Abstract
Previous studies have suggested dopamine to be involved in error monitoring/processing, possibly through impact on reinforcement learning. The current study tested whether methylphenidate (MPH), an indirect dopamine agonist, modulates brain and behavioral responses to error, and whether such modulation is more pronounced in cocaine-addicted individuals, in whom dopamine neurotransmission is disrupted. After receiving oral MPH (20 mg) or placebo (counterbalanced), 15 healthy human volunteers and 16 cocaine-addicted individuals completed a task of executive function (the Stroop color word) during functional magnetic resonance imaging (fMRI). During MPH, despite not showing differences on percent accuracy and reaction time, all subjects committed fewer total errors and slowed down more after committing errors, suggestive of more careful responding. In parallel, during MPH all subjects showed reduced dorsal anterior cingulate cortex response to the fMRI contrast error>correct. In the cocaine subjects only, MPH also reduced error>correct activity in the dorsolateral prefrontal cortex (controls instead showed lower error>correct response in this region during placebo). Taken together, MPH modulated dopaminergically innervated prefrontal cortical areas involved in error-related processing, and such modulation was accentuated in the cocaine subjects. These results are consistent with a dopaminergic contribution to error-related processing during a cognitive control task.
Keywords: anterior cingulate cortex, cerebellum, cocaine addiction, dopamine, dorsolateral prefrontal cortex, executive function, fMRI, methylphenidate, norepinephrine, Stroop
Introduction
Error monitoring is a core executive function that allows for successful identification and correction of discrepancies between an intended and executed response, vital for flexible adaptation in complex, dynamic environments (Taylor et al. 2007). Recent efforts aimed at uncovering the neurochemistry of error monitoring have pointed to an important contribution of dopamine (Jocham and Ullsperger 2009; Barnes et al. 2011). This contribution has been suggested by previous studies that have used dopaminergic challenges to modulate error-related processing [e.g., error-related negativity (de Bruijn et al. 2004; Zirnheld et al. 2004)], or that have included relevant disease states that impact dopamine neurotransmission [e.g., Parkinson's disease (Stemmer et al. 2007), attention deficit/hyperactivity disorder (ADHD) (Shiels and Hawk 2010), or drug addiction (Hester et al. 2009)]. The current study combined these approaches, employing a novel, joint pharmacological manipulation/disease state design.
Our first goal was to test whether methylphenidate (MPH), an indirect dopamine and norepinephrine agonist [that blocks both respective transporters (Kuczenski and Segal 1997)], modulates brain and behavioral responses to error on a classical executive function task. After receiving oral MPH or placebo, human volunteers performed the Stroop color-word task (Stroop 1935) while undergoing event-related functional magnetic resonance imaging (fMRI) (Leung et al. 2000). The Stroop color-word task consistently activates dopaminergically innervated prefrontal cortex (PFC) regions that mediate error-related processing, especially the anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex (DLPFC). Although the precise functions of these regions continue to be debated, the dorsal ACC (dACC) has been implicated in performance monitoring (van Veen and Carter 2002), conflict monitoring (Egner et al. 2008), error detection (Swick and Turken 2002), and the prediction of posterror adaptation (slowing) (Danielmeier et al. 2011), whereas the DLPFC has been implicated in nonemotional conflict resolution (Egner et al. 2008) and the implementation of cognitive control (Kerns et al. 2004). MPH is thought to decrease the energy requirements needed to perform a cognitive task (i.e., by enhancing signal-to-noise ratio of neuronal activity) (Swanson et al. 2011). Indeed, MPH has produced PFC signal reductions during executive function tasks when healthy controls underwent positron emission tomography (Mehta et al. 2000; Volkow et al. 2008) or fMRI (Dodds et al. 2008; Marquand et al. 2011), and when individuals with ADHD underwent fMRI (Schweitzer et al. 2004; Peterson et al. 2009). Therefore, we hypothesized that MPH, received during performance of the Stroop color-word task, would enhance task performance (reduce errors and increase posterror slowing) and reduce PFC (e.g., dACC and DLPFC) response to error.
Our second goal was to test whether MPH exerts more pronounced effects in individuals with potential compromises in dopamine functioning. For this purpose, in addition to healthy individuals we studied a sample of cocaine-addicted individuals, who show drug-mediated decreases in dopamine release and receptor availability (Volkow et al. 2004), and functional impairments in PFC areas innervated by dopamine (Goldstein and Volkow 2011). Given dopamine's inverted U-shaped effects on cognition (e.g., Seamans and Yang 2004; Arnsten 2009), we hypothesized stronger response to MPH in the cocaine subjects. Also supporting this hypothesis are studies of individuals with impulsivity, ADHD, and cocaine use disorder (CUD). In one prior study of healthy individuals, trait impulsivity, associated with decreased levels of striatal dopamine D2 receptors (Lee et al. 2009) and decreased activity in PFC (Volkow et al. 2011), modulated response to MPH (the higher the impulsivity, the lower the reversal learning errors during MPH compared with placebo) (Clatworthy et al. 2009). Similarly, MPH more prominently affected neural response (during tasks of inhibitory control/executive function) in ADHD subjects than in healthy controls (Rubia, Halari, Cubillo et al. 2011; Rubia, Halari, Mohammad et al. 2011); a recent study showed that treatment-naïve ADHD subjects with the greatest response in a number of subcortical and cortical regions (including DLPFC) during an MPH challenge were also the ones most likely to show inattention symptom improvement with a regimented treatment dose of MPH (Volkow et al. 2012). In CUD, intravenous MPH improved inhibitory control (decreased stop signal reaction time [RT] during the stop signal task) as associated with higher DLPFC fMRI response (Li et al. 2010); oral MPH also selectively increased fMRI response in CUD (compared with controls) in the ACC during an emotionally salient cognitive control task (Goldstein et al. 2010).
Materials and Methods
Subjects
Subjects were 15 healthy human volunteers and 16 individuals with CUD, recruited from advertisements in local newspapers and by word of mouth. All were right-handed and native English speakers, and were able to understand all study procedures and to provide written consent in accordance with Stony Brook University's Institutional Review Board. All subjects underwent a full physical examination and clinical interview (see Supplementary Information for interview components). CUD were current users of cocaine, meeting criteria for current cocaine dependence but otherwise healthy and not currently taking medications. Although 1 subject also met criteria for current heroin dependence (a factor we inspected in Supplementary Information), the primary drug of choice in all CUD was cocaine. All other comorbidities (except for nicotine dependence: N = 12; Table 1) were in fully sustained remission, including marijuana abuse (N = 5), alcohol use disorders (N = 8), and heroin dependence (N = 1). Exclusion criteria were 1) history of head trauma or loss of consciousness (>30 min) or other neurological disease of central origin (including seizures); 2) abnormal vital signs at time of screening; 3) history of major medical conditions, encompassing cardiovascular (high blood pressure, cardiac arrhythmias apart from sinus bradycardia, or an abnormal electrocardiography at time of screening), endocrinological (including metabolic), oncological, or autoimmune diseases; 4) history of major psychiatric disorder (other than substance abuse or dependence for CUD and/or nicotine dependence for both groups); 5) pregnancy (urine test); 6) contraindications to MRI; 7) history of glaucoma; and 8) except for cocaine in CUD, positive urine screens for psychoactive drugs or their metabolites (amphetamine or methamphetamine, phencyclidine, benzodiazepines, cannabis, opiates, barbiturates, or inhalants). Thus, subjects did not test positive for any psychoactive drugs other than cocaine on study day. Positive urine screens for cocaine did not differ by medication administration (10/16 CUD tested positive on MPH day; 8/16 CUD tested positive on placebo day; P > 0.3).
Table 1.
Demographics and drug use of all study subjects (16 cocaine subjects and 15 controls)
| Test (between) | Cocaine (N = 16) | Control (N = 15) | |
|---|---|---|---|
| Gender: male/female | χ2 = 0.0 | 15/1 | 14/1 |
| Race: African-American / Caucasian/other | χ2 = 2.8 | 14/2/0 | 10/3/2 |
| Age (years) | t = 2.7* | 46.3 ± 7.8 | 38.9 ± 7.1 |
| Education (years) | t = 2.0 | 12.8 ± 1.8 | 13.9 ± 1.2 |
| Verbal IQ: Wide Range Achievement Test III—Reading Scale Grade Equivalent (Wilkinson 1993) | t = 1.1 | 11.7 ± 1.9 | 12.4 ± 1.3 |
| Nonverbal IQ: WASI—Matrix Reasoning Scale (Wechsler 1999) | t = 0.2 | 9.9 ± 3.0 | 9.7 ± 3.7 |
| Depression: Beck Depression Inventory II (Beck et al. 1996) | Z = −3.1* | 8.2 ± 5.4 | 2.4 ± 4.1 |
| Socioeconomic status: Hollingshead index | t = 0.1 | 36.3 ± 8.5 | 36.5 ± 8.9 |
| Cigarette smokers (current or past/nonsmokers) | χ2 = 11.6* | 13/3 | 3/12 |
| Daily cigarettes (current smokers: N = 12/0) | 8.1 ± 3.7 | — | |
| Time since last use (within 4 h/>4 h): Methylphenidate | Placebo | 4/8 | 2/10 | — | |
| Cocaine age of onset (years) | 27.4 ± 7.2 | — | |
| Cocaine duration of use (years) | 15.6 ± 8.3 | — | |
| Cocaine past month use: days/week | 3.0 ± 2.3 | — | |
| Cocaine current abstinence (min–max, median) | 0–25, 2 | — | |
| Cocaine urine status (yes/no): Methylphenidate | Placebo | 10/16 | 8/16 | ||
| Severity of Dependence scale (0–15): Methylphenidate | Placeboa | 7.4 ± 2.4 | 6.6 ± 2.8 | — | |
| Withdrawal symptoms: 18-item CSSA (0–126): Methylphenidate | Placebo | 21.0 ± 8.4 | 16.9 ± 8.5 | — | |
| Cocaine craving: 5-item Questionnaire (0–45): methylphenidate | Placeboa | 25.4 ± 11.2 | 18.9 ± 11.8 | — |
All demographics were completed before or after all methylphenidate procedures, therefore not reflective of any methylphenidate effects; WASI, Wechsler Abbreviated Scale of Intelligence; CSSA, Cocaine Selective Severity Assessment Scale; values are frequencies or means ± standard deviation.
aSignificantly different between methylphenidate and placebo sessions.
*P < 0.01.
Methylphenidate Administration
Oral MPH (20 mg) or placebo (lactose) was administered in a counterbalanced fashion across all subjects on 2 separate study days. We aimed for 1 week in between scans to minimize carry-over effects, and all subjects (but especially CUD) were scheduled as close to this target date as possible. Only 3 subjects completed their second scanning session before the 7-day benchmark (1 CUD: 6 days; 2 controls: 5 days, 6 days). Subjects underwent fMRI 90-min postmedication administration, within the window of peak MPH effects (60–120 min) (Volkow et al. 1998) (see Supplementary Information for additional considerations of MPH administration such as blinding procedures, and description and analyses of cardiovascular functioning and self-reported ratings of MPH effects). Study groups did not differ in number of days between the MPH and placebo scans (CUD: 9.4 ± 4.6; control: 14.1 ± 13.1; P > 0.2).
Task
Subjects performed an event-related Stroop color-word task, adapted from previously published neuroimaging studies that have used a similar design (Leung et al. 2000; Peterson et al. 2002; Blumberg et al. 2003; Potenza et al. 2003; Harrison et al. 2005; Brewer et al. 2008; Devito et al. 2012; Moeller et al. 2012). During this task, subjects pressed for ink color of color words printed in their congruent (e.g., “blue” in blue ink) or incongruent (e.g., “blue” in red ink) colors. After training, which consisted of at least 2 complete task runs with different color randomizations, once outside and once inside the scanner, subjects completed 3 runs of the Stroop color-word task while undergoing fMRI. A continuous series of color words (red, blue, yellow, and green) was presented through fMRI compatible goggles. Ninety-four percent of words were displayed in their congruent colors, while 6% of words were displayed in their incongruent colors (Fig. 1). With these task parameters, each task run contained 12 incongruent events, therefore totaling 36 such events per subject. The total number of task errors across all subjects (i.e., summed across both the congruent and incongruent trials, and averaged across MPH and placebo days and all 3 task runs) was 16.7 ± 7.8 (see Table 2 for additional details). Owing to medication time constraints (i.e., to ensure peak effects of MPH throughout), our task did not include a neutral control condition [e.g., rows of colored strings of Xs (Roesch-Ely et al. 2005)]. Consequently, we were unable in this study to inspect for potential facilitation effects [i.e., a decrease in RT that occurs when color words are presented in their congruent colors], although it has been noted that such facilitation effects are not uniformly observed (MacLeod 1991). To avoid a priming effect, no word or color of an incongruent stimulus mirrored the preceding congruent color word; otherwise, stimuli were presented randomly and were used in all possible combinations to form the incongruent stimuli. Incongruent events were pseudorandomly spaced by at least 5 stimuli (range: 5–31 stimuli apart; median: 14 stimuli). Each word was presented for 1300 ms, with an intertrial interval of 350 ms. Each run lasted 5.6 min (4.3 min for all stimuli, 1.2 min for all preceding fixation slides, and 3.2 s for a terminating fixation slide) (Fig. 1). Remuneration for task completion was fixed to $25. Accuracy and RT, and postconflict and posterror slowing were collected using E-prime (see Supplemental Information on how the latter 2 variables were calculated).
Figure 1.
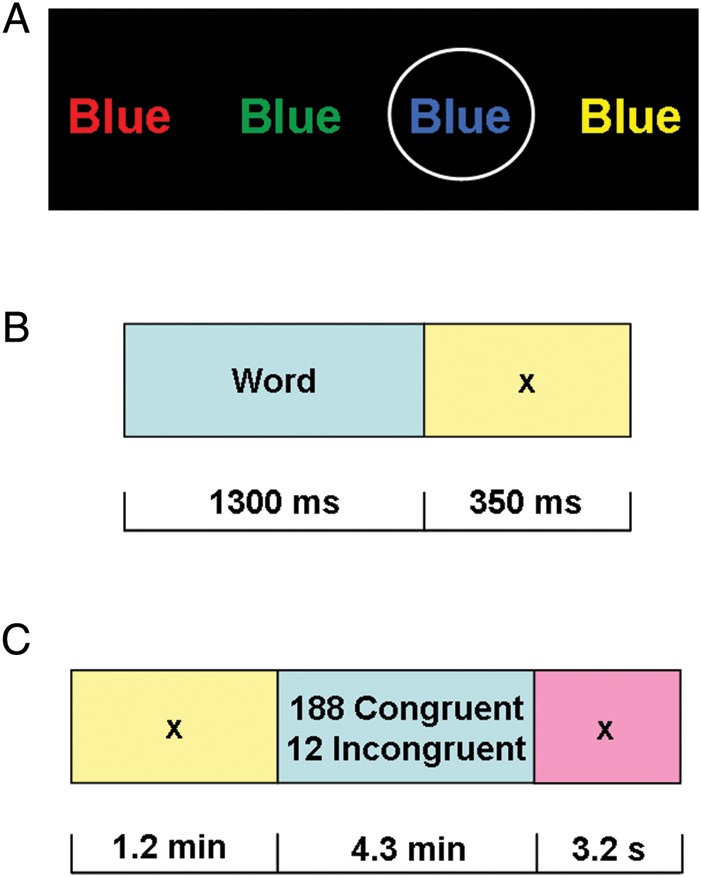
fMRI Stroop color-word task. Subjects pressed for ink color as quickly and accurately as possible (performance was recorded throughout). fMRI response to conflict trials (all incongruent), error trials (all error), and their interaction were each compared with active baselines (all congruent trials, all correct trials, and congruent correct trials, respectively). (A) Examples of color words: the circled (blue) stimulus is congruent; all others are incongruent. (B) Individual trial, comprised of a 1300-ms color-word stimulus and 350-ms interstimulus interval. (C) Individual run, comprised 200 individual trials and a 3200-ms interval to separate runs.
Table 2.
Performance on the Stroop color-word fMRI task across all study subjects and across 3 task runs
| t (between) | Cocaine (N = 16) | Control (N = 15) | |
|---|---|---|---|
| Methylphenidate | |||
| Accuracy (raw errors) | |||
| Congruent (max per run: 188) | min, max | 0.8 | 8.3 ± 4.7a* | 1.0, 17.0 | 9.8 ± 6.1 | 0.3, 20.0 |
| Incongruent (max per run: 12) | min, max | −1.2 | 5.0 ± 2.7 | 0.7, 10.7 | 3.9 ± 2.5 | 0.7, 9.3 |
| (Incongruent minus congruent): interference | min, max | −1.5 | −3.3 ± 4.9a* | −14.7, 3.0 | −5.9 ± 5.0 | −17.0, 0.3 |
| Reaction time, all trials (ms) | |||
| Congruent | −0.3 | 671.4 ± 56.5a* | 679.7 ± 81.0 |
| Incongruent | 1.4 | 933.3 ± 106.4 | 885.4 ± 85.6 |
| (Incongruent minus congruent): interference | 2.0 | 261.9 ± 80.4 | 205.7 ± 77.3 |
| Reaction time, correct trials only (ms) | |||
| Congruent | −0.3 | 671.4 ± 56.3a* | 679.5 ± 81.5 |
| Incongruent | 1.7 | 946.4 ± 111.5 | 885.5 ± 82.7 |
| (Incongruent minus congruent): interference | 2.3* | 275.1 ± 89.9 | 206.1 ± 78.6 |
| Behavior adjustment (ms) | |||
| Postconflict adjustment | 1.1 | 961.5 ± 204.3 | 891.4 ± 147.3 |
| Posterror adjustment congruent trials | −0.8 | 74.4 ± 67.8 | 95.8 ± 79.1 |
| Posterror adjustment all trials | −0.5 | 75.8 ± 73.5 | 90.0 ± 83.0 |
| Placebo | |||
| Accuracy (raw errors) | |||
| Congruent (max per run: 188) | min, max | −1.0 | 16.4 ± 11.7b* | 1.5, 37.7 | 12.9 ± 7.8 | 2.0, 27.0 |
| Incongruent (max per run: 12) | min, max | −1.0 | 5.7 ± 3.3 | 0.3, 11.0 | 4.6 ± 2.4 | 0.7, 8.3 |
| (Incongruent minus congruent): interference | min, max | 0.8 | −10.7 ± 9.7b* | −26.7, 2.7 | −8.3 ± 6.3 | −20.3, 1.3 |
| Reaction time, all trials (ms) | |||
| Congruent | 0.7 | 704.7 ± 79.5b* | 683.0 ± 85.5 |
| Incongruent | 1.1 | 935.6 ± 106.0 | 896.0 ± 85.3 |
| (Incongruent minus congruent): interference | 0.6 | 230.9 ± 89.7 | 213.3 ± 79.6 |
| Reaction time, correct trials only (ms) | |||
| Congruent | 0.8 | 705.9 ± 81.4b* | 682.0 ± 86.0 |
| Incongruent | 1.3 | 943.7 ± 111.6 | 895.6 ± 84.0 |
| (Incongruent minus congruent): interference | 0.8 | 237.9 ± 99.4 | 213.6 ± 78.6 |
| Behavior adjustment ms) | |||
| Postconflict adjustment | 0.3 | 944.3 ± 170.3 | 928.5 ± 75.4 |
| Posterror adjustment congruent trials | 0.6 | 56.5 ± 75.2 | 42.3 ± 51.9 |
| Posterror adjustment all trials | −1.0 | 30.5 ± 115.8 | 65.2 ± 67.1 |
Numbers are M ± SD, reflecting averages across 3 task runs (e.g., each subject had 12 × 3 = 36 incongruent events that contributed to the respective incongruent task performance average); error minimums and maximums are also presented given their centrality to the fMRI results; the relatively high postconflict adjustment scores likely reflect the fact that “iI” events did not occur in this task (see Methods section).
aDifferent from the parallel variable during placebo.
bDifferent from the parallel variable during methylphenidate.
*P < 0.05.
MRI Data Acquisition
Magnetic resonance imaging scanning was performed on a 4T whole-body Varian/Siemens MRI scanner. The blood-oxygenation-level-dependent (BOLD) fMRI responses were measured as a function of time using a T2*-weighted single-shot gradient-echo planar sequence (TE/TR = 20/1600 ms, 4 mm slice thickness, 1-mm gap, typically 33 coronal slices, 20-cm FOV, 64 × 64 matrix size, 90°-flip angle, 200-kHz bandwidth with ramp sampling, 207 time points, and 4 dummy scans to avoid nonequilibrium effects in the fMRI signal). Padding was used to minimize subject motion, which was also monitored immediately after each fMRI run (Caparelli et al. 2003). Earplugs (28 dB sound attenuation; Aearo Ear TaperFit 2; Aearo Company) and headphones (30 dB sound attenuation; Commander XG MRI Audio System, Resonance Technology, Inc.) were used to minimize the interference effect of scanner noise during fMRI (Tomasi et al. 2005). Anatomical images were collected using a T1-weighted 3D-MDEFT (3D modified driven equilibrium Fourier transform) sequence (Lee et al. 1995) and a modified T2-weighted hyperecho sequence (Hennig and Scheffler 2001), and were reviewed by a neurologist to rule out gross morphological abnormalities that could affect the BOLD-fMRI signal.
MRI Data Processing
Analyses were performed with Statistical Parametric Mapping (SPM2) (Wellcome Trust Centre for Neuroimaging, London, UK). Image reconstruction was performed using an iterative phase correction method that produces minimal signal-loss artifacts in echo-planar images (Caparelli and Tomasi 2008). A six-parameter rigid body transformation (3 rotations, 3 translations) was used for image realignment and correction of head motion. Criteria for acceptable motion were 2-mm displacement and 2° rotation. After implementing these criteria, CUD had data available from 5.0 ± 1.2 scans; controls subjects had data available from 4.8 ± 1.0 scans [between group t(29) = 0.5, P > 0.6; max scans per subject was 6: 2 medication conditions × 3 task runs]. The realigned datasets were spatially normalized to the standard stereotactic space of the Montreal Neurological Institute (MNI) using a 12-parameter affine transformation (Ashburner et al. 1997) and a voxel size of 3 × 3 × 3 mm. An 8-mm full-width-half-maximum Gaussian kernel was used to spatially smooth the data.
BOLD-fMRI Analyses
Three general linear models (Friston et al. 1995), each with six motion regressors (3 translation and 3 rotation) and up to three task conditions (incongruent correct events, congruent error events, and/or incongruent error events) convolved with a canonical hemodynamic response function and low-pass and high-pass (cut-off frequency: 1/90 s) filters, were used to calculate individual BOLD-fMRI maps. Contrast maps were calculated for all available runs for all subjects (who met all motion criteria as described above), with each contrast reflecting percent signal change from baseline. Because of a short intertrial interval of 350 ms (Fig. 1), the baselines of these 3 models consisted of all the task events that were not modeled in the relevant design matrices, and at minimum included the fourth (and most frequent) type of task event (congruent correct events). Although the correct congruent trials were included in the error variance (i.e., serving as the implicit baseline), they nonetheless account for the correct congruent effect.
Design Matrix 1 included 1 regressor collapsed across both incongruent trials (Incongruent Correct and Incongruent Incorrect), leaving out both congruent trials (Congruent Correct and Congruent Incorrect) to serve as the baseline. Design Matrix 2 included 1 regressor collapsed across both error trials (Congruent Incorrect and Incongruent Incorrect), leaving out both correct trials (Congruent Correct and Incongruent Correct) to serve as the baseline. Design Matrix 3 included 3 regressors: Incongruent Correct trials, Congruent Incorrect trials, and Incongruent Incorrect trials, leaving out the Congruent Correct trials to serve as the baseline. Using these 3 separate design matrices, we calculated the following first level main and interaction contrasts. 1) Using Design Matrix 1, we tested for a main effect of “congruency”, defined as (Incongruent Error + Incongruent Correct) − (Congruent Error + Congruent Correct). 2) Using Design Matrix 2, we tested for a main effect of “correctness”, defined as (Incongruent Error + Congruent Error) − (Incongruent Correct + Congruent Correct). 3) Using Design Matrix 3, we tested for a “correctness × congruency” interaction, defined as [(Incongruent Correct − Congruent Correct) − (Incongruent Error − Congruent Correct)] + (Congruent Error − Congruent Correct). Note that due to the active baselines for each of these contrasts, a BOLD signal below zero does not necessarily reflect deactivations. At the second level, we tested how each of these first level contrasts differed as a function of medication and group; for this purpose, we estimated 3 separate 2 (medication: MPH, placebo) × 2 (group: CUD, control) mixed analyses of variance (ANOVAs) at the whole-brain level in SPM.
Brain activation clusters were corrected for multiple comparisons using the continuous random field calculation (Adler 1981). In the present study, the random field calculation was based on the expected Euler characteristics of the regions above a Pcorr < 0.05 threshold [false discovery rate (FDR), voxel level corrected], with 5 contiguous voxels; we also flagged the voxels that were significant using the more conservative FWE voxel-level correction. Because we had a priori hypotheses about the ACC and DLPFC, we used region of interest (ROI) analyses for these regions (with the same significance criteria). These ROIs were 20-mm spheres around peak coordinates of the ACC and DLPFC, taken from the study that guided our task development (Leung et al. 2000) and therefore independent from the current results. Our 4-T MRI scanner provides excellent signal in these regions, increasing confidence that we were able to reliably estimate the magnitude of the error>correct and incongruent > congruent BOLD signals with the current number of respective trials. For these 2 ROIs, across all subjects and medication conditions, the average signal-to-noise ratio was 235.3 ± 48.0 (range: 107.0–380.4). For all analyses, anatomical specificity was corroborated with the MRIcron software.
All brain activation and deactivation peak coordinates were further extracted and evaluated to identify outliers and to report average values in a volume comparable to the image smoothness [e.g., the volume of the resolution elements or “resels” (Worsley et al. 1992)], rather than single-voxel peak values. Thus, 9-mm isotropic cubic masks were created and centered at the exact coordinates in Table 4 and were kept fixed across subjects and conditions. The mean and standard deviation of the BOLD-fMRI signals were computed using a custom program written in IDL (IDL, ITT Visual Information Solutions, Boulder, CO). These extracted BOLD signals, which give precise spatial localization of the functional responses (Tomasi et al. 2007a, b), were used in SPSS correlation analyses between select BOLD-fMRI activations (regions that showed significant effects of medication as reported in Results section) and select behavioral measures (task measures that showed significant effects of medication as also reported in Results section). Accordingly, these correlations were conducted using change scores, such that placebo was subtracted from MPH for both the behavioral and brain measures; the constituent MPH and placebo scores were examined as well. Only in CUD, we also inspected correlations with the drug use variables listed in Table 1. Brain–behavior correlations were considered significant at P < 0.01 to minimize Type I error.
Table 4.
Medication and group effects during conflict and error on the Stroop color-word task
| Region | BA | Side | Voxels | Peak Z | Voxel-level corrected P values (FDR) | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Congruency: (Incongruent Error + Incongruent Correct) − (Congruent Error + Congruent Correct) | ||||||||
| Cocaine > Control | ||||||||
| Cerebellum | 19 | R | 1067 | 5.2 | 0.001b | 18 | −60 | −12 |
| Calcarine fissure | 17 | L | 4.9 | 0.001b | −3 | −87 | −9 | |
| Lingual gyrus | 18 | R | 4.4 | 0.003 | 18 | −84 | −12 | |
| Insula | 13 | R | 37 | 4.9 | 0.001a,b | 42 | 9 | 3 |
| Insula | 13 | L | 96 | 4.4 | 0.003a | −45 | 9 | 3 |
| Putamen | — | L | 3.7 | 0.012a | −33 | −3 | −3 | |
| Superior frontal gyrus: DLPFC | 9 | L | 54 | 4.8 | 0.002b | −21 | 45 | 39 |
| Cuneus | 19 | R | 73 | 3.9 | 0.008 | 15 | −81 | 42 |
| Superior occipital | 19 | R | 3.2 | 0.033a | 24 | −78 | 33 | |
| Middle frontal gyrus: DLPFC | 46 | R | 15 | 3.8 | 0.009a | 27 | 45 | 33 |
| Lingual gyrus | 27 | L | 29 | 3.8 | 0.010 | −9 | −39 | 0 |
| Fusiform gyrus | 19 | L | 42 | 3.7 | 0.012 | −39 | −69 | −12 |
| Inferior occipital | 19 | L | 3.5 | 0.028 | −42 | −66 | −9 | |
| Superior medial frontal gyrus: DLPFC | 9 | M | 9 | 3.6 | 0.014a | 0 | 54 | 45 |
| Correctness: (Incongruent Error + Congruent Error) − (Incongruent Correct + Congruent Correct) | ||||||||
| Cocaine > Control | ||||||||
| Precuneus | 7 | R | 5 | 5.0 | 0.013a,b | 9 | −63 | 63 |
| MPH < PL | ||||||||
| dACC | 24, 32 | R | 5 | 3.8 | 0.042b,c | 3 | 24 | 39 |
| Group × Medication | ||||||||
| Middle frontal gyrus: DLPFC | 46 | L | 30 | 3.9 | 0.015b,c | −24 | 33 | 21 |
| Interaction: [(Incongruent Correct − Congruent Correct) − (Incongruent Error − Congruent Correct)] + (Congruent Error − Congruent Correct) | ||||||||
| None | ||||||||
dACC, dorsal anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; MPH, methlyphenidate; PL, placebo.
All results were significant at P < 0.05 FDR corrected, 5 voxels min.
aNo longer significant (P > 0.05) after either correction for covariates, or after subject exclusions (see Results section).
bRegion also significant at P < 0.05 FWE corrected.
cP < 0.05 FDR corrected using ROI analysis as implemented with PickAtlas (see Methods section).
Results
Task Performance
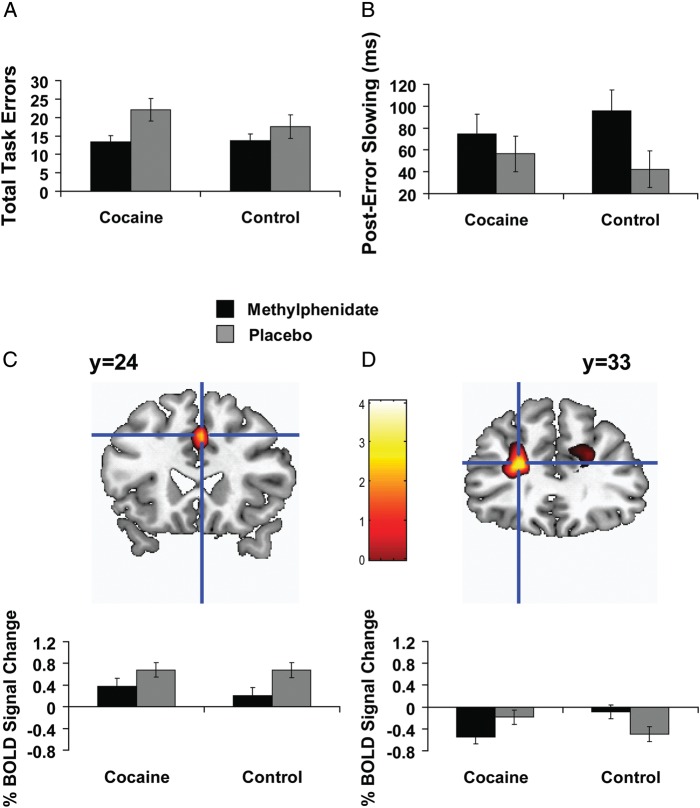
Behavioral data (percent accuracy and RT) were separately analyzed with mixed 2 (medication: MPH, placebo) × 2 (trial: congruent, incongruent) × 2 (group: CUD, control) ANOVAs (Table 2). These analyses revealed the reliable Stroop interference effect: higher task accuracy [F(1,29) = 100.5, P < 0.001] and faster RT [F(1,29) = 301.3, P < 0.001] on congruent trials than on incongruent trials in all subjects, validating the task. Medication main effects for accuracy and RT were in anticipated directions (higher percent accuracy and faster RT during MPH across both trial types), but did not reach significance (accuracy: P > 0.06; RT: P > 0.08). There were no group effects or interactions. In addition to the percent accuracy scores, we analyzed total raw errors (summed across the congruent and incongruent trials) through a 2 (medication: MPH, placebo) × 2 (group: CUD, control) ANOVA. This analysis indeed revealed a medication main effect [MPH<placebo; F(1,29) = 8.7, P < 0.01] (Fig. 2A) [note that although the medication × group interaction was not significant (P > 0.2), the restorative effects of MPH were more prominently observed in CUD as expected (significant medication effect only in this group: paired t(15) = 2.4, P < 0.05)] (see also Table 2).
Figure 2.
The impact of oral methylphenidate (20 mg) on brain and behavior during the Stroop color-word task in 15 healthy individuals and 16 individuals addicted to cocaine. Compared with placebo, and in all subjects, methylphenidate (A) decreased task-related errors, (B) increased posterror slowing, and (C) decreased right dorsal anterior cingulate cortex (dACC) response to the contrast error > correct. (D) There was also a medication × group interaction in the left DLPFC (lower error > correct DLPFC response during MPH in the cocaine subjects, but lower error > correct DLPFC response during placebo in the controls; note that the correct congruent baseline means that BOLD response below zero does not necessarily indicate deactivations as also indicated in Methods). For (C) and (D), Figure shows mean percent blood-oxygen-level-dependent (BOLD) signal change during methylphenidate and placebo, with associated means and standard errors separately for cocaine subjects and control subjects. For display purposes, Figure activations are thresholded at P < 0.005 voxel-level uncorrected. Anatomical images are presented in neurological convention (L = L).
Postconflict and posterror slowing were separately analyzed with 2 (medication: MPH, placebo) × 2 (group: CUD, control) ANOVAs. Of all possible main effects or interactions, there was a medication main effect on posterror slowing (when the trial after the committed error was a congruent event) [F(1,29) = 4.2, P < 0.05] (Fig. 2B) (Table 2), such that all subjects increased their posterror slowing (i.e., initiated more careful behavior) during MPH. There was a trend for the MPH-induced increase in posterror slowing to negatively correlate with the MPH-induced decrease in errors in all subjects (r = −0.43, P < 0.05) [i.e., fewer errors associated with more posterror slowing during MPH as directly compared with placebo].
SPM
Task-Related Activations
The Stroop color-word task produced activations in regions previously reported (Leung et al. 2000) (Table 3). For the “congruency” main effect (computed using Design Matrix 1, which affords inspection of incongruent > congruent effects) (Table 4), 2nd Level whole-brain SPM analyses revealed only group main effects. In particular, CUD showed higher incongruent > congruent activations than controls in regions that included the left DLPFC, right cerebellum, and various regions relevant to visual processing. However, given our a priori interest in the error > correct contrast and given that no medication main effects or interactions emerged for the incongruent > congruent contrast (including when using ROI analyses of the dACC and DLPFC), we focused the remainder of our analyses on response to the contrast error > correct.
Table 3.
Color word Stroop SPM activations across all study subjects and medication conditions
| Region | BA | Side | Voxels | Peak Z | Voxel-level corrected P values (FDR) | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Congruency: (Incongruent Error + Incongruent Correct) − (Congruent Error + Congruent Correct) | ||||||||
| Inferior frontal gyrus | 45 | L | 12 998 | 7.5 | 0.000* | −48 | 30 | 21 |
| Inferior frontal gyrus | 44 | R | >7.7 | 0.000* | 33 | 12 | 30 | |
| Middle frontal gyrus: DLPFC | 46 | R | 7.3 | 0.000* | 33 | 36 | 27 | |
| Middle frontal gyrus | 6 | L | 6.9 | 0.000* | −36 | 6 | 60 | |
| Insula | 13 | L | 7.5 | 0.000* | −39 | 15 | 3 | |
| Putamen | — | R | 6.5 | 0.000* | 30 | 18 | 3 | |
| Precentral gyrus | 6 | L | >7.7 | 0.000* | −45 | 3 | 39 | |
| Inferior parietal lobule | 40 | L | 7.6 | 0.000* | −42 | −51 | 57 | |
| Inferior parietal lobule | 40 | R | 7.0 | 0.000* | 45 | −51 | 48 | |
| Superior parietal lobule | 7 | L | 7.5 | 0.000* | −21 | −69 | 54 | |
| Superior parietal lobule | 7 | R | 7.5 | 0.000* | 36 | −60 | 54 | |
| Middle occipital | 7 | R | 6.2 | 0.000* | 33 | −66 | 39 | |
| Fusiform gyrus | 19 | L | 7.0 | 0.000* | −42 | −63 | −12 | |
| Fusiform gyrus | 37 | R | 6.3 | 0.000* | 27 | −57 | −15 | |
| Precuneus | 7 | M | 6.9 | 0.000* | 0 | −69 | 51 | |
| Inferior occipital | 19 | R | 6.9 | 0.000* | 42 | −63 | −15 | |
| Cerebellum | 18 | R | 6.7 | 0.000* | 21 | −84 | −18 | |
| Lingual gyrus | 18 | L | 7.4 | 0.000* | −18 | −84 | −12 | |
| Correctness: (Incongruent Error + Congruent Error) − (Incongruent Correct + Congruent Correct) | ||||||||
| Superior frontal gyrus | 10 | R | 9633 | 5.4 | 0.000* | 15 | 57 | 24 |
| Inferior frontal gyrus | 45 | L | 5.3 | 0.000* | −45 | 27 | 24 | |
| Medial frontal gyrus | 32 | L | >7.7 | 0.000* | −6 | 24 | 42 | |
| Supplementary motor area | 6 | R | >7.7 | 0.000* | 3 | 12 | 54 | |
| Insula | 13 | L | 7.7 | 0.000* | −45 | 12 | 3 | |
| Insula | 13 | R | 7.4 | 0.000* | 42 | 15 | 6 | |
| Precuneus | 7 | R | 7.5 | 0.000* | 9 | −69 | 51 | |
| Precuneus | 7 | L | 6.1 | 0.000* | −9 | −75 | 54 | |
| Precentral | 44 | L | 7.4 | 0.000* | −39 | 6 | 33 | |
| Supramarginal gyrus | 40 | R | 6.9 | 0.000* | 54 | −36 | 39 | |
| Superior parietal lobule | 7 | L | 6.6 | 0.000* | −24 | −60 | 45 | |
| Middle frontal gyrus | 8 | R | 6.4 | 0.000* | 27 | 12 | 48 | |
| Middle frontal gyrus: DLPFC | 46 | L | 5.7 | 0.000* | −30 | 48 | 24 | |
| Midcingulate | 23 | M | 6.0 | 0.000* | 0 | −15 | 36 | |
| Inferior parietal lobule | 40 | R | 5.9 | 0.000* | 48 | −48 | 48 | |
| Inferior parietal lobule | 40 | L | 5.8 | 0.000* | −51 | −45 | 39 | |
| Midddle Temporal | 21 | R | 4.9 | 0.000* | 54 | −48 | 6 | |
| Middle occipital | 7 | R | 4.4 | 0.000 | 33 | −63 | 39 | |
| Cerebellum | 37 | R | 1321 | 5.6 | 0.000* | 33 | −57 | −27 |
| Cerebellum | 37 | L | 4.7 | 0.000* | −27 | −57 | −27 | |
| Fusiform gyrus | 37 | L | 5.3 | 0.000* | −39 | −60 | −15 | |
| Fusiform gyrus | 18 | L | 4.6 | 0.000 | −24 | −69 | −15 | |
| Inferior occipital | 19 | R | 3.8 | 0.001 | 42 | −66 | −15 | |
| Middle temporal | 37 | L | 3.7 | 0.001 | −51 | −60 | 3 | |
| Interaction: [(Incongruent Correct − Congruent Correct) − (Incongruent Error − Congruent Correct)] + (Congruent Error − Congruent Correct) | ||||||||
| None | ||||||||
DLPFC, dorsolateral prefrontal cortex, ACC, anterior cingulate cortex; L, left side, R, right side, B, bilateral, M, medial.
All results were significant at P < 0.05 FDR corrected, 5 voxels min.
*Region also significant at P < 0.05 FWE corrected.
For the “correctness” main effect (computed using Design Matrix 2, which affords inspection of error > correct effects) (Table 4), second level whole-brain analyses again revealed only a group main effect, such that CUD showed higher error>correct BOLD response than controls in the precuneus (although this effect did not survive subject exclusions as marked in Table 4; see Supplementary Information for analyses). Of greater interest for our purposes, ROI analyses revealed a (within-subjects) medication main effect in the right dACC, such that all subjects showed higher error > correct BOLD response in this region during placebo than MPH (Fig. 2C). There was also a medication × group interaction in the left DLPFC (Fig. 2D): CUD showed lower error > correct BOLD response in the left DLPFC during MPH than placebo, whereas controls showed the opposite pattern. There were no significant second level effects for the “correctness × congruency” interaction contrast (computed using Design Matrix 3), indicating that there were no regions in which error > correct activations were further modulated by the incongruent trials. For all SPM analyses, the results in Table 4 account for correction for covariates (i.e., those variables that differed between the groups or between the MPH and placebo study sessions) and subject exclusions (see Supplementary Information for these additional analyses).
Brain–Behavior Correlations
We correlated MPH, placebo, and the MPH > placebo difference scores in the dACC and DLPFC with the respective scores for task-related errors and posterror slowing (i.e., the behavioral variables that showed MPH effects). In all subjects during placebo, higher error > correct activity in the DLPFC correlated with more task-related errors (r = 0.54, P < 0.01) and [and a trend for less posterror slowing (r = −0.36, P < 0.05)], suggesting that MPH-induced decreases in this region could be beneficial in this context. Correlations with the drug use variables listed in Table 1 were nonsignificant in CUD.
Discussion
After receiving oral MPH, an indirect dopamine (and norepinephrine) agonist, healthy individuals and CUD performed an event-related Stroop color-word task while undergoing fMRI. Consistent with our hypotheses, MPH main effects (behavior and brain) were observed in all subjects. In particular, MPH improved task performance (i.e., reduced task errors and increased posterror slowing) while concurrently reducing dACC BOLD response to the contrast error > correct. These results are consistent with the view that MPH enhanced the efficiency of processing in all subjects (Swanson et al. 2011), in agreement with previous research (Mehta et al. 2000; Volkow et al. 2008; Marquand et al. 2011; Tomasi et al. 2011). For example, MPH reduced the amount of brain glucose (by about 50%, as measured by positron emission tomography with [18F]fluorodeoxyglucose) needed to perform numerical calculations (Volkow et al. 2008) (note that this decrease in brain glucose indeed reflected decreased activity in task-relevant regions, including the ACC). In a recent study, MPH increased the difference (relative to placebo) in dACC activity elicited to aware > unaware errors, an effect that appeared to be driven by less activity during MPH to unaware errors (Hester et al. 2012). Although some studies have reported increased activation during MPH (e.g., Tomasi et al. 2011; Costa et al. 2012; Pauls et al. 2012), it is important to note differences in the task requirements and/or activated regions [i.e., while the current Stroop task engaged conflict/error processing and implicated the dorsal subregion of the ACC specifically, Tomasi et al. (2011) included tasks of working memory and visual attention, Costa et al. (2012) found increased activity in the putamen, and Pauls et al. (2012) found activity in a larger portion of the ACC (that also included its rostral subregion)]. Despite these acknowledged inconsistencies in the literature, and although we cannot conclusively establish specificity of our results to error (versus interference) in dACC (Supplementary Information), the current study's finding that MPH modulated error-related processing supports the influential hypothesis that the error-processing system is orchestrated by dopamine (Holroyd and Coles 2002).
The current study also juxtaposed MPH effects in healthy controls with those in CUD, a population characterized by perturbed dopaminergic functioning. An interesting medication × group interaction emerged in the DLPFC, such that only CUD showed lower error > correct DLPFC response during MPH. By focusing response in the DLPFC, MPH may have facilitated CUD's ability to initiate cognitive control (Kerns et al. 2004), an executive function that is disrupted in addiction (Kalivas and Volkow 2005). In particular, the nature of this interaction raises the intriguing possibility that while MPH may have had a salubrious effect on CUD, it may have had an opposite, detrimental effect on controls; in the latter group, MPH might have increased dopamine levels in these putatively intact individuals beyond optimal levels (e.g., to a point located on the downward side of the dopamine U-shaped curve). If higher doses of MPH had been used, it is possible that a similar deterioration also would have been discernable in behavior. Although this idea would need to be empirically verified in future studies, it is nonetheless bolstered by current supporting analyses: 1) although the group × medication interaction on total behavioral errors did not reach significance, MPH significantly improved this measure of task performance only in CUD; and 2) the correlation in all subjects between reduced error > correct DLPFC response with fewer task errors suggests that reduced DLPFC response could be adaptive in this context—suggesting that MPH may have been more beneficial to CUD, consistent with the effect for behavioral errors in this group. Taken together, our DLPFC finding supports the important hypothesis that MPH, which increases extracellular dopamine by blocking the dopamine transporters, most benefitted the individuals with dopaminergic compromises (here, CUD), consistent with previous studies of individuals with impulsivity (Clatworthy et al. 2009), ADHD (Rubia, Halari, Cubillo et al. 2011; Rubia, Halari, Mohammad et al. 2011; Volkow et al. 2012), and CUD (Goldstein et al. 2010; Li et al. 2010). A differing direction of BOLD response between the current results and prior studies of CUD might be explained by differences in the respective task baselines. In particular, results obtained when using an emotionally salient cognitive control task employed a fixation baseline (Goldstein et al. 2010); results obtained when using the stop signal task results employed a stop success>stop error baseline (Li et al. 2010), with the latter contrast in particular being directly opposite in direction to the current study. Importantly, because reduced BOLD-fMRI response in the DLPFC during the Stroop color-word task predicted better clinical outcome (treatment retention) in a prospective study of CUD (Brewer et al. 2008), DLPFC normalization via MPH may benefit addiction treatment as remains to be tested in future longitudinal studies.
Whereas medication effects were observed when analyzing the error > correct contrast, group effects (CUD > control) were largely observed when analyzing the incongruent > congruent contrast. One region of note, activated during incongruent > congruent but not error > correct, was the cerebellum. Cerebellar hyperactivity during performance of executive function tasks has previously been observed in abusers of cocaine (Hester and Garavan 2004), alcohol (Desmond et al. 2003), and opiates (Yücel et al. 2007). Cerebellar hyperactivity was also reported in a study of adult ADHD, such that greater subcortical and cerebellar blood flow during an executive function task was observed in ADHD subjects compared with healthy controls during both MPH and unmedicated conditions (Schweitzer et al. 2004). Taken together, CUD's higher cerebellar activity in the current study, in combination with increased activity during incongruent > congruent trials in frontal regions such as the DLPFC, may reflect higher cognitive effort that was needed (even with MPH) to resolve cognitive interference comparably to the healthy controls.
This study has several limitations that could be remediated in future studies. First, although the number of cigarettes smoked in CUD did not correlate with any ROIs in Table 4, future studies should nonetheless recruit actively smoking control subjects. A related concern is that, to minimize the possibly confounding impact of cigarette deprivation/withdrawal on brain function (Xu et al. 2005; Wang et al. 2007), we did not exert direct control over subjects' cigarette smoking. A potential interaction between MPH and cigarette smoking in currently smoking CUD remains to be addressed in future studies with larger sample sizes. Such a study could extend recent investigations that have documented an increase in smoking behavior during acute MPH administration (Stoops et al. 2011; Vansickel et al. 2011) [although MPH may actually protect against smoking onset among youth with ADHD (Hammerness et al. 2012)]. Nevertheless, an important consideration for the current study is that there were no differences between the MPH and placebo days in whether currently smoking CUD smoked a cigarette within 4 h of scanning (Table 1). Second, future studies should verify these results while 1) including more incongruent trials, 2) eliciting more errors (e.g., via a shorter response window), and 3) incorporating a lower level baseline (e.g., colored symbols) that would enable modeling the correct congruent trials as explicit events. Despite the excellent signal-to-noise ratio in our ROIs (and, accordingly, presumably sufficient power; see Materials and Methods section), these task modifications could be useful to verify the magnitude of the current parameter estimates. These task modifications could also yield more precise conclusions vis-à-vis whether the correct congruent trials contributed to our results [i.e., in CUD, MPH could have increased activity during error trials, decreased activity during correct trials, or both (with an opposite pattern of effects in controls)]. While this issue does not change our central conclusion that MPH decreased error > correct BOLD signal in CUD—and that decreased DLPFC activity to error > correct is associated with decreased task-related (behavioral) errors, therefore indicating a potential beneficial effect of such relatively lowered activation—the precise nature of this effect remains to be clarified with future tasks designed to elicit higher error rates. Such a task could be especially interesting in light of a previous pharmacological fMRI study in healthy controls that showed that MPH reduced deactivations to correct responses (Dodds et al. 2008). Third, because MPH also blocks the norepinephrine transporter (Hannestad et al. 2010), future studies could include more targeted dopamine or norepinephrine agonists or antagonists [MPH was chosen for this study in part because it has potential therapeutic value in CUD (Levin et al. 2007), although this is a topic of debate (Grabowski et al. 1997; Schubiner et al. 2002)]. However, even if effects are due to norepinephrine transporter blockade, the underlying mechanism of optimization could still be dopaminergic because norepinephrine transporters also have affinity for dopamine (Hannestad et al. 2010).
In conclusion, MPH enhanced Stroop task performance and posterror slowing in health and in cocaine addiction. In parallel, MPH reduced error-related activity in dopaminergically innervated PFC regions relevant to error-related processing: dACC activity in all subjects and DLPFC activity uniquely in CUD. This pharmacological fMRI study, which manipulated dopaminergic functioning and localized the resulting functional changes, helps address a previously identified void on the neurochemistry of error monitoring (Jocham and Ullsperger 2009), while also contributing to a long-standing effort of using the Stroop color-word test to interrogate neural impairments in drug addiction (Bolla et al. 2004; Eldreth et al. 2004; Gruber and Yurgelun-Todd 2005; Salo et al. 2009; Azizian et al. 2010; Marinkovic et al. 2012). In addition, if our findings are subsequently replicated in treatment-seeking CUD, these brain regions could then become potential therapeutic targets in future longitudinal intervention studies. Similar to its restorative effects on brain function in ADHD (Rubia, Halari, Cubillo et al. 2011; Rubia, Halari, Mohammad et al. 2011), the supervised and controlled administration of oral MPH could potentially be used to enhance cognitive function (Sofuoglu et al. 2013), as well as possibly ameliorate inflexible patterns of behavior and optimize brain response to error, in cocaine addiction.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This study was supported by grants from the National Institute on Drug Abuse (to R.Z.G.: 1R01DA023579; to S.J.M.: 1F32DA030017-01). This manuscript has been authored by Brookhaven Science Associates, LLC under Contract No. DE-AC02-98CHI-886 with the US Department of Energy. The United States Government retains, and the publisher, by accepting the article for publication, acknowledges, a worldwide license to publish or reproduce the published form of this manuscript, or allow others to do so, for the US Government purposes.
Supplementary Material
Notes
The authors gratefully acknowledge the contributions of Thomas Maloney, Nelly Alia-Klein, Juntian Shan, Dimitris Samaras, Ruiliang Wang, Frank Telang, and Gene-Jack Wang. Conflict of Interest: None declared.
References
- Adler RJ. The Geometry of Random Fields. Chichester: John Wiley & Sons; 1981. [Google Scholar]
- Arnsten AF. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs. 2009;23(Suppl 1):33–41. doi: 10.2165/00023210-200923000-00005. doi:10.2165/00023210-200923000-00005. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Neelin P, Collins DL, Evans A, Friston K. Incorporating prior knowledge into image registration. Neuroimage. 1997;6:344–352. doi: 10.1006/nimg.1997.0299. doi:10.1006/nimg.1997.0299. [DOI] [PubMed] [Google Scholar]
- Azizian A, Nestor LJ, Payer D, Monterosso JR, Brody AL, London ED. Smoking reduces conflict-related anterior cingulate activity in abstinent cigarette smokers performing a Stroop task. Neuropsychopharmacology. 2010;35:775–782. doi: 10.1038/npp.2009.186. doi:10.1038/npp.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JJ, Dean AJ, Nandam LS, O'Connell RG, Bellgrove MA. The molecular genetics of executive function: role of monoamine system genes. Biol Psychiatry. 2011;69:e127–143. doi: 10.1016/j.biopsych.2010.12.040. doi:10.1016/j.biopsych.2010.12.040. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- Blumberg HP, Leung HC, Skudlarski P, Lacadie CM, Fredericks CA, Harris BC, Charney DS, Gore JC, Krystal JH, Peterson BS. A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry. 2003;60:601–609. doi: 10.1001/archpsyc.60.6.601. doi:10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, et al. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. doi:10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biol Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. doi:10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caparelli E, Tomasi D. K-space spatial low-pass filters can increase signal loss artifacts in Echo-Planar Imaging. Biomed Signal Process Control. 2008;3:107–114. doi: 10.1016/j.bspc.2007.11.003. doi:10.1016/j.bspc.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caparelli EC, Tomasi D, Arnold S, Chang L, Ernst T. k-Space based summary motion detection for functional magnetic resonance imaging. Neuroimage. 2003;20:1411–1418. doi: 10.1016/S1053-8119(03)00339-2. doi:10.1016/S1053-8119(03)00339-2. [DOI] [PubMed] [Google Scholar]
- Clatworthy PL, Lewis SJ, Brichard L, Hong YT, Izquierdo D, Clark L, Cools R, Aigbirhio FI, Baron JC, Fryer TD, et al. Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J Neurosci. 2009;29:4690–4696. doi: 10.1523/JNEUROSCI.3266-08.2009. doi:10.1523/JNEUROSCI.3266-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Riedel M, Pogarell O, Menzel-Zelnitschek F, Schwarz M, Reiser M, Moller HJ, Rubia K, Meindl T, Ettinger U. Methylphenidate effects on neural activity during response inhibition in healthy humans. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs107. doi:10.1093/cercor/bhs107. [DOI] [PubMed] [Google Scholar]
- Danielmeier C, Eichele T, Forstmann BU, Tittgemeyer M, Ullsperger M. Posterior medial frontal cortex activity predicts post-error adaptations in task-related visual and motor areas. J Neurosci. 2011;31:1780–1789. doi: 10.1523/JNEUROSCI.4299-10.2011. doi:10.1523/JNEUROSCI.4299-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn ER, Hulstijn W, Verkes RJ, Ruigt GS, Sabbe BG. Drug-induced stimulation and suppression of action monitoring in healthy volunteers. Psychopharmacology (Berl) 2004;177:151–160. doi: 10.1007/s00213-004-1915-6. doi:10.1007/s00213-004-1915-6. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Chen SH, DeRosa E, Pryor MR, Pfefferbaum A, Sullivan EV. Increased frontocerebellar activation in alcoholics during verbal working memory: an fMRI study. Neuroimage. 2003;19:1510–1520. doi: 10.1016/s1053-8119(03)00102-2. doi:10.1016/S1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Devito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Kober H, Potenza MN. A preliminary study of the neural effects of behavioral therapy for substance use disorders. Drug Alcohol Depend. 2012;122:228–235. doi: 10.1016/j.drugalcdep.2011.10.002. doi:10.1016/j.drugalcdep.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds CM, Muller U, Clark L, van Loon A, Cools R, Robbins TW. Methylphenidate has differential effects on blood oxygenation level-dependent signal related to cognitive subprocesses of reversal learning. J Neurosci. 2008;28:5976–5982. doi: 10.1523/JNEUROSCI.1153-08.2008. doi:10.1523/JNEUROSCI.1153-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb Cortex. 2008;18:1475–1484. doi: 10.1093/cercor/bhm179. doi:10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage. 2004;23:914–920. doi: 10.1016/j.neuroimage.2004.07.032. doi:10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general approach. Hum Brain Mapp. 1995;2:189–210. doi:10.1002/hbm.460020402. [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. doi:10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Woicik PA, Maloney T, Tomasi D, Alia-Klein N, Shan J, Honorio J, Samaras D, Wang R, Telang F, et al. Oral methylphenidate normalizes cingulate activity in cocaine addiction during a salient cognitive task. Proc Natl Acad Sci USA. 2010;107:16667–16672. doi: 10.1073/pnas.1011455107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski J, Roache JD, Schmitz JM, Rhoades H, Creson D, Korszun A. Replacement medication for cocaine dependence: methylphenidate. J Clin Psychopharmacol. 1997;17:485–488. doi: 10.1097/00004714-199712000-00008. doi:10.1097/00004714-199712000-00008. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Res Cogn Brain Res. 2005;23:107–118. doi: 10.1016/j.cogbrainres.2005.02.016. doi:10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Hammerness P, Joshi G, Doyle R, Georgiopoulos A, Geller D, Spencer T, Petty CR, Faraone SV, Biederman J. Do stimulants reduce the risk for cigarette smoking in youth with attention-deficit hyperactivity disorder? A Prospective, Long-Term, Open-Label Study of Extended-Release Methylphenidate. J Pediatr. 2012 doi: 10.1016/j.jpeds.2012.06.046. doi:10.1016/j.peds.2012.06.046. [DOI] [PubMed] [Google Scholar]
- Hannestad J, Gallezot JD, Planeta-Wilson B, Lin SF, Williams WA, van Dyck CH, Malison RT, Carson RE, Ding YS. Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol Psychiatry. 2010;68:854–860. doi: 10.1016/j.biopsych.2010.06.017. doi:10.1016/j.biopsych.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Shaw M, Yucel M, Purcell R, Brewer WJ, Strother SC, Egan GF, Olver JS, Nathan PJ, Pantelis C. Functional connectivity during Stroop task performance. Neuroimage. 2005;24:181–191. doi: 10.1016/j.neuroimage.2004.08.033. doi:10.1016/j.neuroimage.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Hennig J, Scheffler K. Hyperechoes. Magn Reson Med. 2001;46:6–12. doi: 10.1002/mrm.1153. doi:10.1002/mrm.1153. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. doi:10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Nandam LS, O'Connell RG, Wagner J, Strudwick M, Nathan PJ, Mattingley JB, Bellgrove MA. Neurochemical enhancement of conscious error awareness. J Neurosci. 2012;32:2619–2627. doi: 10.1523/JNEUROSCI.4052-11.2012. doi:10.1523/JNEUROSCI.4052-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009;34:2450–2458. doi: 10.1038/npp.2009.67. doi:10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. doi:10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Jocham G, Ullsperger M. Neuropharmacology of performance monitoring. Neurosci Biobehav Rev. 2009;33:48–60. doi: 10.1016/j.neubiorev.2008.08.011. doi:10.1016/j.neubiorev.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. doi:10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. doi:10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J Neurochem. 1997;68:2032–2037. doi: 10.1046/j.1471-4159.1997.68052032.x. doi:10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- Lee JH, Garwood M, Menon R, Adriany G, Andersen P, Truwit CL, Ugurbil K. High contrast and fast three-dimensional magnetic resonance imaging at high fields. Magn Reson Med. 1995;34:308–312. doi: 10.1002/mrm.1910340305. doi:10.1002/mrm.1910340305. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, et al. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. doi:10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HC, Skudlarski P, Gatenby JC, Peterson BS, Gore JC. An event-related functional MRI study of the stroop color word interference task. Cereb Cortex. 2000;10:552–560. doi: 10.1093/cercor/10.6.552. doi:10.1093/cercor/10.6.552. [DOI] [PubMed] [Google Scholar]
- Levin FR, Evans SM, Brooks DJ, Garawi F. Treatment of cocaine dependent treatment seekers with adult ADHD: double-blind comparison of methylphenidate and placebo. Drug Alcohol Depend. 2007;87:20–29. doi: 10.1016/j.drugalcdep.2006.07.004. doi:10.1016/j.drugalcdep.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Li CS, Morgan PT, Matuskey D, Abdelghany O, Luo X, Chang JL, Rounsaville BJ, Ding YS, Malison RT. Biological markers of the effects of intravenous methylphenidate on improving inhibitory control in cocaine-dependent patients. Proc Natl Acad Sci USA. 2010;107:14455–14459. doi: 10.1073/pnas.1002467107. doi:10.1073/pnas.1002467107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. doi:10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Rickenbacher E, Azma S, Artsy E. Acute alcohol intoxication impairs top-down regulation of Stroop incongruity as revealed by blood oxygen level-dependent functional magnetic resonance imaging. Hum Brain Mapp. 2012;33:319–333. doi: 10.1002/hbm.21213. doi:10.1002/hbm.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquand AF, De Simoni S, O'Daly OG, Williams SC, Mourao-Miranda J, Mehta MA. Pattern classification of working memory networks reveals differential effects of methylphenidate, atomoxetine, and placebo in healthy volunteers. Neuropsychopharmacology. 2011;36:1237–1247. doi: 10.1038/npp.2011.9. doi:10.1038/npp.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000;20:RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Tomasi D, Honorio J, Volkow ND, Goldstein RZ. Dopaminergic involvement during mental fatigue in health and cocaine addiction. Translational Psychiatry. 2012 doi: 10.1038/tp.2012.110. 2:e176; doi:10.1038/tp.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls AM, O'Daly OG, Rubia K, Riedel WJ, Williams SC, Mehta MA. Methylphenidate effects on prefrontal functioning during attentional-capture and response inhibition. Biol Psychiatry. 2012;72:142–149. doi: 10.1016/j.biopsych.2012.03.028. doi:10.1016/j.biopsych.2012.03.028. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Kane MJ, Alexander GM, Lacadie C, Skudlarski P, Leung HC, May J, Gore JC. An event-related functional MRI study comparing interference effects in the Simon and Stroop tasks. Brain Res Cogn Brain Res. 2002;13:427–440. doi: 10.1016/s0926-6410(02)00054-x. doi:10.1016/S0926-6410(02)00054-X. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Potenza MN, Wang Z, Zhu H, Martin A, Marsh R, Plessen KJ, Yu S. An FMRI study of the effects of psychostimulants on default-mode processing during Stroop task performance in youths with ADHD. Am J Psychiatry. 2009;166:1286–1294. doi: 10.1176/appi.ajp.2009.08050724. doi:10.1176/appi.ajp.2009.08050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Leung HC, Blumberg HP, Peterson BS, Fulbright RK, Lacadie CM, Skudlarski P, Gore JC. An FMRI Stroop task study of ventromedial prefrontal cortical function in pathological gamblers. Am J Psychiatry. 2003;160:1990–1994. doi: 10.1176/appi.ajp.160.11.1990. doi:10.1176/appi.ajp.160.11.1990. [DOI] [PubMed] [Google Scholar]
- Roesch-Ely D, Scheffel H, Weiland S, Schwaninger M, Hundemer HP, Kolter T, Weisbrod M. Differential dopaminergic modulation of executive control in healthy subjects. Psychopharmacology (Berl) 2005;178:420–430. doi: 10.1007/s00213-004-2027-z. doi:10.1007/s00213-004-2027-z. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Smith AB, Mohammad AM, Brammer M, Taylor E. Methylphenidate normalizes fronto-striatal underactivation during interference inhibition in medication-naive boys with attention-deficit hyperactivity disorder. Neuropsychopharmacology. 2011;36:1575–1586. doi: 10.1038/npp.2011.30. doi:10.1038/npp.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Halari R, Mohammad AM, Taylor E, Brammer M. Methylphenidate normalizes frontocingulate underactivation during error processing in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;70:255–262. doi: 10.1016/j.biopsych.2011.04.018. doi:10.1016/j.biopsych.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Ursu S, Buonocore MH, Leamon MH, Carter C. Impaired prefrontal cortical function and disrupted adaptive cognitive control in methamphetamine abusers: a functional magnetic resonance imaging study. Biol Psychiatry. 2009;65:706–709. doi: 10.1016/j.biopsych.2008.11.026. doi:10.1016/j.biopsych.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubiner H, Saules KK, Arfken CL, Johanson CE, Schuster CR, Lockhart N, Edwards A, Donlin J, Pihlgren E. Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp Clin Psychopharmacol. 2002;10:286–294. doi: 10.1037//1064-1297.10.3.286. doi:10.1037/1064-1297.10.3.286. [DOI] [PubMed] [Google Scholar]
- Schweitzer JB, Lee DO, Hanford RB, Zink CF, Ely TD, Tagamets MA, Hoffman JM, Grafton ST, Kilts CD. Effect of methylphenidate on executive functioning in adults with attention-deficit/hyperactivity disorder: normalization of behavior but not related brain activity. Biol Psychiatry. 2004;56:597–606. doi: 10.1016/j.biopsych.2004.07.011. doi:10.1016/j.biopsych.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. doi:10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Shiels K, Hawk LW., Jr Self-regulation in ADHD: the role of error processing. Clin Psychol Rev. 2010;30:951–961. doi: 10.1016/j.cpr.2010.06.010. doi:10.1016/j.cpr.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Devito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64:452–463. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmer B, Segalowitz SJ, Dywan J, Panisset M, Melmed C. The error negativity in nonmedicated and medicated patients with Parkinson's disease. Clin Neurophysiol. 2007;118:1223–1229. doi: 10.1016/j.clinph.2007.02.019. doi:10.1016/j.clinph.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Poole MM, Vansickel AR, Hays KA, Glaser PE, Rush CR. Methylphenidate increases choice of cigarettes over money. Nicotine Tob Res. 2011;13:29–33. doi: 10.1093/ntr/ntq198. doi:10.1093/ntr/ntq198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. doi:10.1037/h0054651. [Google Scholar]
- Swanson J, Baler RD, Volkow ND. Understanding the effects of stimulant medications on cognition in individuals with attention-deficit hyperactivity disorder: a decade of progress. Neuropsychopharmacology. 2011;36:207–226. doi: 10.1038/npp.2010.160. doi:10.1038/npp.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Turken AU. Dissociation between conflict detection and error monitoring in the human anterior cingulate cortex. Proc Natl Acad Sci USA. 2002;99:16354–16359. doi: 10.1073/pnas.252521499. doi:10.1073/pnas.252521499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Stern ER, Gehring WJ. Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist. 2007;13:160–172. doi: 10.1177/1073858406298184. doi:10.1177/1073858406298184. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Caparelli EC, Chang L, Ernst T. fMRI-acoustic noise alters brain activation during working memory tasks. Neuroimage. 2005;27:377–386. doi: 10.1016/j.neuroimage.2005.04.010. doi:10.1016/j.neuroimage.2005.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Thalamo-cortical dysfunction in cocaine abusers: implications in attention and perception. Psychiatry Res. 2007a;155:189–201. doi: 10.1016/j.pscychresns.2007.03.002. doi:10.1016/j.pscychresns.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Widespread disruption in brain activation patterns to a working memory task during cocaine abstinence. Brain Res. 2007b;1171:83–92. doi: 10.1016/j.brainres.2007.06.102. doi:10.1016/j.brainres.2007.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang GJ, Wang R, Telang F, Caparelli EC, Wong C, Jayne M, Fowler JS. Methylphenidate enhances brain activation and deactivation responses to visual attention and working memory tasks in healthy controls. Neuroimage. 2011;54:3101–3110. doi: 10.1016/j.neuroimage.2010.10.060. doi:10.1016/j.neuroimage.2010.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansickel AR, Stoops WW, Glaser PE, Poole MM, Rush CR. Methylphenidate increases cigarette smoking in participants with ADHD. Psychopharmacology (Berl) 2011;218:381–390. doi: 10.1007/s00213-011-2328-y. doi:10.1007/s00213-011-2328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. doi:10.1016/S0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. doi:10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Wong C, Ma J, Pradhan K, Benveniste H, Swanson JM. Methylphenidate decreased the amount of glucose needed by the brain to perform a cognitive task. PLoS ONE. 2008;3:e2017. doi: 10.1371/journal.pone.0002017. doi:10.1371/journal.pone.0002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, Hitzemann R, Pappas N. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155:1325–1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Quantification of Behavior Sackler Colloquium: Addiction: Beyond dopamine reward circuitry. Proc Natl Acad Sci USA. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Kollins SH, Wigal TL, Newcorn JH, Telang FW, Fowler JS, Logan J, Wong CT, et al. Methylphenidate-elicited dopamine increases in ventral striatum are associated with long-term symptom improvement in adults with attention deficit hyperactivity disorder. J Neurosci. 2012;32:841–849. doi: 10.1523/JNEUROSCI.4461-11.2012. doi:10.1523/JNEUROSCI.4461-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, Detre JA, Lerman C. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J Neurosci. 2007;27:14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. doi:10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Wilkinson G. The Wide-Range Achievement Test 3- Administration Manual. Wilmington, DE: Wide Range Inc; 1993. [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. doi:10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Rodriguez P, Simon SL, Brody A, Jarvik M, Domier CP, Olmstead R, et al. Brain activity in cigarette smokers performing a working memory task: effect of smoking abstinence. Biol Psychiatry. 2005;58:143–150. doi: 10.1016/j.biopsych.2005.03.028. doi:10.1016/j.biopsych.2005.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel M, Lubman DI, Harrison BJ, Fornito A, Allen NB, Wellard RM, Roffel K, Clarke K, Wood SJ, Forman SD, et al. A combined spectroscopic and functional MRI investigation of the dorsal anterior cingulate region in opiate addiction. Mol Psychiatry. 2007;12:611, 691–702. doi: 10.1038/sj.mp.4001955. [DOI] [PubMed] [Google Scholar]
- Zirnheld PJ, Carroll CA, Kieffaber PD, O'Donnell BF, Shekhar A, Hetrick WP. Haloperidol impairs learning and error-related negativity in humans. J Cogn Neurosci. 2004;16:1098–1112. doi: 10.1162/0898929041502779. doi:10.1162/0898929041502779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.