The acute glucose-lowering effect of certain bariatric procedures—before any significant weight loss has occurred—has been known for decades (1). In a comprehensive meta-analysis by Buchwald et al. (2), type 2 diabetes remission rates after the most common bariatric procedure, Roux-en-Y gastric bypass (RYGB), were reported to be 80%. Applying the 2009 consensus criteria for the definition of diabetes remission has been reported to show complete remission of diabetes in “only” 41% of 160 RYGB-treated obese patients with type 2 diabetes (3). Importantly, bariatric surgery seems to improve several components of the metabolic syndrome, and type 2 diabetes–specific mortality rates have been demonstrated to be up to 90% lower in RYGB-treated subjects compared with nontreated control subjects (2,4). In comparison with medical therapy alone, recent clinical trials have shown that RYGB or biliopancreatic diversion resulted in better glucose control (5), RYGB achieved glycemic control in significantly more patients (6), and sleeve gastrectomy resolved the diabetic state more effectively (7). Thus, it is well established that bariatric procedures improve glycemic control or even resolve the type 2 diabetic state. However, the mechanisms by which these operations bring about their glucose-lowering effects remain much debated. Obviously, the body weight–lowering effect of these operations contributes to the long-term improvements in glucose metabolism (primarily via increased hepatic and peripheral insulin sensitivity [8]), but it is striking that amelioration of hyperglycemia after bariatric surgery occurs within days of the surgery, pointing to immediate, weight loss–independent mechanisms possibly related to surgery-induced changes in food intake (caloric restriction), gastrointestinal (GI) anatomy, or transit of nutrients.
The predominant hypotheses on the physiological background for the metabolic advantages (specifically, the glucose-lowering effects) after bariatric surgery include changed release of GI hormones (increased secretion of hormones with antidiabetes properties and reduced secretion of “diabetogenic” hormones) and surgery-induced restriction of food intake. Here, the evidence for these two hypotheses will be scrutinized and compared to provide a robust analysis of current knowledge.
It’s all GI factors
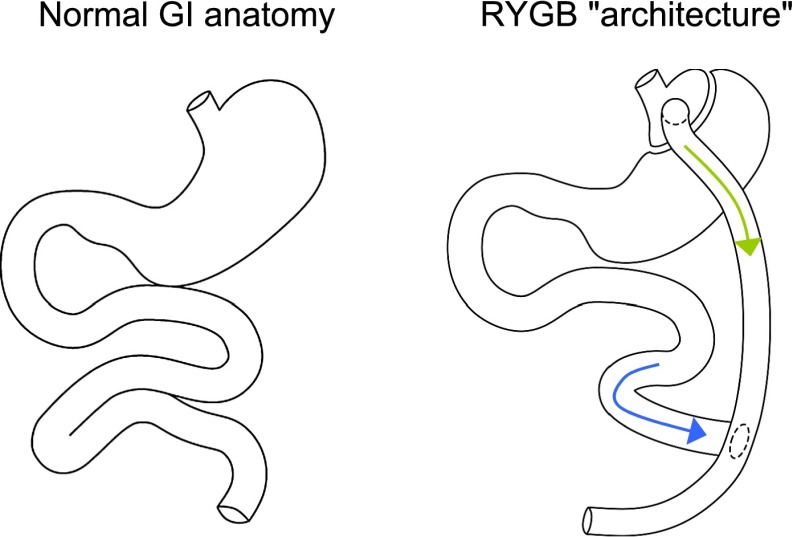
During recent years, it has become clear that the GI tract constitutes the largest and most varied endocrine organ of the body. More than 40 hormones originate from the GI tract, and several of these exert considerable impact on glucose metabolism and appetite regulation. The different GI hormones originate from specialized enteroendocrine cells scattered throughout the GI tract, with certain enteroendocrine cells located in specific regions and others more widespread and some with different density along the GI tract. The most important tasks of this complicated system of diverse enteroendocrine cells integrated amid muscle, nerve, and exocrine tissues are as follows: 1) to sense the amount and type of nutritional content in the lumen of the gut and 2) to signal to tissues and organs involved in metabolism and deposition of nutrients, making them react in harmonious concert to provide optimal short- and long-term conditions for handling of nutrients. The recent characterization of postprandial metabolism after bariatric surgical procedures (entailing anatomical rerouting of nutrients through the GI tract), combined with the rapid and frequent remission of type 2 diabetes after these operations, has more so put the small intestine center stage as an endocrine organ of significance in the regulation of appetite and metabolism. Most bariatric surgery procedures have been developed based on either restrictive ideas (e.g., gastric banding restricting the stomach using an adjustable silicone band or vertical-banded gastroplasty creating a smaller prestomach pouch) or malabsorptive ideas (e.g., biliopancreatic diversion connecting the distal part of the small intestine to the ventricle, bypassing the duodenum and jejunum, or jejunoileal bypass in which all but ∼40 cm of the small bowel is detached and set to the side) or a combination (e.g., RYGB). (See below.) Currently, the most commonly used form of bariatric surgery is RYGB. As illustrated in Fig. 1, RYGB includes surgical formation of a small stomach pouch using a stapler device. The small intestine is divided 75 cm distally from the ligament of Treitz, and the distal end is connected to the newly formed small stomach pouch (gastrojejunostomy). The upper part of the small intestine is then reattached in a Y-shaped configuration ~125 cm distally to the gastrojejunostomy, thus forming a “secretory limb” where gastric juices, bile, and pancreatic exocrine products enter duodenum and flow onward to the jejuno-jejunostomy. In contrast, nutrients pass directly from the small stomach pouch into the “alimentary limb” (distal jejunum). In this way, mostly undigested food passes through ~125 cm of jejunum before being mixed with bile and pancreatic and gastric juices (which have traveled alone from the duodenum through ~75 cm of jejunum).
Figure 1.
In the left panel, the normal GI anatomy is shown. In the right panel, the anatomy after RYGB is shown. The green arrow indicates the passage of nutrients from the gastric pouch directly to the distal part of the jejunum (through the alimentary limb). The blue arrow indicates the passage of gastric, pancreatic, and bile fluids to jejunum (through the secretory limb).
The observed remission of type 2 diabetes before any significant weight loss has taken place (9) suggests that the operation itself, including rerouting of ingested food (bypassing significant parts of the small intestine) and digestive fluids, changes handling of nutrients. In support of this theory, the purely restrictive procedure of gastric banding, which gives rise to long-term metabolic advantages associated with weight loss (10), has no acute effect on postprandial glucose metabolism (or gut hormones) (11). Rerouting of mainly undigested food from the stomach directly into the distal part of the small intestine (ileum) after RYGB (Fig. 1) directly stimulates nutrient-sensing enteroendocrine cells, with no stimulation in the bypassed proximal small intestine. In line with this, RYGB-induced changes in gut endocrinology with the potential to improve overeating and compromised glucose homeostasis include increased secretion of the incretin (insulinotropic) hormone glucagon-like peptide(GLP)-1 and the anorexic hormones peptide YY and oxyntomodulin from L cells (with a high density in the distal small intestine) (8,12). In addition to the strong but strictly glucose-dependent insulinotropic effect of GLP-1, the hormone has also been shown to enhance important steps of insulin biosynthesis and gene transcription, upregulate genes for the cellular machinery involved in insulin secretion, stimulate β-cell proliferation, enhance the differentiation of new β-cells from pancreatic progenitor cells, reduce apoptosis of β-cells, inhibit pancreatic glucagon secretion, and reduce appetite and gastrointestinal motility (13). Observation of these powerful effects led to the development of GLP-1 receptor agonists for the treatment of type 2 diabetes (13). Fasting plasma GLP-1 levels have consistently been shown to be preserved after bariatric surgery, whereas postprandial secretion increases by several fold (∼20) after operations that entail rerouting of nutrients to the lower part of the small intestine (e.g., RYGB) (8,12). In line with this, the incretin effect, i.e., the greater insulin secretion in response to an oral glucose stimulus compared with an isoglycemic intravenous glucose stimulus, was shown to increase (by approximately fivefold) in patients with type 2 diabetes 4 weeks after RYGB (no increase in incretin effect was observed in a group of matched patients obtaining weight loss via caloric restriction) (14).
Recently, the role of RYGB-induced elevated postprandial GLP-1 levels in humans has been examined in studies using the specific GLP-1 receptor antagonist exendin(9-39) (15,16). Salehi, Prigeon, and D’Alessio found that GLP-1 receptor blockade eliminated approximately one-half of the greatly elevated postprandial insulin responses in RYGB-treated subjects independently of glucose levels during ingestion of a meal (16). Jørgensen et al. (15) investigated postprandial glucose metabolism in obese patients with type 2 diabetes on two separate occasions with concomitant infusion of exendin(9-39) or placebo before and again ∼1 week after RYGB. GLP-1 receptor blockade blunted postprandial β-cell responses more after than before RYGB, which in turn resulted in a greater impact of exendin(9-39) on glucose tolerance after the operation compared with preoperatively. This suggests a greater role of GLP-1 in maintaining glucose tolerance and insulin secretion in patients with type 2 diabetes after versus before RYGB. Together, these observations support the notion that elevated postoperative levels of GLP-1, which in turn stimulate insulin secretion, constitute an important part of the glucose-lowering effect of RYGB.
A fascinating case report points to the importance of changed delivery of nutrients to the distal gut after RYGB for improving postprandial glucose metabolism (17). In a preoperatively diabetic patient, a standardized mixed-liquid meal test was performed on two separate occasions after RYGB: one meal was administered through the mouth (entering the small newly formed stomach pouch and from there flowing directly into the lumen of the L cell–rich ileum) and the other via a gastrostomy catheter into the remnant gastric pouch and onward through the duodenum and jejunum (approximating the preoperative nutritional pathway). When the meal was administered orally, typical postoperative exaggerated postprandial insulin and GLP-1 responses were found, while feeding through the gastric catheter resulted in insulin and GLP-1 responses similar to those seen preoperatively, and postprandial glucose intolerance reemerged. Thus, RYGB seems to have a direct beneficial effect on postprandial glucose metabolism, most likely owing to the greater exposure of L cells in the distal small intestine to ingested nutrients.
A common argument against RYGB-induced elevations of GLP-1 secretion constituting an important determinant for postoperative metabolic advantages is that subcutaneous administration of GLP-1 receptor agonists, resulting in peripheral plasma concentrations equal to those of endogenous GLP-1 observed after RYGB, only improves glycemic control modestly (18). However, because of extensive degradation by the ubiquitous enzyme dipeptidyl peptidase 4, only 10–15% of endogenously secreted GLP-1 reaches the peripheral circulation (13). Thus, after RYGB, levels of GLP-1 in the intestinal and portal circulation need to be grossly elevated to prompt ∼20-fold higher-than-normal peripheral concentrations. Interestingly, important GLP-1 effects are elicited in these particular areas via activation of local afferent sensory nerve fibers (arising from the nodose ganglion), sending impulses to the nucleus of the solitary tract and onward to the hypothalamus, which in turn signal, for example, pancreatic insulin secretion (13). These potentially very important mechanisms may not be elicited during GLP-1 receptor agonist treatment, explaining the different metabolic effects of GLP-1–based medical therapy and RYGB.
In addition to increased L-cell secretion, reduced secretion of the other incretin hormone, glucose-dependent insulinotropic polypeptide, which also is adipogenic and glucagonotropic (19), from enteroendocrine K cells primarily located in the bypassed proximal part of the small intestine (duodenum and jeunum) and suppression of ghrelin secretion have been described (8). However, the precise role these hormones play in the glucose lowering and other metabolic advantages imposed by bariatric surgery remains to be established, as does RYGB-induced alterations in the recirculation of bile acids (increasingly being recognized as metabolic regulators, e.g., via activation of TGR5 on GLP-1–secreting L cells [20]).
Whether bariatric surgery per se improves metabolic function via modifications of GI factors or purely via the low caloric intake in the first days after surgery remains controversial (8). However, data recently presented at the Annual Meeting of the European Association for the Study of Diabetes (21) showed that caloric restriction for a week (600 kcal/day resulting in 2.1 kg weight loss) or gastric banding had no effect on either hepatic or peripheral insulin sensitivity, whereas RYGB significantly improved both measures.
It’s all food restriction
Over the last few years, detailed observations have been made on change in plasma glucose after bariatric surgery—procedures that involve sudden change in food intake—as well as surgery to the GI tract. Importantly, Guidone et al. (22) showed that in people with type 2 diabetes, normal plasma glucose was restored within 7 days of biliopancreatic diversion surgery. As the group studied had a mean body weight of 152 kg, calorie balance and maintenance of steady weight would require intake of at least 3,200 kcal/day. The cataclysmic metabolic effect commencing on the day of surgery was that of sudden and profound negative calorie balance. The flow of carbon energy out of triglyceride stores must abruptly increase, and as ectopic fat stores are drawn on first in times of need, it could be predicted that liver fat levels would fall rapidly. This has previously been observed during modest calorie restriction (23). Liver fat level is closely related to hepatic insulin sensitivity for control of glucose production (23,24). It is therefore to be expected that fasting plasma glucose concentration would fall in step with liver fat. These predictions about pathophysiologic steps during the reversal of type 2 diabetes helped identify the time sequence of steps during the development of the condition, and this led directly to the twin cycle hypothesis of the etiology of type 2 diabetes (25). Hypotheses are testable.
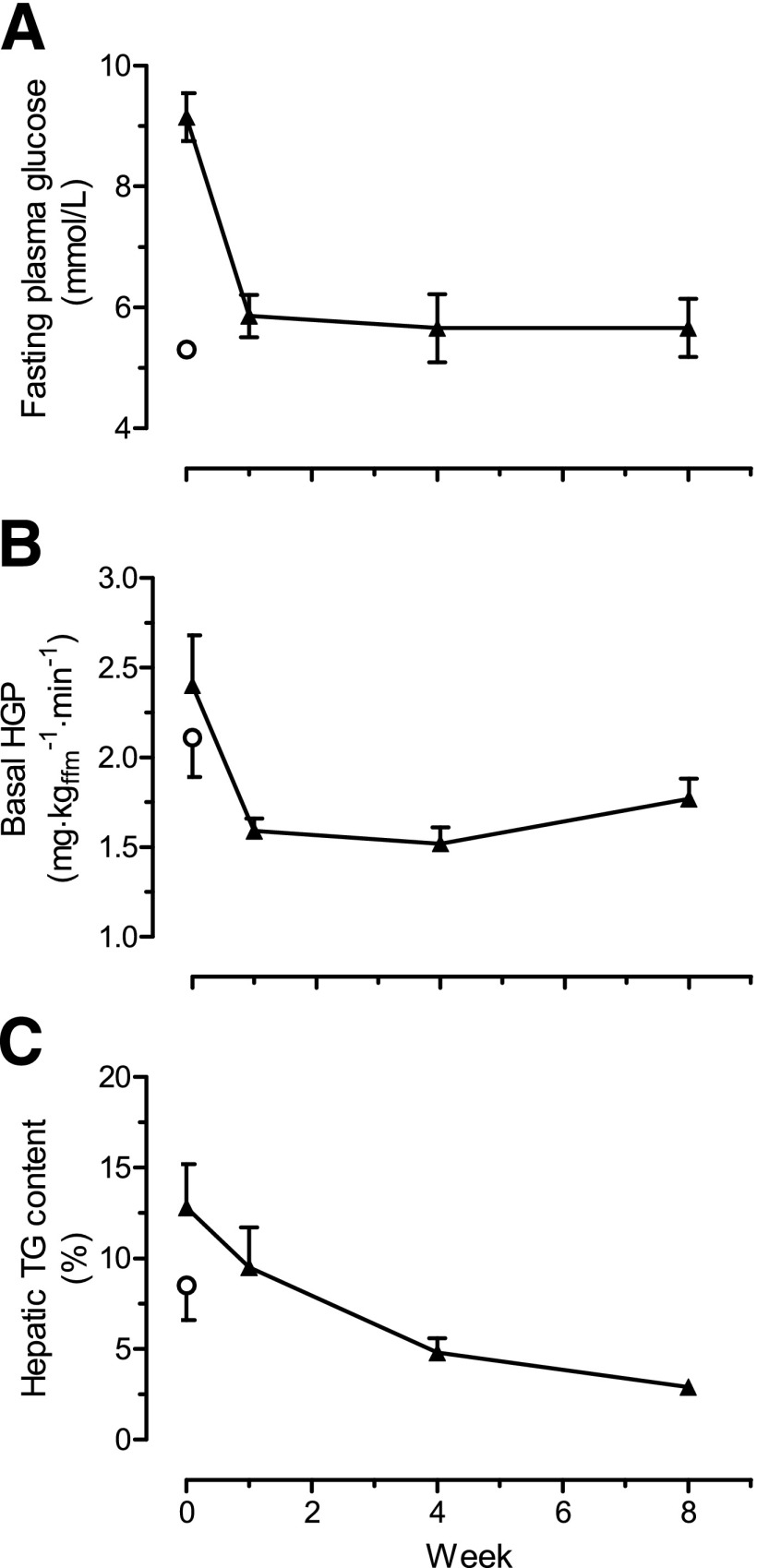
For testing of the aspect of the twin cycle hypothesis relating to negative calorie balance, the Counterpoint study was conducted (26). The effects of a 600 kcal/day diet were examined in persons with up to 4 years’ duration of type 2 diabetes. In the first 7 days, mean fasting plasma glucose fell from 9.2 ± 0.4 to 5.9 ± 0.4 mmol/L. Simultaneously, liver fat levels fell 30% to the same level as those in nondiabetic control subjects and hepatic glucose production normalized (Fig. 2). An 8-week period of less severe calorie restriction has been shown to produce generally similar changes (23). The insulin sensitivity of the liver returned to normal in the 7-day period, but it is important to emphasize that muscle insulin sensitivity, reflected by the clamp technique, did not change at all. Acute, major negative calorie balance normalizes plasma glucose in type 2 diabetes.
Figure 2.
Effect of 8 weeks of a very-low-calorie diet on plasma glucose (A), hepatic glucose production (HGP) (B), and hepatic triacylglycerol (TG) content (C) for diabetic subjects (▲). ○, mean of a weight-matched nondiabetic control group. Data are shown as means ± SE. ffm, fat-free mass. Reprinted with permission from Lim et al. (26).
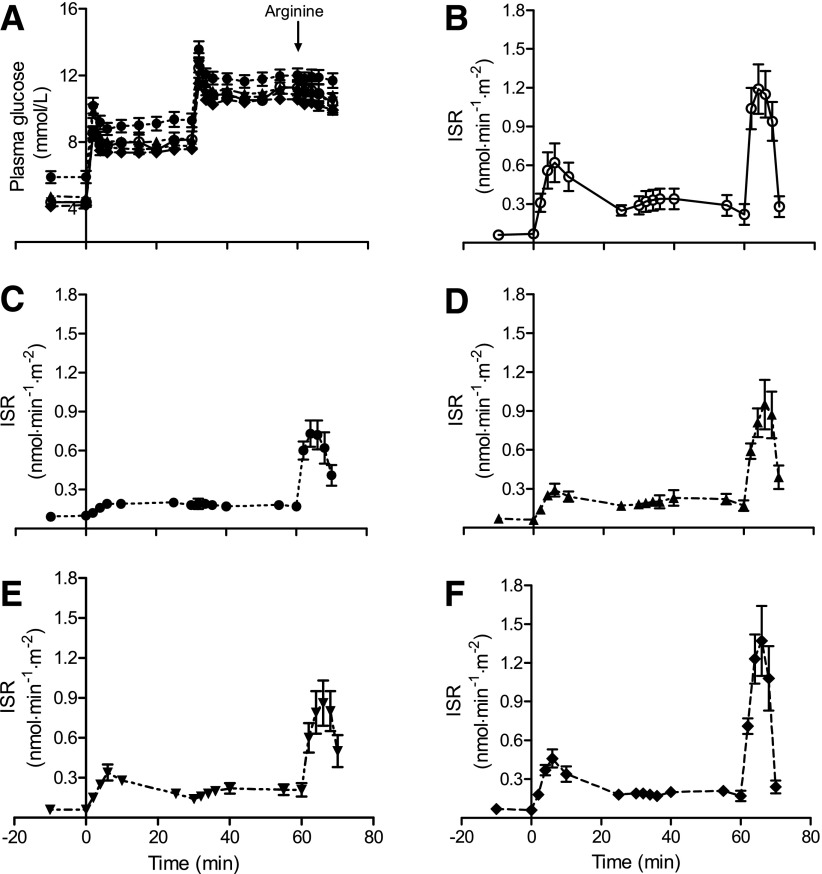
Even more interestingly from the perspective of the etiology of type 2 diabetes, over the 8-week study period the pancreas fat level gradually fell to normal levels and both first-phase and total insulin response, measured by a gold standard method, gradually returned to normal (Fig. 3). Hence, the Counterpoint study established that two separate time courses in the reversal of type 2 diabetes could be identified: a rapid return of fasting metabolism to normal in step with a fall in liver fat and a slower return of β-cell function to normal in step with a fall in pancreatic fat. During the 8 weeks of a very-low-calorie diet, mean body weight fell by 15.3 kg. No ongoing dietary input was provided after this intervention study, and mean body weight rose by 4 kg over the subsequent 12 weeks. At the end of this period, only 3 of 10 subjects retested had returned to a diabetic state based on oral glucose tolerance test criteria despite the weight gain (26). The study demonstrated that reversal of type 2 diabetes and restoration of normal β-cell function depend simply on reduction in intraorgan fat in liver and pancreas, and this can be produced by dietary means alone.
Figure 3.
Insulin secretion test data in control and diabetic subjects at each time point showing the complete normalization of first-phase and total insulin response after 8 weeks of dieting. A: Achieved plasma glucose levels in each group. B: Insulin section rates (ISR) in the nondiabetic control group. C: Diabetic group at baseline. D: Diabetic group at 1 week of diet. E: Diabetic group at 4 weeks. F: Diabetic group at 8 weeks. Data are shown as means ± SE. Reproduced with permission from Lim et al. (26).
It has been suggested that surgical bypass of the foregut is responsible for normalization of the incretin response, which in turn brings about normalization of plasma glucose levels (5,19). However, any surgical process that allows direct and sudden delivery of nutrients into the distal part of small intestine will cause supranormal secretion of incretins, especially when magnified by the typical use of either glucose or a liquid meal for such tests. The lack of relevance of the GI hormones in decreasing plasma glucose levels after bariatric surgery can be appreciated by comparing the effect of restrictive procedures such as gastric banding or sleeve gastrectomy with that of RYGB. All achieve similar effects on diabetes, although in proportion to degree of calorie restriction as reflected by ultimate weight loss (6,10).
The incretins are important secondary regulator hormones and exert modest effects compared with, for example, insulin itself. This can be seen upon considering the extent of decrease in plasma glucose achieved by administration of pharmacological doses of GLP-1 receptor agonists or of dipeptidyl peptidase 4 inhibitors. Blood glucose control is typically slightly improved, e.g., with exenatide therapy (27). In this study, mean HbA1c fell from 8.4 to 7.6%, thus remaining distinctly abnormal despite pharmacological levels of GLP-1 receptor agonist. Fasting plasma glucose was decreased by a smaller degree (from 9.3 to 8.7 mmol/L) as expected owing to the very nature of incretins, which predominantly affect the postprandial period. Incretins are most unlikely to bring about normalization of plasma glucose in diabetes, but they are interesting hormones concerned with fine-tuning, not primary control, of metabolism.
The major mechanism of the decrease in plasma glucose after bariatric surgery or hypocaloric dieting is not in doubt. Acute negative calorie balance is all that is needed to reverse type 2 diabetes. One forgotten observation is that of Walter Pories, the father of bariatric surgery. He pointed out decades ago that an intended gastric bypass operation that had to be abandoned (because when the abdominal cavity was opened, the stomach was found to contain food) produced an effect on blood glucose in the days after surgery that was the same as that produced by gastric bypass itself, as food intake had to be severely restricted post–abdominal surgery (28).
Conclusions
Two incontrovertible conclusions can be derived, and several areas can be identified as requiring clarification. First, surgically induced direct delivery of nutrients to the small intestine will increase the GLP-1 response to a meal and enhance the insulin response. Second, induction of sudden negative calorie balance by any means in type 2 diabetes normalizes plasma glucose levels within days, and this is the predominant mechanism underlying the early metabolic changes after bariatric surgery. It remains to be established what proportion of the enhanced meal-related insulin secretion is dependent on change in incretin secretion and also what difference on long-term β-cell function results from surgically induced increased GLP-1 secretion.
It would be of considerable practical utility to identify in advance individuals likely to return to normal blood glucose control after bariatric surgery or sustained dietary intervention. Until recently, the best prognostic index has been duration of diabetes (29). However, a recent study on people with BMI <35 kg/m2 dramatically demonstrated that RYGB achieved complete remission in 58 of 66 people even though the median duration of diabetes was 8 years (range 1–19 years). This is a far higher percentage than observed in previously reported subgroups with comparable duration but higher initial BMI (30). It would appear that if intraorgan fat mass is not greatly elevated, there is a substantially greater chance of success of reducing it below the threshold level for diabetes for the individual. In these less obese subjects, there was no recurrence of diabetes over a median of 5 years of follow-up.
There is no debate as to whether bariatric surgery is much more likely to achieve long-term weight loss when applied prospectively to groups of people than is dietary advice. That is an enormously important clinical consideration. However, this should not be confused with the mechanism of how reversal of type 2 diabetes comes about. Aspects of diabetes can be modulated by a number of physiological modulators including the incretin system. But type 2 diabetes is basically simple: if an individual is too heavy, the risk of diabetes is high, and if populations are too heavy then type 2 diabetes will be prevalent.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
F.K.K. and R.T. researched data and wrote the manuscript. F.K.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This publication is based on the presentations from the 4th World Congress on Controversies to Consensus in Diabetes, Obesity and Hypertension (CODHy). The Congress and the publication of this supplement were made possible in part by unrestricted educational grants from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Ethicon Endo-Surgery, Janssen, Medtronic, Novo Nordisk, Sanofi, and Takeda.
References
- 1.Ackerman NB. Observations on the improvements in carbohydrate metabolism in diabetic and other morbidly obese patients after jejunoileal bypass. Surg Gynecol Obstet 1981;152:581–586 [PubMed] [Google Scholar]
- 2.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724–1737 [DOI] [PubMed] [Google Scholar]
- 3.Pournaras DJ, Aasheim ET, Søvik TT, et al. Effect of the definition of type II diabetes remission in the evaluation of bariatric surgery for metabolic disorders. Br J Surg 2012;99:100–103 [DOI] [PubMed] [Google Scholar]
- 4.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007;357:753–761 [DOI] [PubMed] [Google Scholar]
- 5.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577–1585 [DOI] [PubMed] [Google Scholar]
- 6.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leonetti F, Capoccia D, Coccia F, et al. Obesity, type 2 diabetes mellitus, and other comorbidities: a prospective cohort study of laparoscopic sleeve gastrectomy vs medical treatment. Arch Surg 2012;147:694–700 [DOI] [PubMed] [Google Scholar]
- 8.Dirksen C, Jørgensen NB, Bojsen-Møller KN, et al. Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass. Diabetologia 2012;55:1890–1901 [DOI] [PubMed] [Google Scholar]
- 9.Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg 2005;15:474–481 [DOI] [PubMed] [Google Scholar]
- 10.Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008;299:316–323 [DOI] [PubMed] [Google Scholar]
- 11.Usinger L, Hansen KB, Kristiansen VB, Larsen S, Holst JJ, Knop FK. Gastric emptying of orally administered glucose solutions and incretin hormone responses are unaffected by laparoscopic adjustable gastric banding. Obes Surg 2011;21:625–632 [DOI] [PubMed] [Google Scholar]
- 12.Rhee NA, Vilsboll T, Knop FK. Current evidence for a role of GLP-1 in Roux-en-Y gastric bypass-induced remission of type 2 diabetes. Diabetes Obes Metab 2012;14:291–298 [DOI] [PubMed]
- 13.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 2007;87:1409–1439 [DOI] [PubMed] [Google Scholar]
- 14.Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 2008;93:2479–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jørgensen NB, Dirksen C, Jacobsen SH, et al. Glucagon-like-peptide-1 (GLP-1) is important for the improved β-cell function in type 2 diabetic subjects after Roux-en-Y gastric bypass (RYGB) (Abstract). Diabetes 2012;61(Suppl. 1):A474 [Google Scholar]
- 16.Salehi M, Prigeon RL, D’Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes 2011;60:2308–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dirksen C, Hansen DL, Madsbad S, et al. Postprandial diabetic glucose tolerance is normalized by gastric bypass feeding as opposed to gastric feeding and is associated with exaggerated GLP-1 secretion: a case report. Diabetes Care 2010;33:375–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 2012;344:d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knop FK. Resolution of type 2 diabetes following gastric bypass surgery: involvement of gut-derived glucagon and glucagonotropic signalling? Diabetologia 2009;52:2270–2276 [DOI] [PubMed] [Google Scholar]
- 20.Knop FK. Bile-induced secretion of glucagon-like peptide-1: pathophysiological implications in type 2 diabetes? Am J Physiol Endocrinol Metab 2010;299:E10–E13 [DOI] [PubMed] [Google Scholar]
- 21.Iaconelli A, Gaggini M, Gastaldelli A, Mingrone G. Short term effects of laparoscopic adjustable gastric banding (LAGB) and Roux-en-Y gastric bypass (RYGB) vs very low calorie diet (VLCD) (Abstract). Diabetologia 2012;55(Suppl. 1):584 [Google Scholar]
- 22.Guidone C, Manco M, Valera-Mora E, et al. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes 2006;55:2025–2031 [DOI] [PubMed] [Google Scholar]
- 23.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 2005;54:603–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravikumar B, Gerrard J, Dalla Man C, et al. Pioglitazone decreases fasting and postprandial endogenous glucose production in proportion to decrease in hepatic triglyceride content. Diabetes 2008;57:2288–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor R. Pathogenesis of type 2 diabetes: tracing the reverse route from cure to cause. Diabetologia 2008;51:1781–1789 [DOI] [PubMed] [Google Scholar]
- 26.Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011;54:2506–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005;28:1092–1100 [DOI] [PubMed] [Google Scholar]
- 28.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995;222:339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen RV, Pinheiro JC, Schiavon CA, Salles JE, Wajchenberg BL, Cummings DE. Effects of gastric bypass surgery in patients with type 2 diabetes and only mild obesity. Diabetes Care 2012;35:1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall TC, Pellen MG, Sedman PC, Jain PK. Preoperative factors predicting remission of type 2 diabetes mellitus after Roux-en-Y gastric bypass surgery for obesity. Obes Surg 2010;20:1245–1250 [DOI] [PubMed] [Google Scholar]