The pandemic of type 2 diabetes necessitates early and effective treatment to delay or prevent micro- and macrovascular complications associated with diabetes (1–3). However, the majority of patients with type 2 diabetes do not reach their therapeutic goals as a result of insufficient treatment. The cardiovascular mortality risk is increased, and 75% of patients with type 2 diabetes die of cardiovascular events. Type 2 diabetes is the main cause of end-stage renal disease and dialysis in many countries (4). The vascular complication risk can be lowered by improved metabolic control (2,3). However, further important treatment goals such as body weight reduction or the prevention of hypoglycemia are seldom accomplished.
In type 2 diabetes, a stepwise escalation of therapy is suggested along the course and progression of the disease. Metformin is widely accepted as the first-line therapy, but owing to partly lacking evidence, with regard to further therapeutic steps there is room for various individual treatment choices. Recently, a Position Statement by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) tried to value and position second- and third-line therapies according to their efficacy, side effects, hypoglycemia incidence, body weight development, and costs (5).
Adding another oral agent to metformin in order to escalate therapy when necessary presently gives the choice of sulfonylureas, glinides, dipeptidyl peptidase (DPP)-4 inhibitors, pioglitazone, and α-glucosidase inhibitors. The efficacy of these agents as add-on medication to metformin is only modest, and on average an additional HbA1c reduction of ~0.5–1% can be expected. Furthermore, the treatment with sulfonylureas, glinides, and pioglitazone is associated with body weight gain. Sulfonylurea and glinide therapy is also associated with a risk of hypoglycemia. α-Glucosidase inhibitors predominantly lower postprandial hyperglycemia and therefore show less HbA1c reduction in most studies. DPP-4 inhibitors as incretin-based therapies have demonstrated less efficacy in lowering glycemic parameters in head-to-head comparisons with glucagon-like peptide (GLP)-1 receptor agonists (GLP-1 RAs). In addition, they are body weight neutral, whereas GLP-1 RAs allow weight loss (5).
Failing oral antidiabetes therapy, an additional injectable treatment option comprises either the start of an insulin therapy (often basal insulin as the first step of insulin treatment) with dose titration to the individual glycemic goal or the injection of a GLP-1 RA as incretin-based therapy. While insulin therapy has been established for decades, treatment options with a GLP-1 RA first became available in 2005. In the meantime, therapy with GLP-1 RA has become more widely used and is seen as an alternative to insulin in certain patients, especially when obesity is also present. In this article, we will debate these two options as possible treatment escalations when oral therapy fails in light of the results of recently published studies on the topic, e.g., the Exenatide Versus Glimepiride for Prevention of Glycemic Deterioration Among Those with Type 2 Diabetes and Metformin Failure (EUREXA) study (for GLP-1 RA) (6) and the Outcome Reduction With Initial Glargine Intervention (ORIGIN) Trial for insulin (7).
Incretin side of the debate
GLP-1 RAs: developments and mode of action.
GLP-1 RAs selectively bind to the GLP-1 receptor, a seven transmembrane–spanning receptor coupled to G-proteins. Activation of the pancreatic GLP-1 receptor on β-cells leads to a glucose-dependent stimulation of insulin secretion and, via indirect pathways, to an also glucose-dependent inhibition of glucagon secretion. These are the two main effects of the glucoregulatory action of GLP-1 RA. Owing to the glucose dependency of these actions, the intrinsic hypoglycemia risk of the GLP-1 RA is very low. Studies in rodents and in isolated human islets have shown an improvement of islet function after GLP-1 exposure as well as an increase in β-cell mass—predominantly the result of an inhibition of β-cell apoptosis. Besides that, extrapancreatic effects of GLP-1 have been observed: in the central nervous system, GLP-1 is a neurotransmitter in hypothalamic areas responsible for the mediation of satiety signals; in the gastrointestinal tract, GLP-1 slows gastric emptying; and in the cardiovascular system, GLP-1 has been found to decrease blood pressure, increase the pulse rate, and improve cardiac function measured by echocardiography. In animal studies, GLP-1 and GLP-1 RA were able to decrease infarct sizes in experimental myocardial ischemia (8,9).
Exenatide was the first GLP-1 RA to be introduced into type 2 diabetes therapy in 2005. It is the synthetic form of exendin-4, a reptilian peptide from the glucagon family with a 53% sequence similarity to GLP-1. It was found to be a good agonist at the GLP-1 receptor and to be resistant to degradation by DPP-4, the enzyme cleaving native GLP-1 within 1–2 min. Exenatide therefore has a half-life of ~3.5 h, making it suitable for twice-daily injections (10).
Liraglutide, the first human GLP-1 analog for once-daily injection, was introduced in 2009. It has a fatty acid side chain covalently bound to the peptide chain leading to aggregation and albumin binding of liraglutide molecules. This prolongs biological half-life and prevents degradation by DPP-4 (11).
The recent further developments of GLP-1 RA have led to longer-acting compounds suitable for once-weekly injection. A weekly formulation of exenatide (exenatide QW, Bydureon; Amylin and Lilly Pharmaceuticals) became available in 2011. Albiglutide (GlaxoSmithKline), a human GLP-1 analog for once-weekly injection, is far advanced in development, as are other compounds on a human GLP-1 basis. Lixisenatide (sanofi-aventis) is a GLP-1 RA similar to exendin-4 for once-daily injection that is far advanced in clinical studies (12).
In the initial clinical studies with exenatide performed in patients failing either on metformin or on sulfonylurea therapy or on a combination of both, additional exenatide treatment demonstrated a decrease of HbA1c versus placebo of 0.9–1.0%, accompanied by a weight loss of 1.6–2.8 kg (13). The patients with metformin as baseline therapy had the lowest rate of hypoglycemia and the best results concerning glycemic improvement and weight loss. In this group, the hypoglycemia incidence was not significantly increased compared with placebo. The higher hypoglycemia incidence in the sulfonylurea-treated patients was due to the use of the sulfonylurea that exerts its insulinotropic action in a glucose-independent manner. The open 3-year study extension of this set of studies demonstrated a sustained HbA1c reduction of 1.0% and a weight loss of 5.3 kg in the study completers (13). Nausea was reported by ~40% of patients. This was mild to moderate and transient and led to a discontinuation of the drug in only ~5% of patients (10). These studies led to the approval of the first GLP-1 RA and to the positioning of this drug class for patients failing on metformin or sulfonyurea monotherapy or on a dual therapy with both agents. In the initial phase III clinical study for liraglutide, this GLP-1 RA was investigated in patients with a more heterogenous disease progression: testing liraglutide efficacy and safety in monotherapy against adding a second oral agent or in a multiple oral drug combination and against the addition of insulin glargine. In these studies, liraglutide was more efficacious in lowering glycemic parameters compared with the comparators and additionally led to a significant weight loss (11). Gastrointestinal side effects were observed less frequently compared with exenatide in a direct head-to-head comparison (14). In recent studies with longer-acting GLP-1 RA, the glycemic efficacy was further improved compared with the shorter-acting agents (8,9,12,15).
Head-to-head studies of GLP-1 RAs with different strategies of insulin therapy.
In several clinical studies, head-to-head comparisons between GLP-1 RA and insulin therapy have been performed, mostly in patients with metformin failure. Exenatide twice daily has been compared head to head against insulin glargine and biphasic premixed insulin aspart 70/30 (16–20). In these studies, exenatide and insulin as add-on therapies achieved similar improvements in overall glycemic control in patients with type 2 diabetes on suboptimal control with oral combination therapy. In all of these studies, exenatide treatment was associated with superior weight reduction but had a higher incidence of gastrointestinal adverse effects than insulin. Exenatide led to better postprandial glycemic control compared with either insulin glargine or premixed insulin aspart 70/30 (16–19); in one study, exenatide was superior regarding hypoglycemia incidence versus biphasic premixed insulin aspart 70/30 (8.0 vs. 20.5% for exenatide vs. insulin, respectively; P < 0.05) (20).
In a 3-year study comparing exenatide and insulin glargine in a similar but smaller patient cohort (36 patients completed the study), exenatide, in addition to showing an advantage in body weight development (−7.9 ± 1.8 kg; P < 0.001), demonstrated a positive effect on parameters of insulin sensitivity and β-cell function after a 4-week drug washout period. Exenatide increased the M value for insulin sensitivity by 39% (P = 0.006), while insulin glargine had no effect (P = 0.647). After the washout period, the disposition index increased with exenatide, while it decreased with insulin glargine compared with the pretreatment values (1.43 ± 0.78 and −0.99 ± 0.65, respectively; P = 0.028). These findings may suggest a beneficial effect on β-cell health (21).
Recently, a further study comparing exenatide QW with insulin glargine over a study period of 84 weeks showed better glycemic control with a lower hypoglycemia incidence and with sustained overall weight loss with exenatide QW. Of the patients on exenatide QW, 31.3% reached an HbA1c <6.5%, whereas only 20.2% patients on insulin glargine met this predefined end point (P = 0.009). The body weight difference was 4.5 kg at the end of the study. The patients on exenatide QW lost 2.1 kg body wt, whereas those on insulin glargine gained 2.4 kg (P < 0.001). Additionally, with regard to hypoglycemia, there was a significantly better outcome in the patients on exenatide QW. Among patients on a dual background medication with metformin plus sulfonylurea, the incidence of minor hypoglycemia was 24% in the exenatide QW group vs. 54% for insulin glargine patients (P < 0.001); among patients taking metformin alone, it was 8% for exenatide QW patients compared with 32% in the insulin glargine group (P < 0.001). Gastrointestinal adverse events occurred more frequently in the exenatide QW group than in the insulin glargine–treated patients (12 vs. 6% with diarrhea and 15 vs. 1% with nausea, respectively; P < 0.05) (22).
Liraglutide was also compared with therapy with insulin glargine in a study with patients on dual oral therapy with a daily dose of 2,000 mg metformin and 4 mg glimepiride. In this study, liraglutide reduced the HbA1c significantly compared with insulin glargine and met the predefined noninferiority criterion (1.33 vs. 1.09% [95% CI 0.08–0.39]; P = 0.0015). Expectedly and in line with the findings for exenatide, greater weight loss was observed with liraglutide compared with insulin glargine (treatment difference −3.43 kg [4.00–2.86]; P < 0.0001). Furthermore, liraglutide reduced systolic blood pressure (−4.0 mmHg) compared with insulin glargine (0.5 mmHg higher; −4.5 mmHg difference [6.8 to −2.2]; P = 0.0001). The rates of hypoglycemic episodes in the liraglutide-treated patients were 0.06 (major hypoglycemia), 1.2 (minor hypoglycemia), and 1.0 (symptoms only) events/patient/year, respectively, in the liraglutide group compared with 0, 1.3, and 1.8 in the insulin glargine–treated group. Nausea occurred in 14% of patients on liraglutide, and 9.8% of participants in the group receiving liraglutide developed antiliraglutide antibodies (23).
Why may GLP-1 RAs be advantageous over insulin therapy in type 2 diabetes?
In the head-to-head studies comparing the add on of GLP-1 RA to insulin therapy in patients on oral background treatment with metformin or dual-drug therapy including a sulfonlyurea, the GLP-1 receptor agonists tested showed noninferiority concerning glycemic parameters compared with insulin. The long-acting GLP-1 RAs as well as liraglutide demonstrate a more sustained effect on fasting plasma glucose, while the effect on postprandial hyperglycemia is modest compared with that of the short-acting exenatide for twice-daily injections (12,24).
In most studies, the observed overall hypoglycemia incidence was also lower in the patients treated with a GLP-1 receptor agonist. A recent meta-analysis investigated the head-to-head studies comparing GLP-1 RA therapy directly with insulin treatment (24). Hypoglycemic episodes were reported by 509 patients in total: 200 of 877 in the GLP-1 RA group and 309 of 855 in the insulin therapy group. A statistically significant decrease in risk of hypoglycemia associated with the use of GLP-1 RA was found based on the random-effect pooling (Mantel-Haenszel odds ratio 0.45 [95% CI 0.27–0.76]; P < 0.01). The trial by Heine et al. (16) comparing insulin glargine with exenatide revealed no statistical difference in the overall incidence of hypoglycemia (events/patient/year). The trial by Nauck et al. (17) comparing exenatide with premixed biphasic insulin aspart 70/30 also showed similar rates at end point. Severe hypoglycemia was rare with GLP-1 RA, with only 10 of 1,130 patients treated with exenatide compared with 15 of 1,103 patients on insulin therapy. No statistically significant increase in risk of severe hypoglycemia was demonstated with GLP-1 RA (Mantel-Haenszel odds ratio 0.65 [0.29–1.45]; P = 0.29). When all data were combined in the meta-analysis, nocturnal hypoglycemia was less frequent in the GLP-1 RA group compared with the insulin-treated group (24).
Weight gain associated with insulin treatment is an additional disadvantage that may affect insulin sensitivity and may be counterproductive for patient motivation toward therapeutic adherence. When given to obese patients with or without diabetes, GLP-1RA results in clinically relevant beneficial effects on body weight. Beneficial effects on blood pressure and cardiovascular surrogate parameters such as a reduction in total cholesterol may also be achieved (10–13). Therefore, GLP-1 RA rather than insulin therapy should be considered in patients with diabetes who are obese or overweight (15). As established thus far, no clinically significant differences seem to exist within the entire group of GLP-1 RAs concerning their effect on body weight reduction (12). Furthermore, the standard doses used for GLP-1 RA treatment may be advantageous in facilitating therapy compared with insulin, where dose titration and additional glucose self-monitoring are necessary. Here, the once-weekly long-acting GLP-1 RA may have additional benefits (10,12,15,24).
The further and future positioning of the GLP-1 RA will of course be dependent on data from ongoing long-term trials investigating not only the durability of glycemic control and body weight reduction but also, and more importantly, cardiovascular end points and safety. In this respect, the results of the LEADER trial with liraglutide (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results - A Long Term Evaluation) and the EXSCEL study with exenatide QW (Exenatide Study of Cardiovascular Event Lowering) will generate important data (9,12,25,26). Additionally, more information as to whether the GLP-1 RA can protect β-cell function and thereby minimize the progression of type 2 diabetes would be an additional important point in the positioning. Here, we have little data proving a direct effect on β-cell function, apoptosis rate, and proliferation in humans in vivo. In a direct comparison in a clinical study, however, the long-term treatment with exenatide showed significant benefits of exenatide versus glimepiride for control of glycemic deterioration in patients with type 2 diabetes as an indirect parameter on β-cell function over time (6).
The recent ADA/EASD Position Statement for the therapy of type 2 diabetes has already changed the positioning of GLP-1 RA treatment from “less validated treatments” in the preceding statement (27) to an equal positioning as second-line therapy with insulin. The novel statement evaluates therapies not only by their efficacy in glucose lowering but also by treatment side effects, hypoglycemia incidence, body weight development, and costs as well as individual considerations concerning patient characteristics (e.g., disease duration, comorbidities, cardiovascular risk) (5). In conclusion, the indications and outcomes for GLP-1 RA treatment compared with insulin therapy seem favorable for patients with type 2 diabetes when oral antidiabetes therapy and lifestyle intervention do not achieve the therapeutic goals. In this case, an injectable therapy with a GLP-1 RA is feasible, especially when body weight reduction and prevention of hypoglycemia are further important therapeutic aims.
Insulin side of the debate
Rationale for early insulin supplementation.
If monotherapy with metformin does not achieve or maintain HbA1c target over ∼3 months, the next step could be to add basal insulin, which has the highest efficacy in lowering HbA1c compared with other options recommended (5). In light of limited data from interventional trials, what could be the rationale for early insulin supplementation?
The principal abnormalities in type 2 diabetes are impaired peripheral insulin action (insulin resistance), insulin secretory dysfunction, and glucagon hypersecretion further promoting hepatic glucose production. An analysis from the Whitehall II study reveals that the pancreatic β-cell can compensate in the long term for declining insulin sensitivity in the metabolic syndrome stage before break down after ~10–12 years (28). At the time of diagnosis of type 2 diabetes, ∼50% of pancreatic β-cell function has been lost, with almost 4–6% further loss of function expected per year thereafter (29,30). Furthermore, there is a significant and progressive loss of pancreatic β-cell mass (31). Recently, a morphometric and β-cell function study designed to examine the pathogenetic relevance of β-cell loss in patients with pancreatic abnormalities undergoing pancreatic surgery revealed that diabetes manifests when β-cell mass declines by ~65% in humans (32).
Insulin therapy in patients with type 2 diabetes corrects glucotoxicity and lipotoxicity, improves peripheral insulin action, and decreases hepatic fat content (33,34). Early implementation of a short course of intensive insulin therapy by continuous subcutaneous or multiple daily injections can induce sustained euglycemia in significantly more patients than those treated with oral hypoglycemic agents (OHAs) (35). Moreover, this short period of intensified insulin therapy had favorable outcomes with regard to recovery and maintenance of β-cell function and prolonged remission (in ~40% of patients) compared with OHA treatment (35,36).
Several studies have found that beyond metabolic effects, insulin may exert nonglycemic effects, in particular pleiotropic anti-inflammatory, antiatherogenic, and antiapoptotic effects (37–39).
The preceding evidence supports the concept of insulin-mediated cessation of disease progression with early insulin intervention to preserve pancreatic β-cells. Based on the improved understanding of the pathophysiology and natural history of type 2 diabetes, insulin therapy should be introduced—not as a last resort but as soon as metformin is inadequate to sustain glycemic targets.
Initiating insulin therapy in type 2 diabetic patients failing on OHAs: the basal-supported oral therapy.
Studying the relationships between fasting plasma glucose levels and insulin secretion during intravenous glucose tolerance tests, Brunzell et al. (40) found that the early insulin secretory response was largely diminished at fasting plasma glucose levels >115 mg/dL. A recent study examined the effects of chronic supplementation of long-acting basal insulin glargine over 8 weeks versus acute intravenous insulin on endogenous β-cell function in hyperglycemic patients with type 2 diabetes on metformin monotherapy (41). Mean age of patients was 55.7 years, and diabetes duration averaged 4.6 years. Chronic reduction but not acute normalization of fasting glycemia improved first- and second-phase insulin secretion during an intravenous glucose tolerance test (41). Interestingly, adding insulin glargine versus NPH insulin to metformin seemed to result in more efficient postprandial β-cell protection in subjects with type 2 diabetes (42).
There are several physician and patient barriers to initiating insulin therapy, particularly concerns about risk of hypoglycemia, weight gain, and inconvenience (43). Large interventional trials with different insulin regimens demonstrated noninferiority of basal insulin analogs (either glargine or detemir) in terms of metabolic control compared with prandial or premixed insulin when added to OHAs (44,45). However, the basal insulin regimens were associated with significantly fewer hypoglycemic events (44,45), less weight gain (44,45), and improved patient treatment satisfaction (45) compared with either prandial or premixed insulin regimens. In a Position Statement on the significance of long-acting insulin analogs (glargine or detemir), 18 published randomized controlled trials (RCTs) and 11 meta-analyses considering the hypoglycemic risk were summarized (46). In 15 of 18 (83%) trials and all 11 meta-analyses, a significantly lower risk of hypoglycemia was described for basal insulin analog therapy compared with the use of NPH basal insulin (46). A favorable action profile of long-acting insulin analogs compared with NPH insulin may at least in part explain the different outcomes observed (47).
Finally, basal insulin analog therapy is more cost-effective than prandial insulin analog therapy (48).
In summary, the addition of basal insulin analogs to oral hypoglycemic agents like metformin is effective, safe, simple, and convenient and may be helpful in overcoming major barriers to timely insulin initiation in both primary and secondary care settings (49,50).
Studies with basal insulin (analogs) added onto metformin.
A meta-analysis of 27 published RCTs with >11,000 type 2 diabetic patients treated with different noninsulin antidiabetes drugs for a mean of 32 weeks added to metformin was recently published (51). The overall effects on HbA1c, body weight, and the relative risk of hypoglycemic events using sulfonylureas, glinides, glitazones, α-glucosidase inhibitors, DPP-4 inhibitors, or GLP-1 RAs were −0.79%, 0.14 kg increase, and 1.43-fold, respectively. Sulfonylureas and glinides were associated with the highest relative risk of hypoglycemia (2.63 and 7.92, respectively).
Should a GLP-1 RA or a basal insulin analog be added to metformin? The meta-analysis mentioned above partly provides an answer: GLP-1 RA therapy lowered HbA1c by 0.99% (mean) and was associated with no risk of hypoglycemia (0.94) and a loss of weight (−1.76 kg) (51). One may argue that the limited efficacy of lowering HbA1c by ~1% of GLP-1 RA therapy is not sufficient to meet glycemic targets in most of the patients failing on metformin monotherapy. By contrast, basal insulin analogs added to metformin provide relatively uniform insulin coverage throughout the day and night, thus making nearly any target of HbA1c achievable, provided insulin dose titration is properly done.
A large, pooled analysis of prospective RCTs in patients with uncontrolled type 2 diabetes on 0–2 OHAs initiating basal insulin glargine after a specific titration algorithm was published last year (52). Of 63 RCTs performed between 1997 and 2007, eleven studies were eligible, meeting the following criteria: 1) studies were phase 3 or later prospective RCTs of ≥24 weeks’ duration; 2) enrolled adult patients with type 2 diabetes with inadequate glycemic control; 3) basal insulin was given once daily, with no concomitant prandial or bolus insulin administration; 4) insulin glargine was initiated at 10 units/day and was administered according to predefined titration algorithms with frequent insulin dose adjustment (from every 1–3 days to every week) to achieve fasting plasma glucose levels <100 mg/dL; and 5) studies were conducted according to good clinical practice and in accordance with the Declaration of Helsinki (52).
A total of 2,171 patients in the 11 selected studies were treated with insulin glargine, of whom 2,154 were included in the final pooled analysis after removal of 17 patients who were taking three or more OHAs at baseline. Mean age of patients was 58.6 years, and diabetes duration averaged 8.9 years. Approximately 2% of patients were taking no OHA, 45% took one OHA, and 52% took two OHAs. Nearly one-half (49.9%) of all patients were taking metformin plus sulfonylurea combination therapy before basal insulin treatment; 36.5% of patients took sulfonylurea only, and 8.5% took metformin only. Overall, at baseline mean HbA1c was 8.77%, fasting plasma glucose 198.8 mg/dL, and body weight 88.5 kg. Study end points included week-24 HbA1c level and change from baseline, the percentage of patients reaching a target HbA1c level of ≤7.0%, change in body weight from baseline, insulin dose at end point, and symptomatic and severe hypoglycemic incidence and event rates during the treatment period (52).
Interestingly, patients on no or one OHA and those on metformin monotherapy at baseline had the largest 24-week reductions in HbA1c after the addition of basal insulin glargine (∼0.44 units/kg body wt): ΔHbA1c −1.8% (mean at baseline 8.87% to mean at 24 weeks 7.1%) and ΔHbA1c −2.0% (mean at baseline 9.08% to mean at 24 weeks 6.9%), respectively. Mean ΔHbA1c in patients on two OHAs, sulfonylurea, and metformin plus sulfonylurea at baseline was –1.7%. Of patients failing on metformin or sulfonylurea monotherapy and metformin plus sulfonylurea in combination, 68.1, 50.4, and 56.4% achieved HbA1c ≤7.0%, respectively (P = 0.0006) (52).
Weight gain was lowest when basal insulin glargine was added to metformin (mean 1.6 kg) compared with a mean of 2.3 and 2.0 kg in patients on sulfonylurea only and metformin plus sulfonylurea, respectively (52).
Patients on no or one OHA at baseline had significantly less symptomatic hypoglycemia when basal insulin glargine was added than those on two OHAs (P = 0.0007). The mean insulin dose per kilogram in patients on metformin only was higher than that for patients on sulfonylurea only or on sulfonylurea plus metformin combination therapy (0.54 vs. 0.43 vs. 0.43 units/kg, respectively). However, despite higher insulin doses, those taking metformin alone had less hypoglycemia than those taking sulfonylurea or metformin plus sulfonylurea: 1.81, 4.88, and 7.30 (mean event rate/subject year), respectively, for symptomatic hypoglycemia and 0.67, 1.05, and 1.56 for confirmed symptomatic hypoglycemia. Overall, the incidence of severe symptomatic hypoglycemia after addition of basal insulin glargine in patients on metformin only, sulfonylurea only, and metformin plus sulfonylurea was very low: 2 of 278 (1.1%), 10 of 792 (1.3%), and 14 of 1,030 (1.4%), respectively (52).
The article included a meta-analysis of studies to further explore the effects of the addition of basal insulin glargine in participants who were uncontrolled on no or one versus two OHAs and any significant differences when added to metformin versus metformin plus sulfonylurea or sulfonylurea only. This meta-analysis functioned as a sensitivity analysis to assess the robustness of the pooled analysis while controlling for effects such as sample size. The authors performed a literature search of PubMed using the search terms “insulin glargine” and “type 2 diabetes” for articles published before 18 June 2010. Of 977 articles, only 5 met the strict criteria for inclusion in their meta-analysis (45,53–56). Overall, the results from the meta-analysis were consistent with the pooled analysis in that adding basal insulin glargine to metformin monotherapy provided beneficial glycemic control (52).
The authors concluded that, in particular, adding insulin glargine to metformin monotherapy was well tolerated and resulted in a significant proportion of patients achieving the glycemic goal of HbA1c ≤7.0% with a low risk of hypoglycemia and weight gain, in spite of a higher insulin dose used on average. They further concluded that some patients may benefit from the initiation of basal insulin earlier in the management of type 2 diabetes and supported the inclusion of insulin as a second step in the ADA/EASD treatment algorithm (5,27). The authors confess that one major limitation of the analysis was that only studies of insulin glargine were evaluated in both the pooled and meta-analysis; thus, applicability to other basal insulin formulations (e.g., NPH insulin and basal insulin analog detemir) is unknown.
Some doctors often argue that RCTs do not reflect “real-life” of type 2 diabetes management in daily practice. Therefore, it is of particular interest that in a predominantly primary care setting, addition of insulin glargine using a simple algorithm achieved significant improvements in glycemic control in patients with type 2 diabetes in all four study arms of the Glycemic Optimization with Algorithms and Labs at Point of Care (GOAL A1C) trial (57). Furthermore, two large open, multicenter, prospective observational studies up to 32 months performed in Germany with 12,216 type 2 diabetic patients failing on OHAs (58,59) and 1,438 type 2 diabetic patients failing on metformin only (60) demonstrated that timely addition of basal insulin glargine was effective and safe also in everyday clinical practice. Recently, the first study to explore the management of insulin initiation using basal insulin analogs (insulin detemir or insulin glargine) in a real-world clinical setting in France was published (61). This 3-month longitudinal observational study was conducted across 761 French centers in insulin-naive type 2 diabetic patients managed under routine clinical care conditions in either primary or secondary care. End points included changes in HbA1c, fasting plasma glucose, rate of hypoglycemia, weight, and adverse events. Of 2,541 patients included, 1,970 (78%) patients were taking insulin detemir, 549 (22%) patients were taking insulin glargine, and the vast majority were prescribed a once-daily dose (97%). The final analysis was performed on 1,863 patients. Based on their results, the authors concluded that insulin initiation can be successfully managed in both primary and secondary care, with most physicians maintaining some use of OHAs alongside the basal insulin analog (61). Most patients (93%) were satisfied or very satisfied with their insulin; insulin detemir and insulin glargine offered equivalent glycemic control combined with an associated low rate of hypoglycemia. Insulin detemir offered the additional benefit of a small weight loss (−0.5 kg as a mean after 3 months), whereas insulin glargine proved weight neutral. Finally, a recent study (Add-on Lantus to Oral Hypoglycemic Agents [ALOHA] study) demonstrated that initiation of insulin glargine to achieve treatment target in Japanese type 2 diabetic patients (n = 3,180) on a basal-supported oral therapy (BOT) regimen is possible under real-life conditions using an appropriate starting dosage and subsequent dose adjustment (62).
Early insulin therapy in type 2 diabetes and prediabetes: outcomes research (the ORIGIN Trial).
The ORIGIN Trial is the largest and longest worldwide prospective intervention trial to assess whether basal insulin therapy to normalize fasting plasma glucose levels may reduce cardiovascular events in people ≥50 years of age with impaired fasting glucose, impaired glucose tolerance, or early type 2 diabetes in addition to other cardiovascular risk factors (63). The trial also tested the effect of n-3 fatty acid supplements versus placebo on cardiovascular outcomes, which will not be discussed in this article.
A total of 12,612 people in 40 countries were randomized during a 2-year period ending December 2005. The trial tested the effect of titrated once-daily basal insulin glargine to target a FPG ≤95 mg/dL versus standard care. The median follow-up was 6.2 years; at study end, the primary outcome status (death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke) was known for 12,443 participants of a total of 12,537 (mean age 63.5 years [35% female]) enrolled (99%). The study ended in December 2011, and the outcome results were just published (7). More than 50% of 6,264 participants randomized to insulin glargine achieved an FPG level 95 mg/dL, and 75% of these participants achieved FPG levels <108 mg/dL for most of the trial. The median insulin dose for glycemic control was 0.40 units/kg body wt by year 6. Long-term glycemic control was sustained with insulin glargine achieving a HbA1c of 6.2% at study end compared with 6.5% under standard care (difference not significant). However, achieving optimal metabolic control did not affect cardiovascular outcomes in these participants with early dysglycemia during the study period: for first coprimary end point, hazard ratio 1.02 (P = 0.63; NS) and second coprimary end point 1.04 (P = 0.27; NS). There was also no difference between the two arms in the incidence of other outcomes like microvascular lesions (kidney or eye disease), all-cause mortality, and cancers. The risk of severe hypoglycemia was 0.7% higher in the insulin glargine group (1 vs. 0.3% per year), and participants randomized to insulin glargine gained a mean of 1.6 kg (340 g per year) during >6 years of follow-up compared with 0.5 kg weight loss with standard care.
In people with prediabetes (impaired fasting glucose or impaired glucose tolerance) at baseline, the development of new diabetes was significantly reduced by 28% (hazard ratio 0.72; P = 0.006) with insulin glargine compared with standard care (7).
The authors concluded the following: 1) Insulin glargine had a neutral effect on cardiovascular outcomes and cancer when used to target fasting plasma glucose levels for >6 years. 2) It significantly reduced new-onset diabetes. 3) Moreover, this therapy maintained near-normal glycemic control and slowed progression of dysglycemia, but it was associated with a modest increase in hypoglycemic episodes and weight gain. 4) Whether the glycemic benefit will affect future microvascular or other outcomes remains unknown. 5) In the meantime, the findings of the ORIGIN Trial do not support changing standard therapies for early dysglycemia (7).
Conclusions
All three components of hyperglycemia—elevated fasting blood glucose; elevated postprandial blood glucose; and, as a consequence, elevated HbA1c—contribute to and correlate with the development and progression of microvascular and macrovascular complications in subjects with diabetes (64). Glycemic targets from the European Society of Cardiology/EASD guidelines are <108 mg/dL for fasting blood glucose, 2 h after beginning eating postprandial blood glucose <135 mg/dL, and ≤6.5% for HbA1c in patients with type 2 diabetes (65). Recently, the ADA/EASD guidelines have recommended new HbA1c treatment goals in their patient-centered approach (5).
In patients not reaching therapeutic goals on metformin only to achieve/maintain an HbA1c target over ∼3 months, the next step recommended was to add either a second OHA, a GLP-1 RA, or basal insulin (5). Basal insulins, especially basal insulin analogs (glargine or detemir), added to OHAs (in particular metformin) alone at bedtime, have the highest efficacy lowering HbA1c and fasting blood glucose with consecutively improved postprandial blood glucose levels (44,45). In principle, insulin therapy has a high risk of hypoglycemia and is associated with weight gain compared with the other options recommended on level 2 of the treatment algorithm (5). However, BOT using either insulin glargine or insulin detemir was associated with significantly less risk comparing BOT using NPH insulin with other insulin regimens like prandial insulin or premixed insulin. Insulin therapy theoretically allows reaching glycemic goals from high baseline glycemic values by individual and consequent dose titration to the desired target. Basal insulin analogs in that respect provide relatively uniform insulin coverage throughout the whole day and night.
On the other hand, GLP-1 RAs added to metformin have a high efficacy in lowering HbA1c and postprandial blood glucose with improvement of fasting blood glucose levels alongside (8,9). By contrast, GLP-1 RA treatment confers no risk of hypoglycemia with further advantage of weight loss and a relative low risk of gastrointestinal side effects (10,12,15).
Two head-to-head studies of GLP-1 RAs, using either exenatide once per week or liraglutide once a day, found superiority or noninferiority in terms of lowering HbA1c compared with basal insulin glargine (21,22). In both studies, GLP-1 RA therapy was associated with a lower risk of hypoglycemia and loss of weight compared with insulin glargine therapy. Both basal insulin therapy and GLP-1 RA therapy are more effective but still more expensive than OHA treatment.
Recently, a systematic review of the efficacy of eight classes of diabetes medications used in current clinical practice (metformin, sulfonylureas, α-glucosidase inhibitors, thiazolidinediones, glinides, DPP-4 inhibitors, GLP-1 RAs, and insulin analogs) to reach the HbA1c target <7.0% in type 2 diabetes was performed (66). A total of 218 RCTs (339 arms and 77,950 patients) were identified in MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials through April 2011, which included at least 30 subjects in every arm with a follow-up of at least 12 weeks.
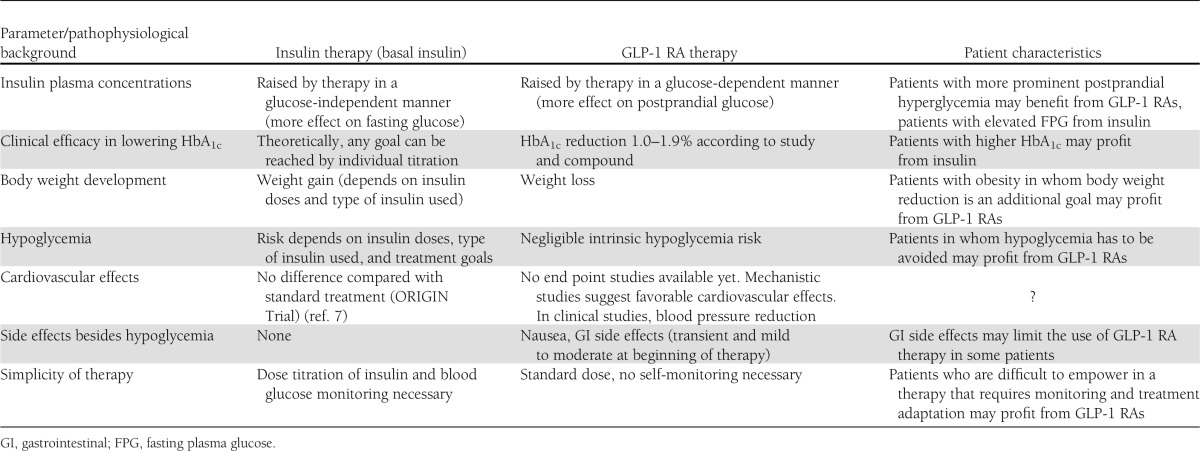
For GLP-1 RAs (n = 5,783 patients) and basal insulins (21,615 patients), overall rates of patients achieving a target of HbA1c <7.0% were 45.7 and 38.9%, respectively. In a much smaller group of only 668 patients treated with long-acting release exenatide, a rate of 63.2% was found. Outcomes for insulins and noninsulin drugs (including GLP-1 RAs) divided by different strata of baseline HbA1c clearly demonstrated a much lower efficacy of noninsulin drugs compared with insulins beyond a baseline HbA1c of ~9.5%.; i.e., this review emphasizes that in most of the patients failing on OHA treatment, adding either a basal insulin analog or a GLP-1 RA is meaningful and effective, with a preference for insulin therapy when baseline HbA1c is already >9.5%. But even with both of these best monotherapy options added to OHA therapy, an average of 40–60% of the patients will not achieve a <7.0% HbA1c target. Table 1 summarizes the characteristics and advantages for various patient types of either therapeutic option of injectable agents when therapeutic goals are not reached with OHA therapy alone.
Table 1.
Characteristics of insulin and GLP-1 RA therapy for distinct patient types not meeting therapeutic goals on oral diabetes treatment alone
What next after incretin or insulin: potential for a combination of GLP-1 RA with insulin?
In type 2 diabetic patients failing on metformin plus basal insulin, adding an injection of prandial insulin (“basal plus”) or a GLP-1 RA could be an appropriate next step (67). The combination of a GLP-1 RA with a basal insulin analog, probably in the near future in a single injectable preparation, should provide synergetic metabolic effects with additional beneficial nonglycemic cardiovascular effects (68) along with a low risk of side effects. A combination therapy comprising basal insulin with a GLP-1 RA would have the advantage of a standard dose of the GLP-1 RA rather than an additional individual dose titration with a short-acting prandial insulin.
The proof of concept of such a combination therapy (GLP-1 RA added to insulin) has already been demonstrated, showing less weight gain and less hypoglycemia than an insulin therapy (69–71). However, it has to be shown by large long-term intervention trials that such combination therapy given early to type 2 diabetic patients, probably when metformin monotherapy is not sufficient and an injectable therapy is feasable, can prevent the development or halt the progression of diabetic secondary complications. Hopefully, such megatrials will not add further frustration (72). Given the described beneficial effects of setting the pancreatic β-cell at rest by early insulinization and the protective and probably restorative effect of GLP-1 RAs on pancreatic β-cells, the optimal recommendation for treatment should start with a GLP-1 RA combined with basal insulin therapy early in the course of this chronic, progressive disease type 2 diabetes.
Acknowledgments
B.G. has been a consultant for and received honoraria from AstraZeneca, Bristol-Myers Squib, Boehringer Ingelheim, Eli Lilly, Novartis, Novo Nordisk, Merck, Roche, Sanofi, and Takeda. R.G.B. has been a consultant for and received honoraria from AstraZeneca, GlaxoSmithKline, Eli Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, and Sanofi. No other potential conflicts of interest relevant to this article were reported.
B.G. and R.G.B. retrieved the necessary data, wrote the manuscript, discussed it at all stages, and approved the final version. B.G. and R.G.B. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This publication is based on the presentations from the 4th World Congress on Controversies to Consensus in Diabetes, Obesity and Hypertension (CODHy). The Congress and the publication of this supplement were made possible in part by unrestricted educational grants from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Ethicon Endo-Surgery, Janssen, Medtronic, Novo Nordisk, Sanofi, and Takeda.
References
- 1.International Diabetes Federation. Diabetes atlas [article online], 2011. Available from http://www.idf.org/diabetesatlas/5e/the-global-burden Accessed 14 December 2012
- 2.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 3.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580–591 [DOI] [PubMed] [Google Scholar]
- 4.U.S. Renal Data System. USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2011 [Google Scholar]
- 5.Inzucchi SE, Bergenstal RM, Buse JB, et al. American Diabetes Association (ADA) European Association for the Study of Diabetes (EASD) Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallwitz B, Guzman J, Dotta F, et al. Exenatide twice daily versus glimepiride for prevention of glycaemic deterioration in patients with type 2 diabetes with metformin failure (EUREXA): an open-label, randomised controlled trial. Lancet 2012;379:2270–2278 [DOI] [PubMed] [Google Scholar]
- 7.Gerstein HC, Bosch J, Dagenais GR, et al. ORIGIN Trial Investigators Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319–328 [DOI] [PubMed] [Google Scholar]
- 8.Aroda VR, Henry RR, Han J, et al. Efficacy of GLP-1 receptor agonists and DPP-4 inhibitors: meta-analysis and systematic review. Clin Ther 2012;34:1247–1258 [DOI] [PubMed]
- 9.Gallwitz B. Glucagon-like peptide-1 analogues for type 2 diabetes mellitus: current and emerging agents. Drugs 2011;71:1675–1688 [DOI] [PubMed] [Google Scholar]
- 10.Gallwitz B. Benefit-risk assessment of exenatide in the therapy of type 2 diabetes mellitus. Drug Saf 2010;33:87–100 [DOI] [PubMed] [Google Scholar]
- 11.Garber AJ. Liraglutide in oral antidiabetic drug combination therapy. Diabetes Obes Metab 2012;14(Suppl. 2):13–19 [DOI] [PubMed] [Google Scholar]
- 12.Madsbad S, Kielgast U, Asmar M, Deacon CF, Torekov SS, Holst JJ. An overview of once-weekly glucagon-like peptide-1 receptor agonists—available efficacy and safety data and perspectives for the future. Diabetes Obes Metab 2011;13:394–407 [DOI] [PubMed] [Google Scholar]
- 13.Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008;24:275–286 [DOI] [PubMed] [Google Scholar]
- 14.Buse JB, Rosenstock J, Sesti G, et al. LEAD-6 Study Group Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009;374:39–47 [DOI] [PubMed] [Google Scholar]
- 15.Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 2012;344:d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG, GWAA Study Group Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005;143:559–569 [DOI] [PubMed] [Google Scholar]
- 17.Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007;50:259–267 [DOI] [PubMed] [Google Scholar]
- 18.Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007;29:2333–2348 [DOI] [PubMed] [Google Scholar]
- 19.Bergenstal R, Lewin A, Bailey T, Chang D, Gylvin T, Roberts V, NovoLog Mix-vs.-Exenatide Study Group Efficacy and safety of biphasic insulin aspart 70/30 versus exenatide in subjects with type 2 diabetes failing to achieve glycemic control with metformin and a sulfonylurea. Curr Med Res Opin 2009;25:65–75 [DOI] [PubMed] [Google Scholar]
- 20.Gallwitz B, Böhmer M, Segiet T, et al. Exenatide twice daily versus premixed insulin aspart 70/30 in metformin-treated patients with type 2 diabetes: a randomized 26-week study on glycemic control and hypoglycemia. Diabetes Care 2011;34:604–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bunck MC, Cornér A, Eliasson B, et al. Effects of exenatide on measures of β-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care 2011;34:2041–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diamant M, Van Gaal L, Stranks S, et al. Safety and efficacy of once-weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes over 84 weeks. Diabetes Care 2012;35:683–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide Effect and Action in Diabetes 5 (LEAD-5) met+SU Study Group Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia 2009;52:2046–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Li L, Yang M, Liu H, Boden G, Yang G. Glucagon-like peptide-1 receptor agonists versus insulin in inadequately controlled patients with type 2 diabetes mellitus: a meta-analysis of clinical trials. Diabetes Obes Metab 2011;13:972–981 [DOI] [PubMed] [Google Scholar]
- 25.Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results - A Long Term Evaluation (LEADER) [article online], 2012. Available from http://clinicaltrials.gov/ct2/show/NCT01179048 Accessed 17 June 2012
- 26.Exenatide Study of Cardiovascular Event Lowering Trial (EXSCEL): a trial to evaluate cardiovascular outcomes after treatment with exenatide once weekly in patients with type 2 diabetes mellitus [article online]. Available from http://www.clinicaltrials.gov/ct2/show/NCT01144338 Accessed 17 June 2012
- 27.Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association. European Association for Study of Diabetes Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabák AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimäki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet 2009;373:2215–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.U.K. Prospective Diabetes Study Group U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 1995;44:1249–1258 [PubMed] [Google Scholar]
- 30.Levy J, Atkinson AB, Bell PM, McCance DR, Hadden DR. Beta-cell deterioration determines the onset and rate of progression of secondary dietary failure in type 2 diabetes mellitus: the 10-year follow-up of the Belfast Diet Study. Diabet Med 1998;15:290–296 [DOI] [PubMed] [Google Scholar]
- 31.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 32.Meier JJ, Breuer TGK, Bonadonna RC, et al. Pancreatic diabetes manifests when beta cell area declines by approximately 65% in humans. Diabetologia 2012;55:1346–1354 [DOI] [PubMed] [Google Scholar]
- 33.Del Prato S. Role of glucotoxicity and lipotoxicity in the pathophysiology of Type 2 diabetes mellitus and emerging treatment strategies. Diabet Med 2009;26:1185–1192 [DOI] [PubMed] [Google Scholar]
- 34.Bolli GB, Lucidi P, Porcellati F, Fanelli CG. Pivotal role of timely basal insulin replacement after metformin failure in sustaining long-term blood glucose control at a target in type 2 diabetes. Diabetes Care 2011;34(Suppl. 2):S220–S224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on β-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet 2008;371:1753–1760 [DOI] [PubMed] [Google Scholar]
- 36.Retnakaran R, Drucker DJ. Intensive insulin therapy in newly diagnosed type 2 diabetes. Lancet 2008;371:1725–1726 [DOI] [PubMed] [Google Scholar]
- 37.Dandona P, Chaudhuri A, Mohanty P, Ghanim H. Anti-inflammatory effects of insulin. Curr Opin Clin Nutr Metab Care 2007;10:511–517 [DOI] [PubMed] [Google Scholar]
- 38.Vehkavaara S, Yki-Järvinen H. 3.5 years of insulin therapy with insulin glargine improves in vivo endothelial function in type 2 diabetes. Arterioscler Thromb Vasc Biol 2004;24:325–330 [DOI] [PubMed] [Google Scholar]
- 39.Kellerer M, Lammers R, Häring HU. Insulin signal transduction: possible mechanisms for insulin resistance. Exp Clin Endocrinol Diabetes 1999;107:97–106 [DOI] [PubMed] [Google Scholar]
- 40.Brunzell JD, Robertson RP, Lerner RL, et al. Relationships between fasting plasma glucose levels and insulin secretion during intravenous glucose tolerance tests. J Clin Endocrinol Metab 1976;42:222–229 [DOI] [PubMed] [Google Scholar]
- 41.Pennartz C, Schenker N, Menge BA, Schmidt WE, Nauck MA, Meier JJ. Chronic reduction of fasting glycemia with insulin glargine improves first- and second-phase insulin secretion in patients with type 2 diabetes. Diabetes Care 2011;34:2048–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forst T, Larbig M, Hohberg C, et al. Adding insulin glargine vs. NPH insulin to metformin results in a more efficient postprandial β-cell protection in individuals with type 2 diabetes. Diabetes Obes Metab 2010;12:437–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vinik A. Advancing therapy in type 2 diabetes mellitus with early, comprehensive progression from oral agents to insulin therapy. Clin Ther 2009;29:1236–1253 [PubMed] [Google Scholar]
- 44.Holman RR, Thorne KI, Farmer AJ, et al. 4-T Study Group Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 2007;357:1716–1730 [DOI] [PubMed] [Google Scholar]
- 45.Bretzel RG, Nuber U, Landgraf W, Owens DR, Bradley C, Linn T. Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet 2008;371:1073–1084 [DOI] [PubMed] [Google Scholar]
- 46.Ludvik B, Brath H, Wascher T, Toplak H, Expertengruppe der Osterreichischen Diabetesgesellschaft The significance of long acting insulin analogues in the treatment of type 2 diabetes mellitus. Wien Klin Wochenschr 2009;121:473–482 [in German] [DOI] [PubMed] [Google Scholar]
- 47.Linn T, Fischer B, Soydan N, et al. Nocturnal glucose metabolism after bedtime injection of insulin glargine or neutral protamine hagedorn insulin in patients with type 2 diabetes. J Clin Endocrinol Metab 2008;93:3839–3846 [DOI] [PubMed] [Google Scholar]
- 48.Bretzel RG, Dippel FW, Linn T, Neilson AR. Comparison of treatment costs in inadequately controlled type 2 diabetes in Germany based on the APOLLO trial with insulin glargine. J Media Econ 2009;12:1–11 [DOI] [PubMed] [Google Scholar]
- 49.Peyrot M, Rubin RR, Lauritzen T, et al. ; The International DAWN Advisory Panel. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care 2005;29:952–953 [DOI] [PubMed] [Google Scholar]
- 50.Bretzel RG, Eckhard M, Landgraf W, Owens DR, Linn T. Initiating insulin therapy in type 2 diabetic patients failing on oral hypoglycemic agents: basal or prandial insulin? The APOLLO trial and beyond. Diabetes Care 2009;32(Suppl. 2):S260–S265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phung OJ, Scholle JM, Talwar M, Coleman CI. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA 2010;303:1410–1418 [DOI] [PubMed] [Google Scholar]
- 52.Fonseca V, Gill J, Zhou R, Leahy J. An analysis of early insulin glargine added to metformin with or without sulfonylurea: impact on glycaemic control and hypoglycaemia. Diabetes Obes Metab 2011;13:814–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riddle MC, Rosenstock J, Gerich J, Insulin Glargine 4002 Study Investigators The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080–3086 [DOI] [PubMed] [Google Scholar]
- 54.Gerstein HC, Yale J-F, Harris SB, Issa M, Stewart JA, Dempsey E. A randomized trial of adding insulin glargine vs. avoidance of insulin in people with Type 2 diabetes on either no oral glucose-lowering agents or submaximal doses of metformin and/or sulphonylureas. The Canadian INSIGHT (Implementing New Strategies with Insulin Glargine for Hyperglycaemia Treatment) Study. Diabet Med 2006;23:736–742 [DOI] [PubMed] [Google Scholar]
- 55.Yki-Järvinen H, Kauppinen-Mäkelin R, Tiikkainen M, et al. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia 2006;49:442–451 [DOI] [PubMed] [Google Scholar]
- 56.Yki-Järvinen H, Juurinen L, Alvarsson M, et al. Initiate Insulin by Aggressive Titration and Education (INITIATE): a randomized study to compare initiation of insulin combination therapy in type 2 diabetic patients individually and in groups. Diabetes Care 2007;30:1364–1369 [DOI] [PubMed] [Google Scholar]
- 57.Kennedy L, Herman WH, Strange P, Harris A, GOAL AIC Team Impact of active versus usual algorithmic titration of basal insulin and point-of-care versus laboratory measurement of HbA1c on glycemic control in patients with type 2 diabetes: the Glycemic Optimization with Algorithms and Labs at Point of Care (GOAL A1C) trial. Diabetes Care 2006;29:1–8 [DOI] [PubMed] [Google Scholar]
- 58.Schreiber SA, Haak T. Insulin glargine benefits patients with type 2 diabetes inadequately controlled on oral antidiabetic treatment: an observational study of everyday practice in 12,216 patients. Diabetes Obes Metab 2007;9:31–38 [DOI] [PubMed] [Google Scholar]
- 59.Schreiber SA, Ferlinz K, Haak T. The long-term efficacy of insulin glargine plus oral antidiabetic agents in a 32-month observational study of everyday clinical practice. Diabetes Technol Ther 2008;10:121–127 [DOI] [PubMed] [Google Scholar]
- 60.Hanefeld M, Fleischmann H, Landgraf W, Pistrosch F. EARLY study: early basal insulin therapy under real-life conditions in type 2 diabetics. Diabetes Stoffw Herz 2012;21:91–97 [Google Scholar]
- 61.Vergès B, Brun JM, Tawil C, Alexandre B, Kerlan V. Strategies for insulin initiation: insights from the French LIGHT observational study. Diabetes Metab Res Rev 2012;28:97–105 [DOI] [PubMed] [Google Scholar]
- 62.Odawara M, Ohtani T, Kadowaki T. Dosing of insulin glargine to achieve the treatment target in Japanese type 2 diabetes on a basal supported oral therapy regimen in real life: ALOHA study subanalysis. Diabetes Technol Ther 2012;14:635–643 [DOI] [PubMed] [Google Scholar]
- 63.The ORIGIN Trial Investigators. Rationale, design, and baseline characteristics for a large international trial of cardiovascular disease prevention in people with dysglycemia: The ORIGIN Trial (Outcome Reduction with an Initial Glargine Intervention). Am Heart J 2008;155:26–32 [DOI] [PubMed]
- 64.Sarwar N, Gao P, Seshasai SR, et al. Emerging Risk Factors Collaboration Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rydén L, Standl E, Bartnik M, et al. Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) European Association for the Study of Diabetes (EASD) Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. Eur Heart J 2007;28:88–136 [DOI] [PubMed] [Google Scholar]
- 66.Esposito K, Chiodini P, Bellastella G, Maiorino MI, Giugliano D. Proportion of patients at HbA1c target <7% with eight classes of antidiabetic drugs in type 2 diabetes: systematic review of 218 randomized controlled trials with 78 945 patients. Diabetes Obes Metab 2012;14:228–233 [DOI] [PubMed] [Google Scholar]
- 67.Raccah D, Bretzel RG, Owens D, Riddle M. When basal insulin therapy in type 2 diabetes mellitus is not enough—what next? Diabetes Metab Res Rev 2007;23:257–264 [DOI] [PubMed] [Google Scholar]
- 68.Lebovitz HE, Banerji MA. Non-insulin injectable treatments (glucagon-like peptide-1 and its analogs) and cardiovascular disease. Diabetes Technol Ther 2012;14(Suppl. 1):S43–S50 [DOI] [PubMed] [Google Scholar]
- 69.Yoon NM, Cavaghan MK, Brunelle RL, Roach P. Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther 2009;31:1511–1523 [DOI] [PubMed] [Google Scholar]
- 70.Arnolds S, Dellweg S, Clair J, et al. Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: a proof-of-concept study. Diabetes Care 2010;33:1509–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes. Ann Intern Med 2011;154:1–11 [DOI] [PubMed] [Google Scholar]
- 72.Del Prato S. Megatrials in type 2 diabetes. From excitement to frustration? Diabetologia 2009;52:1219–1226 [DOI] [PubMed] [Google Scholar]


