Regarding the question of how to use insulin in type 2 diabetes, a systematic review conforming to methods of the Cochrane collaboration was published in 2006 (1). This review included studies published in Medline until May 2004. The analysis compared insulin monotherapy with combination therapy with insulin and oral hypoglycemic agent (OHA) in previously insulin-naive patients. With use of the methods detailed in the review (1), 13 randomized controlled trials (RCTs) could be identified and included 1,811 participants with a mean age of 60 years and duration of diabetes of 10 years. The authors concluded that bedtime NPH insulin combined with oral antihyperglycemic agents provides glycemic control comparable with that provided by insulin monotherapy with twice daily insulin or basal/bolus insulin regimens but is associated with less weight gain if metformin is used. However, since May 2004 there has been an exponential increase in the number of patients participating in RCTs comparing basal insulin plus OHA with other insulin regiments with similar OHA (vide infra). Such studies have become possible thanks to the development of rapid- and long-acting insulin analogs and commercial support for studies addressing insulin therapy.
This review focuses on comparison of different insulin treatment regimens in both insulin-naive (first objective) and previously insulin-treated (second objective) patients with type 2 diabetes. We wished to examine whether there is an advantage (glycemic control, weight gain, or hypoglycemia) of using premixed and basal-bolus regimens with or without OHA compared with basal insulin and OHA. For the first objective, we used the principles outlined in the previous Cochrane review (1) to compare glycemic control between basal insulin/OHA and other regimens. The latter included regimens with premixed insulin twice daily with or without OHA or regimens using prandial insulin three times daily or multiple insulin injection therapy (basal and prandial three times daily) with or without OHA. Although the impact of the number of insulin injections can only be compared if OHA is the same in the two arms, it is still common clinical practice to use premixed insulins and multiple insulin injection regimens without OHA, which is why such comparisons were included. We do not focus on differences between basal insulin analogs, as this information is readily available in meta-analyses (2,3). Data on body weight and hypoglycemia were also analyzed from eligible trials in a simple fashion. The second objective was to analyze trials comparing intensification regimens with twice-daily premixed insulin and prandial or basal-bolus regimens in previously insulin-treated patients. These few studies are discussed individually, as their designs are too heterogeneous to allow meta-analysis.
METHODS
We reviewed the literature with the general objective of defining how insulin should be used in type 2 diabetes when one considers glycemic control, weight gain, and hypoglycemia. We had two specific objectives. First, we aimed to examine, using methods of the earlier Cochrane collaboration, whether insulin-naive type 2 diabetic patients should be treated with basal insulin with OHA, with premixed insulin twice daily with the same or no OHA, or with an insulin given more than two times daily (prandial insulin three times daily or multiple insulin injection therapy) with or without OHA. The second objective was to examine whether previously insulin-treated patients should be treated with twice-daily premixed insulin and prandial or basal-bolus regimens in previously insulin-treated patients.
Criteria for considering studies
Types of studies and patients.
For the first objective, RCTs (any design) with a minimum follow-up of 2 months including >20 insulin-naive type 2 diabetic patients (total for two arms) were included. For the second objective, we searched for trials using the same search criteria in previously insulin-treated patients.
Types of interventions.
The following comparisons were included to meet the first objective: comparison of basal insulin and OHA with premixed or multiple insulin injections and the same OHA or no OHA. Studies with inhaled insulin, which is not on the market, are not included. Comparisons merely focusing on use of OHA in combination with insulin versus use of a similar insulin regimen without OHA are not included. For the second objective, any comparison of insulin regimens we could identify using the same search strategy as for the first objective but that was performed in previously insulin-treated patients was included.
Types of outcome measures.
The main outcome measure was glycemic control measured using HbA1c. Additional outcome measures included insulin dose, hypoglycemia, and weight gain.
Search methods
The search strategy (81 terms) was as listed in Table 01 in the meta-analysis by the Cochrane collaboration (1), and we selected the studies based on our objectives. We focused on RCTs in MEDLINE (1966–October 2012). In addition, we searched PubMed for human trials published in English using the search terms “insulin therapy” and “type 2 diabetes.”
Methods of review
Two independent reviewers (H.Y.-J. and A.K.) scanned titles and abstracts for every record retrieved. The procedures for quality assessment of trials, data extraction, and data analysis have previously been described (1).
Description of studies
The search provided 2,998 citations. After exclusion of studies not meeting the criteria listed above, the remaining abstracts were independently assessed. Of these, 14 fulfilled the criteria for the first objective. Reasons for exclusion of studies (first and second objective) included absence of two different insulin regimens, studies including both insulin-naive and insulin-treated patients without analysis of these groups separately, lack of RCTs, only comparisons between basal insulins, <2 months’ duration or <20 patients in the two treatment arms, use of inhaled insulin, and use of mixed insulins once or more than twice daily. The list is available from the authors if required. For the second objective, a total of six studies comparing different insulin treatment regimens in previously insulin-treated patients was identified. The designs of these studies were too heterogeneous to allow any meta-analysis to be performed.
Data extraction
Data extraction was performed by two reviewers independently (H.Y.-J. and A.K.). Differences in the extracted data were discussed and resolved by referring to the original article.
Data analyses
SDs of changes in HbA1c were derived from published SEMs. If not provided, these values were extracted from graphs. When studies did not provide the change in HbA1c from baseline values and their SDs, the change was calculated by subtracting baseline from posttreatment mean HbA1c values, and the SD of the change was calculated as previously described (1). Adjustments for insulin dose were performed by dividing the mean change in HbA1c by the mean insulin dose in each study at the end of intervention. In Fig. 2, data are given as weighted mean difference with 95% CI. Heterogeneity was calculated using the χ2 test and the I2. Notable heterogeneity was defined as an I2 substantially >50% (4). Calculations and forest plots were made using Review Manager, version 5.0.17 (Copenhagen, Denmark; Nordic Cochrane Centre, Cochrane Collaboration, 2008).
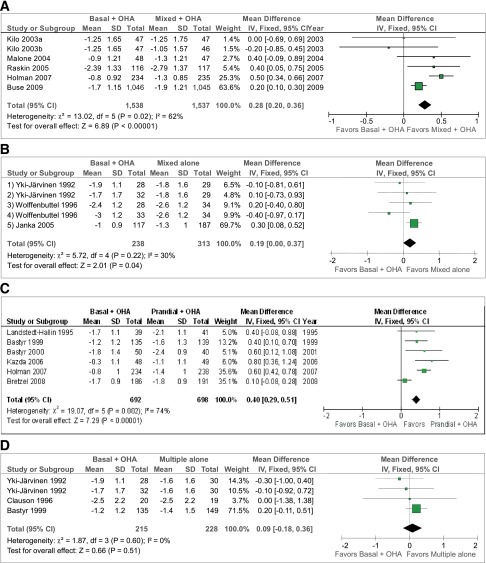
Figure 2.
Mean change in HbA1c (%) and corresponding 95% CIs of each comparison separately and pooled. Comparisons and studies are as described for Fig. 1.
RESULTS
Studies in insulin-naive patients
Comparison of HbA1c between insulin regimens in insulin-naive patients.
A total of 14 studies met the inclusion criteria for the first objective (Supplementary Table 1). Thirteen studies had a parallel, while one had a crossover, design. The mean dropout rate weighted by study size was 9.2%. When weighted by study size, the mean age of the patients was 58 years, duration of diabetes 9.2 years, BMI 30.2 kg/m2, and study duration 27 weeks.
As shown in Fig. 1A–D, in none of the trials performed prior to 2003 was the glycemic target of 7.0% achieved. The mean weighted basal insulin dose was 37 IU/day and HbA1c 7.5% (58 mmol/mol).
Figure 1.
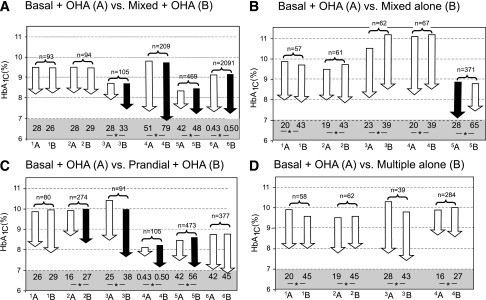
Graphical representation of studies comparing basal insulin plus OHA with premixed insulin plus OHA (A), premixed insulin alone (B), prandial insulin plus OHA (C), and multiple insulin alone (D). Paired comparisons in each panel represent two insulin therapy arms: basal insulin plus OHA (A) and the comparator arm in each pair (B). Arrows indicate baseline and end HbA1c in each study. A black arrow indicates a significant difference in HbA1c compared with the other arm and a white arrow a nonsignificant difference. The numbers below the arrows denote total insulin doses (IU/day). *Significant difference in insulin doses between the two arms. The total number of subjects in the two treatment arms is shown above the arrows. For panel A, the studies are as follows: 1A, Kilo et al. (ref. 20), bedtime NPH × 1 and metformin; 1B, biphasic protaminated aspart/aspart 70/30 × 2 + metformin; 2A, Kilo et al., bedtime NPH × 1 and metformin; 2B, protaminated human insulin/human insulin 70/30 × 2 + metformin (ref. 20); 3A, Malone et al. (ref. 21), bedtime glargine × 1 + metformin; 3B, lispro protamine suspension/lispro 75/25 × 2 + metformin; 4A, Raskin et al. (ref. 22), bedtime glargine × 1 + metformin + PIO (pioglitazone) (∼30%); 4B, biphasic insulin aspart 70/30 × 2 + metformin + PIO (∼30%); 5A, Holman et al. (ref. 17), detemir × 1–2 + metformin + sulfonylurea; 5B, 70/30 aspart × 2 + metformin + sulfonylurea; 6A, Buse et al. (ref. 23), glargine × 1 + OHA; and 6B, protamine suspension 75% and lispro 25% × 2. For panel B, studies are as follows: 1A, Yki-Järvinen et al. (ref. 24), bedtime NPH + metformin + sulfonylurea vs. 1B, NPH/regular 70/30 × 2; 2A, Yki-Järvinen et al. (ref. 24), morning NPH + metformin + sulfonylurea, vs. 2B, NPH/regular 70/30 × 2; 3A, Wolffenbuttel et al. (ref. 25), bedtime NPH × 1 + sulfonylurea; 3B, NPH/regular 70/30 × 2; 4A, Wolffenbuttel et al. (ref. 25), morning NPH × 1 + sulfonylurea, vs. 4B, NPH/regular 70/30 × 2; 5A, Janka et al. (ref. 12), morning glargine × 1 + sulfonylurea + metformin, vs. 5B, NPH/regular 70/30 × 2. For panel C, the studies are as follows: 1A, Landstedt-Hallin et al. (ref. 26), bedtime NPH × 1 + sulfonylurea, vs. 1B, regular × 3 + sulfonylurea; 2A, Bastyr et al. (ref. 27), bedtime NPH + sulfonylurea, vs. 2B, lispro × 3 + sulfonylurea; 3A, Bastyr et al. (ref. 28), bedtime NPH × 1 + sulfonylurea, vs. 3B, lispro × 3 + sulfonylurea; 4A, Kazda et al. (ref. 29), glargine, vs. 4B, lispro × 3; 5A, Holman et al. (ref. 17), detemir × 1–2 + sulfonylurea + metformin, vs. 5B, aspart × 3 + sulfonylurea + metformin; and 6A, Bretzel et al. (ref. 30), glargine × 1 + sulfonylurea + metformin, vs. 6B, lispro × 3 + sulfonylurea + metformin. For panel D, the studies are as follows: 1A, Yki-Järvinen et al. (ref. 24), bedtime NPH + sulfonylurea + metformin, vs. 1B, regular × 3 and NPH; 2A, morning NPH + sulfonylurea + metformin, vs. 2B, regular × 3 and NPH (ref. 24); 3A, Clauson et al. (ref. 31), bedtime NPH and sulfonylurea, vs. 3B, rapid-acting insulin × 3 and NPH; and 4A, Bastyr et al. (ref. 27), bedtime NPH + sulfonylurea, vs. 4B, lispro × 3 + NPH.
Comparison of basal and OHA with premixed insulin and OHA.
Use of premixed insulin and OHA was associated with a significantly lower HbA1c by 0.28% (95% CI −0.20 to −0.36%, P < 0.00001) compared with basal insulin and OHA (Fig. 2A) with notable heterogeneity. Mean weighted insulin doses were higher in the premixed insulin and OHA (42 IU/day) than in the basal insulin and OHA (37 IU/day) arms of the studies. When the mean change in HbA1c was normalized by the insulin dose, the difference in HbA1c between basal and OHA and premixed and OHA regimens was no longer significant (P = 0.56, test for overall effect) (Figs. 1A and 2A).
Comparison of basal insulin and OHA with premixed insulin alone.
There were no differences in the decrease in HbA1c between basal insulin and OHA and premixed insulin alone (Fig. 2B). The weighted mean insulin dose was significantly higher in the premixed insulin alone arms (55 IU/day) in all studies than in the basal insulin and OHA arms (25 IU/day) (Figs. 1B and 2B).
Comparison of basal insulin and OHA with prandial insulin and OHA.
Prandial insulin with OHA was associated with a slightly greater reduction in HbA1c (−0.40 [95% CI −0.29 to −0.51]) compared with basal insulin with OHA (P < 0.0001) (Fig. 2C), with notable significant heterogeneity. In four of six studies, the insulin dose was significantly higher in the prandial compared with the basal insulin group (Fig. 1C). The mean weighted baseline and study end HbA1cs were 9.0% (75 mmol/mol) and 7.4% (57 mmol/mol) and insulin dose 44 IU/day in the prandial and OHA groups (Figs. 1C and 2C).
Comparison of basal insulin and OHA with multiple insulin alone.
There were no differences in the decrease in HbA1c between basal insulin/OHA and multiple insulin injections alone. The mean weighted insulin dose was 84% higher in the insulin alone (33 IU/day) than in the combination therapy (18 IU/day) arms (Figs. 1D and 2D).
Comparison of weight gain in studies in insulin-naive patients.
In 2 of 21 comparisons, data on weight gain (Fig. 3) were not available. In 10 of 19 comparisons reporting data, weight gain was significantly less in the basal and OHA compared with other treatment arms (Fig. 3). In the other 10, weight gain did not differ between the regimens. Mean weighted weight gain in the basal insulin and OHA arms averaged 2.2 kg (1.5 kg per 1% decrease in HbA1c), in the premixed insulin and OHA arms 3.7 kg (2.0 kg per 1% decrease in HbA1c), and in the prandial and OHA arms 3.7 kg (2.4 kg per 1% decrease in HbA1c).
Figure 3.
Weight gains (left panel) and overall rate of hypoglycemia during the whole study (right panel). Open squares indicate that the difference in weight gain or overall rate of hypoglycemia was not significant between the groups, while closed squares indicate that weight gain or rate of hypoglycemia was significantly smaller in the basal plus OHA arm compared with the other treatment arms. Comparisons and studies are as described for Fig. 1.
Comparison of hypoglycemia in studies in insulin-naive patients.
In four comparisons, data on hypoglycemia were not available (Fig. 3). In comparisons between basal insulin/OHA and premixed insulin/OHA or prandial insulin/OHA, the incidence of any hypoglycemia (defined as incidence of hypoglycemia per patient or percent of patients with hypoglycemia if incidence not given) during the whole study was significantly less in the basal insulin and OHA arm than in the premixed or prandial plus OHA arms in 8 of 17 comparisons reporting data. Hypoglycemia was not greater in any study using basal compared with premixed or prandial/multiple insulin injections with or without OHA.
Studies in previously insulin-treated patients
Details of the design and subjects of six studies performed in previously insulin-treated subjects are shown in Supplementary Table 2. Essential results are shown in Table 1.
Three studies compared addition of premixed insulin to OHA with addition of basal insulin to OHA. In two of the three studies, glycemic control was better in the premixed compared with the basal arm. In all three studies, weight gains were significantly higher in the premixed compared with the basal arm. In two of the three studies, hypoglycemic events were more frequent in the premixed compared with the basal arm (Table 1).
Table 1.
Subject characteristics and study design of studies including previously insulin-treated type 2 diabetic patients
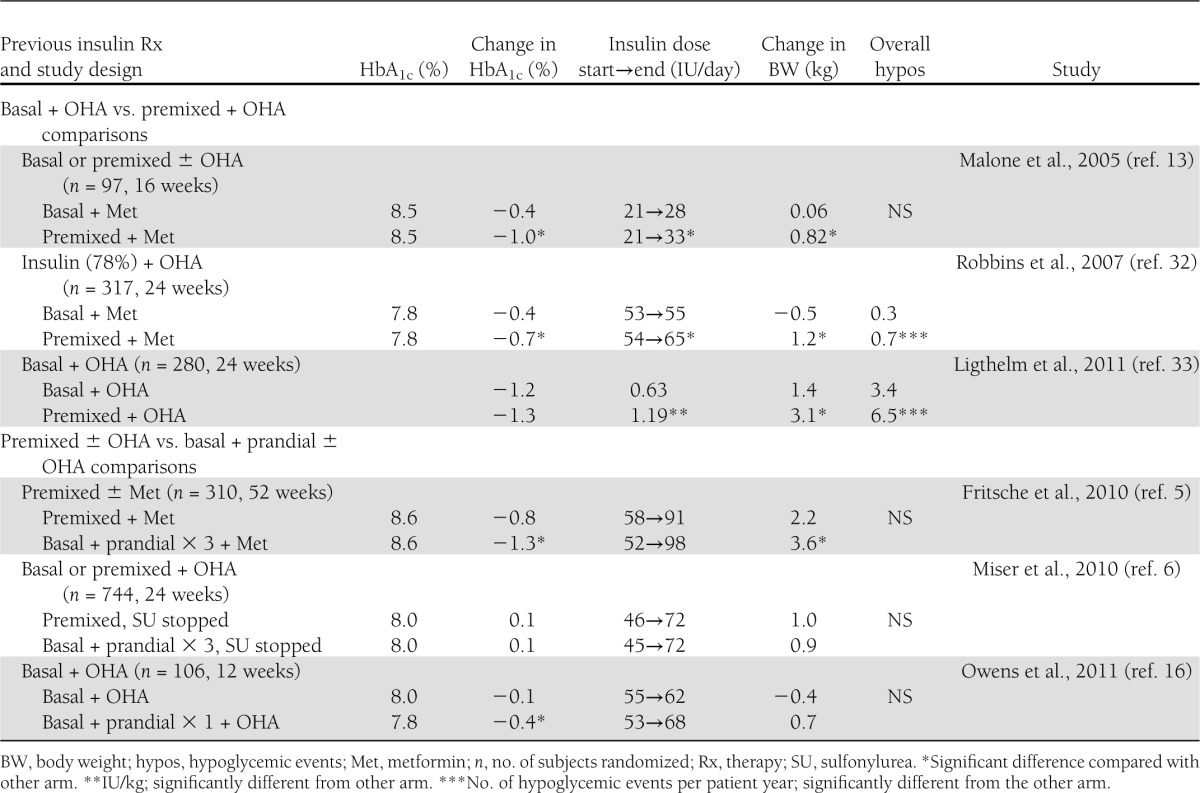
Another three studies in previously insulin-treated patients compared prandial or basal plus prandial insulin with basal or premixed insulins in the face of similar OHA. Fritsche et al. (5) compared premixed with basal plus prandial insulin therapy in the face of similar OHA. Glycemic control was better in the basal plus prandial compared with the premixed group. The patients also gained significantly more weight in the basal plus prandial compared with the premixed insulin group. In the study of Miser et al. (6), there was no improvement in glycemic control from an initial HbA1c of 8.0% (64 mmol/mol) in either the premixed or the prandial insulin group, although insulin doses were increased by 26–27 IU/day. Lack of improvement in glycemic control could have been due to discontinuation of sulfonylureas at randomization. In the study by Owens et al. (16), basal plus prandial insulin and OHA improved HbA1c by 0.4%, which was significantly more than with continued basal insulin and OHA. However, in the latter group HbA1c decreased only by 0.1% from a baseline of 8.0% (64 mmol/mol) (Table 1).
CONCLUSIONS
For the insulin-naive patients, we identified 14 trials with relevant comparisons between basal and OHA and another insulin regimen with or without OHA (Supplementary Table 1). The patient characteristics were comparable across the studies with respect to baseline age, BMI, and diabetes duration. When Figs. 1 and 2 are considered, it is obvious that glycemic targets were mostly not achieved. The meta-analysis suggested that better control has been obtained with premixed compared with basal insulin in the face of similar OHA, as in previous meta-analyses by Lasserson et al. (7) and Giugliano et al. (8). This conclusion was hampered by significant heterogeneity as in the previous analyses. More importantly, the difference in glycemic control disappeared after adjustment for insulin doses, which were consistently higher in patients using premixed rather than basal insulin (Fig. 1). This approach could of course be criticized, as it is the end glycemic control that matters. Thus, analyses after adjustment for insulin doses might be considered irrelevant for clinical practice. The lower insulin doses in basal insulin treatment arms cannot be attributed to hypoglycemia, as rates of hypoglycemia were higher or similar in patients treated with premixed compared with basal insulins (Fig. 3) (8). In all basal insulin and OHA studies in Fig. 1, the weighted mean insulin dose was 37 IU/day and HbA1c 7.5% (58 mmol/mol). This contrasts with the much higher insulin doses in the large studies achieving on average an HbA1c target of 7.0% (53 mmol/mol) with basal insulins (9–11). Thus, inadequate titration of basal insulin appears to be a likely explanation for inferior glycemic control with basal compared with premixed insulin studies.
Comparison of basal insulin and OHA with premixed insulin without OHA (Fig. 1B) or multiple insulin injections alone (Fig. 1D) showed suboptimal glycemic control and no significant differences between the treatment arms except for one study (12). As expected, insulin doses were much lower in the combination therapy than in the premixed or multiple insulin injection alone arms.
Regarding studies in previously insulin-treated patients, glycemic targets were not achieved. In the three studies comparing premixed and OHA with basal insulin and OHA, the insulin doses and weight gains were greater in the premixed compared with basal insulin arm. Basal insulin was inadequately titrated in the two studies in which glycemic control was better with premixed compared with basal insulin, as HbA1c at the end of the studies in the basal insulin arm averaged 8.1% (65 mmol/mol) (13) and 7.4% (57 mmol/mol) (13).
Given that addition of basal insulin to previous OHA has been recommended as the way to initiate insulin therapy in type 2 diabetes (14,15), one would expect to find studies comparing “intensification” of insulin therapy by use of prandial injections or replacing basal insulin by premixed insulin. As shown in Table 1, only one such study has been performed. This was a relatively small proof-of-concept study comparing addition of one prandial injection to basal insulin with continued use of basal insulin. In this study, HbA1c in the basal insulin arm averaged 7.9% (63 mmol/mol), which is much higher than has been observed in large studies adequately titrating basal insulin (9,10). The study of Miser et al. (6) is difficult to interpret, as sulfonylureas were stopped and there was no improvement in glycemic control with either premixed or basal and prandial insulin regimens. Finally, in the study of Fritsche et al. (5), better glycemic control and more weight gain were achieved with the basal plus prandial than with the premixed insulin regimen.
Taken together, the present data in insulin-naive patients do not demonstrate differences in glycemic control when the change in HbA1c is adjusted for the insulin dose used. The present analysis was limited to RCTs, which results may not be applicable to routine practice. Use of premixed or prandial insulin compared with basal insulin is associated with more hypoglycemia and weight gain. These considerations thus support use of basal insulin as an option to initiate insulin therapy, which is in keeping with the joint statements by the American Diabetes Association and European Association for the Study of Diabetes from 2009 and 2012 (14,15). Although most guidelines recommend intensification of insulin treatment by using more than one injection, data are very limited supporting this approach. Indeed, only the small proof-of-concept study (16) included a basal insulin and OHA control arm (Table 1). In the 4-T study (17,18), patients were randomized to receive biphasic insulin aspart twice daily, prandial insulin aspart three times daily, or basal insulin detemir once daily. In this study, median HbA1cs were comparable after 3 years but weight gain and hypoglycemia were less with basal than with the other insulin regimens. In this study, however, 68–82% of the patients were taking a second type of insulin at the end of the study (18). Even in previously insulin-treated patients, the frequency of hypoglycemia and weight gain increases as the number of insulin injections increases (Table 1). This implies that intensification with either continued titration of basal insulin or addition of newer agents such as glucagon-like peptide 1 analogs to basal insulin (19) might be more attractive options than an increase in the number of insulin injections.
Acknowledgments
H.Y.-J. received consultation fees from Merck, Sanofi, and Bristol-Myers Squibb and participated in an investigator-initiated trial by Amylin/Eli Lilly and in company-sponsored trials of Boehringer Ingelheim and Sanofi. H.Y.-J. received honoraria for speaking at meetings organized by Sanofi, Eli Lilly, and Merck Sharp & Dohme. No other potential conflicts of interest relevant to this article were reported.
H.Y.-J. designed the study, searched the literature, performed the statistical analyses, and wrote the manuscript. A.K. searched the literature, performed the statistical analyses, and wrote the manuscript.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dcS13-2026/-/DC1.
This publication is based on the presentations from the 4th World Congress on Controversies to Consensus in Diabetes, Obesity and Hypertension (CODHy). The Congress and the publication of this supplement were made possible in part by unrestricted educational grants from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Ethicon Endo-Surgery, Janssen, Medtronic, Novo Nordisk, Sanofi, and Takeda.
References
- 1.Goudswaard AN, Furlong NJ, Valk GD, Stolk RP, Rutten GEHM. Insulin monotherapy versus combinations of insulin with oral hypoglycaemic agents in patients with type 2 diabetes mellitus. Cochrane Database Syst Rev 2004;CD003418 [DOI] [PMC free article] [PubMed]
- 2.Bazzano LA, Lee LJ, Shi L, Reynolds K, Jackson JA, Fonseca V. Safety and efficacy of glargine compared with NPH insulin for the treatment of Type 2 diabetes: a meta-analysis of randomized controlled trials. Diabet Med 2008;25:924–932 [DOI] [PubMed] [Google Scholar]
- 3.Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev 2007:CD005613. [DOI] [PubMed] [Google Scholar]
- 4.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558 [DOI] [PubMed] [Google Scholar]
- 5.Fritsche A, Larbig M, Owens D, Häring HU, GINGER study group Comparison between a basal-bolus and a premixed insulin regimen in individuals with type 2 diabetes-results of the GINGER study. Diabetes Obes Metab 2010;12:115–123 [DOI] [PubMed] [Google Scholar]
- 6.Miser WF, Arakaki R, Jiang H, Scism-Bacon J, Anderson PW, Fahrbach JL. Randomized, open-label, parallel-group evaluations of basal-bolus therapy versus insulin lispro premixed therapy in patients with type 2 diabetes mellitus failing to achieve control with starter insulin treatment and continuing oral antihyperglycemic drugs: a noninferiority intensification substudy of the DURABLE trial. Clin Ther 2010;32:896–908 [DOI] [PubMed] [Google Scholar]
- 7.Lasserson DS, Glasziou P, Perera R, Holman RR, Farmer AJ. Optimal insulin regimens in type 2 diabetes mellitus: systematic review and meta-analyses. Diabetologia 2009;52:1990–2000 [DOI] [PubMed] [Google Scholar]
- 8.Giugliano D, Maiorino MI, Bellastella G, Chiodini P, Ceriello A, Esposito K. Efficacy of insulin analogs in achieving the hemoglobin A1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Care 2011;34:510–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riddle MC, Rosenstock J, Gerich JE, Insulin Glargine 4002 Study Investigators The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080–3086 [DOI] [PubMed] [Google Scholar]
- 10.Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 2006;29:1269–1274 [DOI] [PubMed] [Google Scholar]
- 11.Yki-Järvinen H, Kauppinen-Mäkelin R, Tiikkainen M, et al. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia 2006;49:442–451 [DOI] [PubMed] [Google Scholar]
- 12.Janka H-U, Plewe G, Riddle MC, Kliebe-Frisch C, Schweitzer MA, Yki-Järvinen H. Comparison of basal insulin added to oral agents versus twice-daily premixed insulin as initial insulin therapy for type 2 diabetes. Diabetes Care 2005;28:254–259 [DOI] [PubMed] [Google Scholar]
- 13.Malone JK, Bai S, Campaigne BN, Reviriego J, Augendre-Ferrante B. Twice-daily pre-mixed insulin rather than basal insulin therapy alone results in better overall glycaemic control in patients with Type 2 diabetes. Diabet Med 2005;22:374–381 [DOI] [PubMed] [Google Scholar]
- 14.Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association. European Association for Study of Diabetes Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inzucchi SE, Bergenstal RM, Buse JB, et al. American Diabetes Association (ADA) European Association for the Study of Diabetes (EASD) Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owens DR, Luzio SD, Sert-Langeron C, Riddle MC. Effects of initiation and titration of a single pre-prandial dose of insulin glulisine while continuing titrated insulin glargine in type 2 diabetes: a 6-month ‘proof-of-concept’ study. Diabetes Obes Metab 2011;13:1020–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holman RR, Thorne KI, Farmer AJ, et al. 4-T Study Group Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 2007;357:1716–1730 [DOI] [PubMed] [Google Scholar]
- 18.Holman RR, Farmer AJ, Davies MJ, et al. 4-T Study Group Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med 2009;361:1736–1747 [DOI] [PubMed] [Google Scholar]
- 19.Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in Basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med 2011;154:103–112 [DOI] [PubMed] [Google Scholar]
- 20.Kilo C, Mezitis N, Jain R, Mersey J, McGill J, Raskin P. Starting patients with type 2 diabetes on insulin therapy using once-daily injections of biphasic insulin aspart 70/30, biphasic human insulin 70/30, or NPH insulin in combination with metformin. J Diabetes Complications 2003;17:307–313 [DOI] [PubMed] [Google Scholar]
- 21.Malone JK, Kerr LF, Campaigne BN, Sachson RA, Holcombe JH, Lispro Mixture-Glargine Study Group Combined therapy with insulin lispro Mix 75/25 plus metformin or insulin glargine plus metformin: a 16-week, randomized, open-label, crossover study in patients with type 2 diabetes beginning insulin therapy. Clin Ther 2004;26:2034–2044 [DOI] [PubMed] [Google Scholar]
- 22.Raskin P, Allen E, Hollander PA, et al. INITIATE Study Group Initiating insulin therapy in type 2 Diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care 2005;28:260–265 [DOI] [PubMed] [Google Scholar]
- 23.Buse JB, Wolffenbuttel BH, Herman WH, et al. DURAbility of basal versus lispro mix 75/25 insulin efficacy (DURABLE) trial 24-week results: safety and efficacy of insulin lispro mix 75/25 versus insulin glargine added to oral antihyperglycemic drugs in patients with type 2 diabetes. Diabetes Care 2009;32:1007–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yki-Järvinen H, Kauppila M, Kujansuu E, et al. Comparison of insulin regimens in patients with non-insulin-dependent diabetes mellitus. N Engl J Med 1992;327:1426–1433 [DOI] [PubMed] [Google Scholar]
- 25.Wolffenbuttel BH, Sels JP, Rondas-Colbers GJ, Menheere PP, Nieuwenhuijzen Kruseman AC. Comparison of different insulin regimens in elderly patients with NIDDM. Diabetes Care 1996;19:1326–1332 [DOI] [PubMed] [Google Scholar]
- 26.Landstedt-Hallin L, Adamson U, Arner P, Bolinder J, Lins P-E. Comparison of bedtime NPH or preprandial regular insulin combined with glibenclamide in secondary sulfonylurea failure. Diabetes Care 1995;18:1183–1186 [DOI] [PubMed] [Google Scholar]
- 27.Bastyr EJ, 3rd, Johnson ME, Trautmann ME, Anderson JHJ, Jr, Vignati L. Insulin lispro in the treatment of patients with type 2 diabetes mellitus after oral agent failure. Clin Ther 1999;21:1703–1714 [DOI] [PubMed] [Google Scholar]
- 28.Bastyr EJ, 3rd, Stuart CA, Brodows RG, et al. IOEZ Study Group Therapy focused on lowering postprandial glucose, not fasting glucose, may be superior for lowering HbA1c. Diabetes Care 2000;23:1236–1241 [DOI] [PubMed] [Google Scholar]
- 29.Kazda C, Hülstrunk H, Helsberg K, Langer F, Forst T, Hanefeld M. Prandial insulin substitution with insulin lispro or insulin lispro mid mixture vs. basal therapy with insulin glargine: a randomized controlled trial in patients with type 2 diabetes beginning insulin therapy. J Diabetes Complications 2006;20:145–152 [DOI] [PubMed] [Google Scholar]
- 30.Bretzel RG, Nuber U, Landgraf W, Owens DR, Bradley C, Linn T. Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet 2008;371:1073–1084 [DOI] [PubMed] [Google Scholar]
- 31.Clauson P, Karlander S, Steen L, Efendic S. Daytime glibenclamide and bedtime NPH insulin compared to intensive insulin treatment in secondary sulphonylurea failure: a 1-year follow-up. Diabet Med 1996;13:471–477 [DOI] [PubMed] [Google Scholar]
- 32.Robbins DC, Beisswenger PJ, Ceriello A, et al. Mealtime 50/50 basal + prandial insulin analogue mixture with a basal insulin analogue, both plus metformin, in the achievement of target HbA1c and pre- and postprandial blood glucose levels in patients with type 2 diabetes: a multinational, 24-week, randomized, open-label, parallel-group comparison. Clin Ther 2007;29:2349–2364 [DOI] [PubMed] [Google Scholar]
- 33.Ligthelm RJ, Gylvin T, DeLuzio T, Raskin P. A comparison of twice-daily biphasic insulin aspart 70/30 and once-daily insulin glargine in persons with type 2 diabetes mellitus inadequately controlled on basal insulin and oral therapy: a randomized, open-label study. Endocr Pract 2011;17:41–50 [DOI] [PubMed] [Google Scholar]
- 34.Clauson PG, Linde B. Absorption of rapid-acting insulin in obese and nonobese NIDDM patients. Diabetes Care 1995;18:986–991 [DOI] [PubMed] [Google Scholar]