys with fragile X syndrome, typically developing boys, and developmentally delayed boys aged 1 to 3 years. Arch Gen Psychiatry. 2008;65:1087–1097. doi: 10.1001/archpsyc.65.9.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsen LM, Dalton KM, Johnstone T, Davidson RJ. Prefrontal social cognition network dysfunction underlying face encoding and social anxiety in fragile X syndrome. Neuroimage. 2008;43:592–604. doi: 10.1016/j.neuroimage.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates WR, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL. Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Res. 1997;75:31–48. doi: 10.1016/s0925-4927(97)00019-x. [DOI] [PubMed] [Google Scholar]
- Kenneson A, Zhang F, Hagedorn CH, Warren ST. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet. 2001;10:1449–1454. doi: 10.1093/hmg/10.14.1449. [DOI] [PubMed] [Google Scholar]
- Keysor CS, Mazzocco MM. A developmental approach to understanding Fragile X syndrome in females. Microsc Res Tech. 2002;57:179–186. doi: 10.1002/jemt.10070. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Summerln JL, Rainey L, Freitas CS, Fox PT. The Talairach Daemon, a database server for Talairach Atlas Labels. NeuroImage. 1997;5:S633. [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Curr Biol. 2007;17:R868–874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Lightbody AA, Reiss AL. Gene, brain, and behavior relationships in fragile X syndrome: evidence from neuroimaging studies. Dev Disabil Res Rev. 2009;15:343–352. doi: 10.1002/ddrr.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesch DZ, Huggins RM, Hagerman RJ. Phenotypic variation and FMRP levels in fragile X. Ment Retard Dev Disabil Res Rev. 2004;10:31–41. doi: 10.1002/mrdd.20006. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, Schopler E. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fmri data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. (WFU Pickatlas, version 2.4) [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Olmos-Serrano JL, Paluszkiewicz SM, Martin BS, Kaufmann WE, Corbin JG, Huntsman MM. Defective GABAergic neurotransmission and pharmacological rescue of neuronal hyperexcitability in the amygdala in a mouse model of fragile X syndrome. J Neurosci. 2010;30:9929–9938. doi: 10.1523/JNEUROSCI.1714-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradee W, Melikian HE, Rasmussen DL, Kenneson A, Conn PJ, Warren ST. Fragile X mouse: strain effects of knockout phenotype and evidence suggesting deficient amygdala function. Neuroscience. 1999;94:185–192. doi: 10.1016/s0306-4522(99)00285-7. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pillay SS, Gruber SA, Rogowska J, Simpson N, Yurgelun-Todd DA. fMRI of fearful facial affect recognition in panic disorder: the cingulate gyrus-amygdala connection. J Affect Disord. 2006;94:173–181. doi: 10.1016/j.jad.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Poline JB, Worsley KJ, Evans AC, Friston KJ. Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage. 1997;5:83–96. doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
- Primerano B, Tassone F, Hagerman RJ, Hagerman P, Amaldi F, Bagni C. Reduced FMR1 mRNA translation efficiency in fragile X patients with premutations. RNA. 2002;8:1482–1488. [PMC free article] [PubMed] [Google Scholar]
- Puig-Antich J, Chambers W. The schedule of affective disorders and schizophrenia for school-aged children. New York: New York Psychiatric Instititute; 1978. [Google Scholar]
- Reiss AL, Dant CC. The behavioral neurogenetics of fragile X syndrome: analyzing gene-brain-behavior relationships in child developmental psychopathologies. Dev Psychopathol. 2003;15:927–968. doi: 10.1017/s0954579403000464. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Freund L. Behavioral phenotype of fragile X syndrome: DSM-III-R autistic behavior in male children. Am J Med Genet. 1992;43:35–46. doi: 10.1002/ajmg.1320430106. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Bauer EP, Farb CR, Schafe GE, LeDoux JE. The group I metabotropic glutamate receptor mGluR5 is required for fear memory formation and long-term potentiation in the lateral amygdala. J Neurosci. 2002;22:5219–5229. doi: 10.1523/JNEUROSCI.22-12-05219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, Lammers CR, Reiss AL, Amaral DG. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24:6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SK, Young AW, Calder AJ, Hellawell DJ, Aggleton JP, Johnson M. Impaired auditory recognition of fear and anger following bilateral amygdala lesions. Nature. 1997;385:254–257. doi: 10.1038/385254a0. [DOI] [PubMed] [Google Scholar]
- Shah SG, Klumpp H, Angstadt M, Nathan PJ, Phan KL. Amygdala and insula response to emotional images in patients with generalized social anxiety disorder. J Psychiatry Neurosci. 2009;34:296–302. [PMC free article] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, Paylor R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav. 2005;4:420–430. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- Suvrathan A, Chattarji S. Fragile X syndrome and the amygdala. Curr Opin Neurobiol. 2011;21:509–515. doi: 10.1016/j.conb.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Suvrathan A, Hoeffer CA, Wong H, Klann E, Chattarji S. Characterization and reversal of synaptic defects in the amygdala in a mouse model of fragile X syndrome. Proc Natl Acad Sci U S A. 2010;107:11591–11596. doi: 10.1073/pnas.1002262107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Ikle DN, Dyer PN, Lampe M, Willemsen R, Oostra BA, Taylor AK. FMRP expression as a potential prognostic indicator in fragile X syndrome. Am J Med Genet. 1999;84:250–261. [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet. 2000;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone F, Pan R, Amiri K, Taylor AK, Hagerman PJ. A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile X (FMR1) gene in newborn and high-risk populations. J Mol Diagn. 2008;10:43–49. doi: 10.2353/jmoldx.2008.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike RL, Hagen EP, Sattler JM. The Stanford-Binet intelligence scale: guide for administering and scoring. Chicago, IL: Riverside; 1986. [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C, Hoeft F, Garrett AS, Hall SS, Reiss AL. Aberrant brain activation during gaze processing in boys with fragile X syndrome. Arch Gen Psychiatry. 2008;65:1315–1323. doi: 10.1001/archpsyc.65.11.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for children. 4th ed. San Antonio, TX: Harcourt Assessment; 2004. [Google Scholar]
- Wechsler D. WAIS-III administration and scoring manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- Wolfensberger SP, Veltman DJ, Hoogendijk WJ, Boomsma DI, de Geus EJ. Amygdala responses to emotional faces in twins discordant or concordant for the risk for anxiety and depression. NeuroImage. 2008;41:544–552. doi: 10.1016/j.neuroimage.2008.01.053. [DOI] [PubMed] [Google Scholar]
- Wright CI, Martis B, McMullin K, Shin LM, Rauch SL. Amygdala and insular responses to emotionally valenced human faces in small animal specific phobia. Biol Psychiatry. 2003;54:1067–1076. doi: 10.1016/s0006-3223(03)00548-1. [DOI] [PubMed] [Google Scholar]