Antihypertensive drug classes are usually classified as 1st, 2nd, 3rd, and 4th or 5th choice to help physicians select the drug most suitable for treatment initiation among the very many classes available to lower blood pressure (BP) in patients with a BP elevation. However, this approach was appropriate decades ago when several drugs had inconveniences that made their use in monotherapy inadvisable. An example was the then widely used vasodilator hydralazine whose sodium-retaining and tachycardic effects made its administration recommendable only with a diuretic or a β-blocker, with, thus, a classification as a 2nd- or 3rd-choice drug (1). This is no longer the case because several current antihypertensive drug classes are characterized by a similar BP-lowering effect (2), a good tolerability profile (2), and evidence of cardiovascular protection in prospective randomized trials (3,4). As recently argued in a document of the European Society of Hypertension (5), this implies that rather than classifying drugs as 1st, 2nd, 3rd, and further choice, it might be more appropriate to help physicians select the drug (or drug combination) that might be preferred for treatment initiation in a given patient or a given clinical condition. This article will discuss the factors that may help physicians move toward this more individualized treatment approach.
Demographic factors
Evidence is available that antihypertensive treatment is protective in either hypertensive males or hypertensive females and that for a similar decrease in BP the reduction of cardiovascular risk is proportionally similar in both sexes (6). Thus, sex does not represent a factor to consider in the choice of antihypertensive treatment except for the need to avoid blockers of the renin-angiotensin system (ACE inhibitors, angiotensin receptor antagonists, and renin inhibitors) in pregnant women because of the suspicion, from animal studies, of teratogenic effects (7).
Although the British guidelines have long maintained that antihypertensive treatment should be different in young and elderly patients (8), there is no substantial basis for an age-related choice of antihypertensive drugs (5). Diuretics, ACE inhibitors, angiotensin receptor antagonists, calcium antagonists, and β-blockers have been shown to have a similar protective effect in patients younger and older than 65 years in a meta-analysis from a large number of randomized trials, with an overall similar ability also to lower an elevated BP (3). An exception might be hypertensive individuals aged ≥80 years. Because in these patients protection against cardiovascular and all-cause death has thus far been documented in only one trial (9), it might be prudent to preferentially use the antihypertensive drugs that this trial adopted, i.e., a diuretic with the addition of an ACE inhibitor, if needed to achieve BP control.
Finally, although reducing an elevated BP is beneficial in all ethnic groups, ACE inhibitors and angiotensin receptor antagonists have been shown to have a limited BP-lowering effect in African Americans (10). Thus, in these patients, and in general in blacks, diuretics or calcium channel blockers are the monotherapy of choice, and the two drugs together represent the preferred combination.
Biochemical markers
Decades ago, the suggestion was made to select antihypertensive treatment by the levels of plasma renin activity and thus by the different degree of activation of the renin-angiotensin system (11). However, plasma renin levels are heavily influenced by the current sodium intake and exhibit a marked increase in patients undergoing treatment with commonly used drugs such as ACE inhibitors and angiotensin receptor antagonists, which means that their assessment requires, to be valid, a washout period under stable conditions—a procedure hardly feasible in clinical practice. Furthermore, although blockers of the renin-angiotensin system may have a somewhat greater BP-lowering effect than other drugs in hypertensive patients with high renin levels (12), in normal– and low–renin level individuals (i.e., the majority of the hypertensive population) no substantial between-drug difference has been consistently reported (13). This explains why after an initial popularity, this procedure was abandoned and is now regarded as obsolete.
Although hypertensive patients are often characterized by sympathetic activation (14), there is also no advantage in selecting treatment based on the level of sympathetic influences on the cardiovascular system because 1) acceptable quantification of sympathetic activity, such as via plasma norepinephrine levels, is hardly possible in the clinical setting and the most modern and precise methods (e.g., microneurography) are only limited to research; 2) simple methods, such as measuring heart rate, are fallible because absolute heart rate values and changes heavily depend also on the vagal influences on the sinus node and because the degree of cardiac sympathetic activation may not go pari passu with the vascular one (15); and 3) drugs that most effectively counteract sympathetic cardiovascular influences, i.e., α-blockers and α- and β-blockers, although capable of effectively reducing BP, have never been tested against placebo in event-based trials and have lost in confrontation with diuretic treatment in the only comparison trial thus far available (16). It should be mentioned, however, that widely used 1st-choice drugs such as blockers of the renin-angiotensin system all have a moderating influence on sympathetic cardiovascular influences because of removal of the stimulating effect of angiotensin II at sympathetic central and peripheral sites (17,18). This is the case also for β-blockers, although their sympatho-moderating effect is mostly evident for the heart. Easier-to-use methods of direct or indirect sympathetic drive quantification (e.g., plasma brain natriuretic peptide levels) may change this negative perspective in the future.
Cardiovascular risk factors
Hypertension is frequently associated with alterations in blood glucose and lipid profile (19), and prevalence of prediabetes, diabetes, dyslipidemias, and metabolic syndrome is much greater in subjects with high than in those with normal BP (20,21). In a recent meta-analysis of Italian observational studies in >52,000 hypertensive patients, diabetes was found in almost 20% and an increased serum cholesterol in >60% of the studied population (22). A quantitative association of plasma lipid and glucose variables with in- and out-of-office BP has also been reported (23). β-Blockers and diuretics have been shown to adversely affect, albeit to a modest degree, serum cholesterol, HDL cholesterol, and triglycerides (24,25). Thus, they should not be considered the preferred drugs in patients with lipid abnormalities unless several agents are required to control BP, as it may not infrequently happen in hypertensives with an unfavorable cardiovascular risk profile (26).
Diuretics and β-blockers have also been found to increase the risk of new-onset diabetes (27,28). On the contrary, although in a randomized trial in individuals with glucose intolerance the ACE inhibitor ramipril did not significantly reduce the development of diabetes compared with placebo (29), a meta-analysis of a large number of studies for a total of ~150,000 patients has shown this drug class, as well as the angiotensin receptor antagonists, to be associated with less new-onset diabetes, particularly compared with treatments based on diuretics and β-blockers (28). Furthermore, compared with diuretics and β-blockers, these drugs have been shown to reduce insulin resistance (30)—a well-known precursor of diabetes (31). This justifies the recommendation of guidelines to avoid isolated or combined administration of diuretics or β-blockers in patients predisposed to diabetes such as those with metabolic syndrome (19) or a blood glucose in the glucose intolerance range, i.e., between 100 and 125 mg/dL (10,13). In these patients, blockers of the renin-angiotensin system should be regarded as the first treatment approach, followed, if needed, by the addition of a calcium channel blocker, which has no adverse effect on glucose metabolism.
This does not mean, however, that in these circumstances diuretics and β-blockers are contraindicated. First, diuretics are frequently needed to control BP, and its diabetogenic influence can be minimized at low doses (27). Second, less or no diabetogenic influence has been reported for vasodilator β-blockers (32). Third, the prognostic impact of new-onset antihypertensive drug-related diabetes, whether it adversely affects outcome like native diabetes or, rather, represents a blood glucose increase of a more cosmetic nature, is still under debate (27,33).
Asymptomatic organ damage
For a similar BP reduction, antihypertensive drugs have been found to have different effects on several asymptomatic organ damages. ACE inhibitors, angiotensin receptor antagonists, and calcium antagonists favor regression of echocardiographic or electrocardiographic left ventricular hypertrophy more effectively than diuretics and β-blockers (34). ACE inhibitors and angiotensin receptor antagonists much more effectively reduce urinary protein excretion than other antihypertensive drugs (35). Blockers of the renin-angiotensin system and calcium channel blockers more effectively regress arteriolar remodeling (i.e., the modification of arteriolar wall structure that increases wall thickness at the expense of the lumen) than other drugs (36). Thus, these drugs should be preferentially used in the presence of these markers of cardiac, renal, and vascular damage, which are all associated with an increased cardiovascular risk (37–39). This is particularly the case for left ventricular hypertrophy and micro- or macroalbuminuria, which can be easily identified and for which there is also evidence, albeit not consistent in all studies (40), that their changes may reflect the effect of treatment on cardiovascular morbid and fatal events (41–43), thus offering an important tool to determine the achieved degree of patients’ protection by treatment.
No conclusive evidence is currently available on whether antihypertensive drugs differ for their ability to favorably affect other markers of cardiac, vascular, or renal damage of prognostic significance (diastolic dysfunction, pulse wave velocity, left atrium dimension, white matter lesions, etc.), with the exception of carotid atherosclerosis, which has been found to be more effectively delayed by calcium channel blockers than by other drugs (44). The advantage of this greater antiatherogenic effect is not so clear, however, because both in hypertension and in other conditions in need of cardiovascular drug treatment the prognostic significance of treatment-related changes in carotid intima-media thickness and plaque number has not been clearly documented (45,46).
Clinical conditions
Evidence is available that in type 2 diabetes diuretics, β-blockers, ACE inhibitors, angiotensin receptor antagonists, and calcium channel blockers have a similar protective effect on the cardiovascular system, presumably because in this condition cardiovascular protection is largely due to BP lowering per se (47). Thus, in diabetic patients physicians can make use of all the above drugs to achieve an effective BP control, i.e., a reduction <140/90 mmHg (5,47,48).
However, because they unfavorably modify insulin resistance (30), diuretics and β-blockers increase the number/doses of hypoglycemic drugs necessary to achieve an adequate blood glucose control (49). Furthermore, β-blockers may blunt the signs and symptoms of hypoglycemia, thereby favoring its potentially harmful consequences. Finally, and most importantly, ACE inhibitors and angiotensin receptor antagonists not only reduce cardiovascular risk (50,51) but also decrease urinary protein excretion, delay appearance of micro- or macroalbuminuria, and slow down progression of renal damage to end-stage renal disease (5,52–54). This nephroprotective effect makes these drugs a mandatory component in the management of this condition both to maximize renal protection and to avoid the increase of cardiovascular risk that occurs when diabetic nephropathy becomes clinically manifest (55). Of note, evidence on the protective properties of blockers of the renin-angiotensin system in diabetes does not extend to renin inhibitors, i.e., aliskiren. Indeed, in diabetic patients administration of this drug on the background of an ACE inhibitor or an angiotensin receptor antagonist has recently been shown to have unfavorable therapeutic effects (56).
The following evidence also exists. In hypertensive patients with a history of heart failure, treatment should avoid calcium channel blockers and include ACE inhibitors, angiotensin receptor antagonists, or diuretics, with those of the loop being necessary if heart failure is clinically manifest or renal function is impaired (10,13). β-Blockers are also drugs of choice in this clinical condition, with those with vasodilating properties (57,58) offering the additional advantage of reducing the marked vasoconstriction characterizing individuals with an inadequate cardiac output. Heart failure also favors the administration of antialdosterone drugs, which in patients with an impaired cardiac function exert a protective effect (59) possibly because of the ability to reduce the elevated aldosterone levels much more effectively than blockers of the renin angiotensin system (60). Antialdosterone drugs should also be considered in resistant hypertension, i.e., when BP fails to be controlled under a three-drug regimen that includes a diuretic, a blocker of the renin-angiotensin system, and a calcium channel blocker, all at effective doses (61). β-Blockers should be preferred in patients with a history of myocardial infarction (in whom they exert a better protection against recurrence of myocardial necrosis and sudden death [10,13]), while β-blockers or calcium channel blockers should be given to patients affected by angina pectoris for their symptomatic benefit. Despite claims to the contrary, there is, on the other hand, no undisputable evidence that some antihypertensive drugs exert a greater prevention of stroke than others and should therefore be preferably used when the risk of stroke is particularly high, as in patients with a history of cerebrovascular disease (4). It is likely that the lesser protective effect against stroke by β-blockers versus calcium channel blockers reported in some meta-analyses (4,62) is accounted for by somewhat lower BP values achieved by patients treated with the latter drugs in a number of studies (63,64). It appears that, given the steep relationship between stroke and BP, strategies to prevent this event should focus on BP control more than on drug selection. Preference to some drugs versus others has been advocated also for control of rate frequency in permanent atrial fibrillation (β-blockers) and for preventing recurrences in paroxysmal atrial fibrillation (blockers of the renin-angiotensin system). Evidence is available for either condition, although the advantages of using blockers of the renin-angiotensin system in paroxysmal atrial fibrillation, supported as it is by pathophysiological data (favorable remodeling of left atrium value and wall structure) and post hoc analyses of randomized trials, have not been confirmed by randomized issue-specific trials (5).
Other criteria of choice
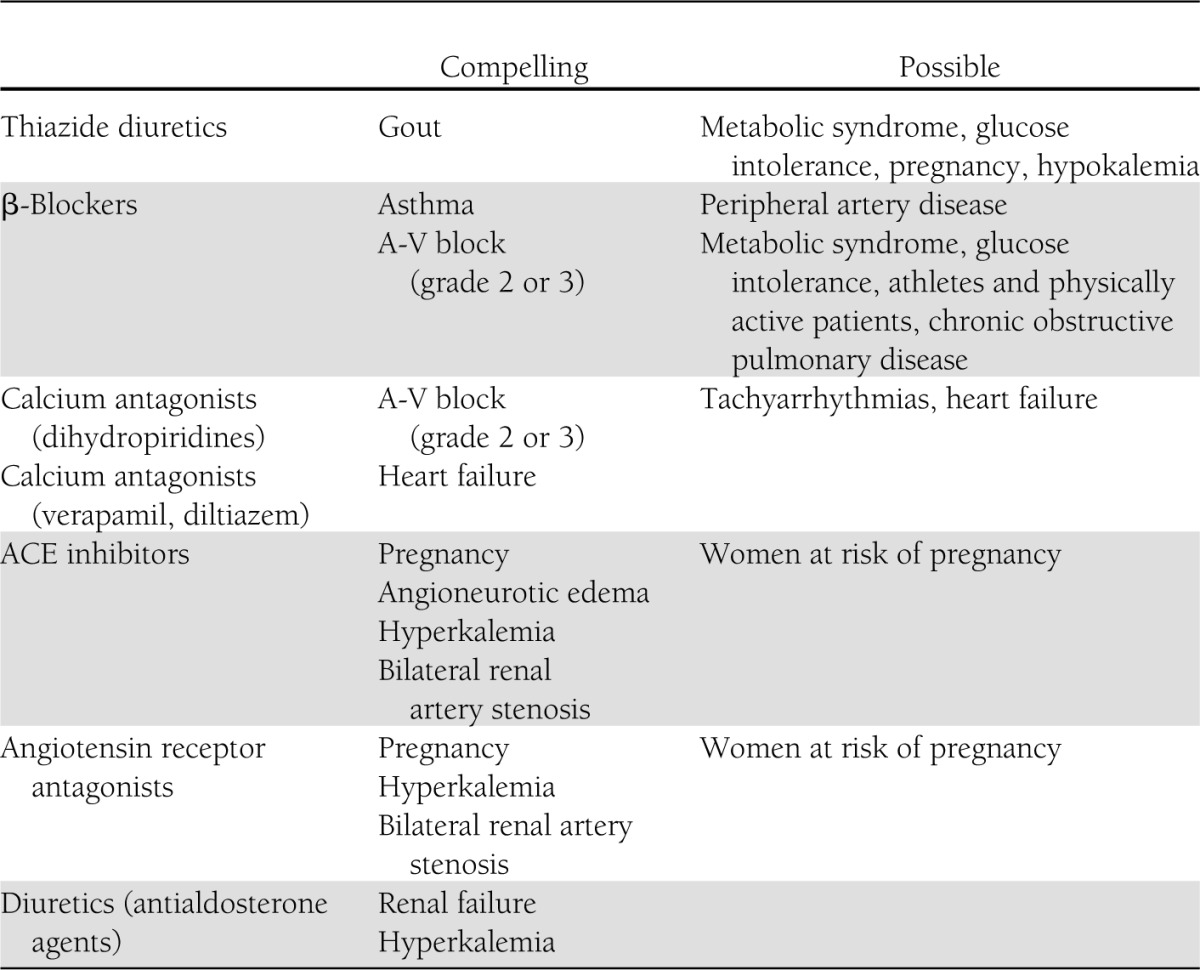
As mentioned by the 2007 European Society of Hypertension–European Society of Cardiology guidelines (13), other criteria that may help selection of appropriate drug treatment are represented by 1) the duration of the BP-lowering effect because drugs that cover the 24-h time interval and thus can be given on a once-a-day basis provide a simplified form of management that helps adherence to the therapeutic regimen (65), 2) the cost of treatment, 3) the contraindications to different drugs as summarized by the European guidelines (Table 1), and 4) the previous experience of the patient with the BP-lowering ability and side effects of a given drug class. Continuing attention to development of side effects is particularly important because treatment-related side effects are the main cause of treatment discontinuation (66), which is accompanied by a marked increase in hypertension-related complications (67).
Table 1.
Major contraindications to antihypertensive drugs
Conclusions
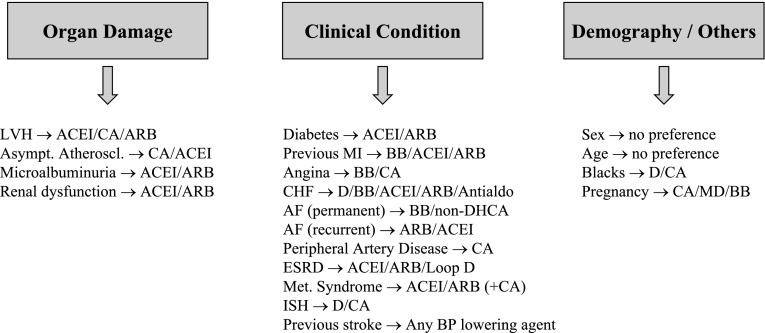
Although BP control remains the fundamental goal of antihypertensive treatment, drugs to be used to achieve this purpose can be selected to better suit the individual patient based on demographic and anthropometric characteristics, concomitant cardiovascular risk factors, asymptomatic organ damage, and clinical conditions (Fig. 1). This allows management of hypertension to be differentiated in many patients, although a central core remains in which no clue exists as to the use of one drug (or drug combination) or another. The trend toward individualization of antihypertensive treatment, however, will unquestionably continue in the future as research will more and more frequently discover differences between different drugs and treatment strategies in different patients and diseases. Hope lies also in genetic studies that could identify, by simple and inexpensive blood tests, polymorphisms associated with the magnitude of the BP response to a given drug as well as the chance of developing side effects.
Figure 1.
Some criteria for selecting drugs for antihypertensive treatment. ACEI, ACE inhibitors; AF, atrial fibrillation; Antialdo, antialdosterone drugs; ARB, angiotensin II receptor blockers; Asymp. Atheroscl., asymptomatic atherosclerosis; BB, β-blockers; CA, calcium antagonists; CHF, congestive heart failure; D, diuretic; DH, dihydropyridine; ESRD, end-stage renal disease; ISH, isolated systolic hypertension; LVH, left ventricular hypertrophy; MD, methyldopa; Met., metabolic; MI, myocardial infarction.
Acknowledgments
G.M. received honoraria as lecturer and chairman in Meeting or Advisory Boards from Bayer, Boehringer Ingelheim, Daiichi Sankyo, Medtronic, Menarini, Novartis, Recordati, Servier, and Takeda. G.G. received honoraria as lecturer and chairman from AstraZeneca, Guidotti, Medtronic, Menarini, and Stroder. No other potential conflicts of interest relevant to this article were reported.
G.M. and G.G. contributed to the discussion and wrote the manuscript. G.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This publication is based on the presentations from the 4th World Congress on Controversies to Consensus in Diabetes, Obesity and Hypertension (CODHy). The Congress and the publication of this supplement were made possible in part by unrestricted educational grants from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Ethicon Endo-Surgery, Janssen, Medtronic, Novo Nordisk, Sanofi, and Takeda.
References
- 1.World Health Organization Arterial Hypertension. Report of a WHO Expert Committee. Geneva, World Health Org., 1978 [PubMed] [Google Scholar]
- 2.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ 2003;326:1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turnbull F, Neal B, Ninomiya T, et al. Blood Pressure Lowering Treatment Trialists’ Collaboration Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. BMJ 2008;336:1121–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009;338:b1665 [DOI] [PMC free article] [PubMed]
- 5.Mancia G, Laurent S, Agabiti-Rosei E, et al. European Society of Hypertension Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens 2009;27:2121–2158 [DOI] [PubMed] [Google Scholar]
- 6.Turnbull F, Woodward M, Neal B, et al. Blood Pressure Lowering Treatment Trialists’ Collaboration Do men and women respond differently to blood pressure-lowering treatment? Results of prospectively designed overviews of randomized trials. Eur Heart J 2008;29:2669–2680 [DOI] [PubMed] [Google Scholar]
- 7.Bos-Thompson MA, Hillaire-Buys D, Muller F, et al. Fetal toxic effects of angiotensin II receptor antagonists. Case report and follow-up after birth. Ann Pharmacol 2005;39:157–161 [DOI] [PubMed]
- 8.National Institute for Health and Clinical Excellence (NICE). Hypertension: clinical management of primary hypertension in adults. Clinical guidelines, CG127 [article online], 2011. Available from www.nice.org.uk/guidance/CG127 Accessed 20 December 2012
- 9.Beckett NS, Peters R, Fletcher AE, et al. HYVET Study Group Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887–1898 [DOI] [PubMed] [Google Scholar]
- 10.Chobanian AV, Bakris GL, Black HR, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National High Blood Pressure Education Program Coordinating Committee The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–2572 [DOI] [PubMed] [Google Scholar]
- 11.Laragh JH, Baer L, Brunner HR, Buhler FR, Sealey JE, Vaughan ED., Jr Renin, angiotensin and aldosterone system in pathogenesis and management of hypertensive vascular disease. Am J Med 1972;52:633–652 [DOI] [PubMed] [Google Scholar]
- 12.Stanton AV, Dicker P, O’Brien ET. Aliskiren monotherapy results in the greatest and the least blood pressure lowering in patients with high- and low-baseline PRA levels, respectively. Am J Hypertens 2009;22:954–957 [DOI] [PubMed] [Google Scholar]
- 13.Mancia G, De Backer G, Dominiczak A, et al. Management of Arterial Hypertension of the European Society of Hypertension. European Society of Cardiology 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2007;25:1105–1187 [DOI] [PubMed] [Google Scholar]
- 14.Grassi G, Cattaneo BM, Seravalle G, Lanfranchi A, Mancia G. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension 1998;31:68–72 [DOI] [PubMed] [Google Scholar]
- 15.Grassi G, Vailati S, Bertinieri G, et al. Heart rate as marker of sympathetic activity. J Hypertens 1998;16:1635–1639 [DOI] [PubMed] [Google Scholar]
- 16.ALLHAT Collaborative Research Group Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA 2000;283:1967–1975 [PubMed] [Google Scholar]
- 17.Saino A, Pomidossi G, Perondi R, et al. Intracoronary angiotensin II potentiates coronary sympathetic vasoconstriction in humans. Circulation 1997;96:148–153 [DOI] [PubMed] [Google Scholar]
- 18.Ferrario CM. Neurogenic actions of angiotensin II. Hypertension 1983;5:V73–V79 [DOI] [PubMed]
- 19.Mancia G, Bombelli M, Corrao G, et al. Metabolic syndrome in the Pressioni Arteriose Monitorate E Loro Associazioni (PAMELA) study: daily life blood pressure, cardiac damage, and prognosis. Hypertension 2007;49:40–47 [DOI] [PubMed] [Google Scholar]
- 20.Kannel WB, Vasan RS. Assessment of cardiovascular risk and choice of antihypertensive therapy. Curr Hypertens Rep 2004;6:346–351 [DOI] [PubMed] [Google Scholar]
- 21.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005;365:1415–1428 [DOI] [PubMed] [Google Scholar]
- 22.Volpe M, Tocci G, Trimarco B, et al. Blood pressure control in Italy: results of recent surveys on hypertension. J Hypertens 2007;25:1491–1498 [DOI] [PubMed] [Google Scholar]
- 23.Mancia G, Facchetti R, Bombelli M, et al. Relationship of office, home, and ambulatory blood pressure to blood glucose and lipid variables in the PAMELA population. Hypertension 2005;45:1072–1077 [DOI] [PubMed] [Google Scholar]
- 24.Pesant Y, Marc-Aurèle J, Bielmann P, et al. Metabolic and antihypertensive effects of nebivolol and atenolol in normometabolic patients with mild-to-moderate hypertension. Am J Ther 1999;6:137–147 [DOI] [PubMed] [Google Scholar]
- 25.Lindholm LH, Persson M, Alaupovic P, Carlberg B, Svensson A, Samuelsson O. Metabolic outcome during 1 year in newly detected hypertensives: results of the Antihypertensive Treatment and Lipid Profile in a North of Sweden Efficacy Evaluation (ALPINE study). J Hypertens 2003;21:1563–1574 [DOI] [PubMed] [Google Scholar]
- 26.Wagner A, Sadoun A, Dallongeville J, et al. High blood pressure prevalence and control in a middle-aged French population and their associated factors: the MONA LISA study. J Hypertens 2011;29:43–50 [DOI] [PubMed] [Google Scholar]
- 27.Mancia G, Grassi G, Zanchetti A. New-onset diabetes and antihypertensive drugs. J Hypertens 2006;24:3–10 [DOI] [PubMed] [Google Scholar]
- 28.Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet 2007;369:201–207 [DOI] [PubMed] [Google Scholar]
- 29.Gerstein HC, Yusuf S, Bosch J, et al. DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006;368:1096–1105 [DOI] [PubMed] [Google Scholar]
- 30.Lithell HO. Effect of antihypertensive drugs on insulin, glucose, and lipid metabolism. Diabetes Care 1991;14:203–209 [DOI] [PubMed] [Google Scholar]
- 31.Jandeleit-Dahm KA, Tikellis C, Reid CM, Johnston CI, Cooper ME. Why blockade of the renon-angiotensin system reduces the incidence of new-onset diabetes. J Hypertens 2005;23:463–473 [DOI] [PubMed]
- 32.Agabiti Rosei EA, Rizzoni D. Metabolic profile of nebivolol, a beta-adrenoceptor antagonist with unique characteristics. Drugs 2007;67:1097–1107 [DOI] [PubMed] [Google Scholar]
- 33.Kostis JB, Wilson AC, Freudenberger RS, Cosgrove NM, Pressel SL, Davis BR, SHEP Collaborative Research Group Long-term effect of diuretic-based therapy on fatal outcomes in subjects with isolated systolic hypertension with and without diabetes. Am J Cardiol 2005;95:29–35 [DOI] [PubMed] [Google Scholar]
- 34.Klingbeil AU, Schneider M, Martus P, Messerli FH, Schmieder RE. A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med 2003;115:41–46 [DOI] [PubMed] [Google Scholar]
- 35.Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med 2008;148:30–48 [DOI] [PubMed] [Google Scholar]
- 36.Rehman A, Schiffrin EL. Vascular effects of antihypertensive drug therapy. Curr Hypertens Rep 2010;12:226–232 [DOI] [PubMed] [Google Scholar]
- 37.Foster MC, Hwang SJ, Larson MG, et al. Cross-classification of microalbuminuria and reduced glomerular filtration rate: associations between cardiovascular disease risk factors and clinical outcomes. Arch Intern Med 2007;167:1386–1392 [DOI] [PubMed] [Google Scholar]
- 38.Bombelli M, Facchetti R, Carugo S, et al. Left ventricular hypertrophy increases cardiovascular risk independently of in-office and out-of-office blood pressure values. J Hypertens 2009;27:2458–2464 [DOI] [PubMed] [Google Scholar]
- 39.Rizzoni D, Porteri E, Boari GE, et al. Prognostic significance of small-artery structure in hypertension. Circulation 2003;108:2230–2235 [DOI] [PubMed] [Google Scholar]
- 40.Haller H, Ito S, Izzo JL, Jr, et al. ROADMAP Trial Investigators Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med 2011;364:907–917 [DOI] [PubMed] [Google Scholar]
- 41.Okin PM, Devereux RB, Jern S, et al. LIFE Study Investigators Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA 2004;292:2343–2349 [DOI] [PubMed] [Google Scholar]
- 42.Schmieder RE, Mann JF, Schumacher H, et al. ONTARGET Investigators Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol 2011;22:1353–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Devereux RB, Wachtell K, Gerdts E, et al. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA 2004;292:2350–2356 [DOI] [PubMed] [Google Scholar]
- 44.Zanchetti A, Bond MG, Hennig M, et al. European Lacidipine Study on Atherosclerosis investigators Calcium antagonist lacidipine slows down progression of asymptomatic carotid atherosclerosis: principal results of the European Lacidipine Study on Atherosclerosis (ELSA), a randomized, double-blind, long-term trial. Circulation 2002;106:2422–2427 [DOI] [PubMed] [Google Scholar]
- 45.Zanchetti A, Hennig M, Hollweck R, et al. Baseline values but not treatment-induced changes in carotid intima-media thickness predict incident cardiovascular events in treated hypertensive patients: findings in the European Lacidipine Study on Atherosclerosis (ELSA). Circulation 2009;120:1084–1090 [DOI] [PubMed] [Google Scholar]
- 46.Costanzo P, Perrone-Filardi P, Vassallo E, et al. Does carotid intima-media thickness regression predict reduction of cardiovascular events? A meta-analysis of 41 randomized trials. J Am Coll Cardiol 2010;56:2006–2020 [DOI] [PubMed] [Google Scholar]
- 47.Turnbull F, Neal B, Algert C, et al. Blood Pressure Lowering Treatment Trialists’ Collaboration Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med 2005;165:1410–1419 [DOI] [PubMed] [Google Scholar]
- 48.Cooper-DeHoff RM, Gong Y, Handberg EM, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA 2010;304:61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pepine CJ, Handberg EM, Cooper-DeHoff RM, et al. INVEST Investigators A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA 2003;290:2805–2816 [DOI] [PubMed] [Google Scholar]
- 50.Heart Outcomes Prevention Evaluation Study Investigators Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 2000;355:253–259 [PubMed] [Google Scholar]
- 51.Yusuf S, Teo KK, Pogue J, et al. ONTARGET Investigators Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008;358:1547–1559 [DOI] [PubMed] [Google Scholar]
- 52.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD, The Collaborative Study Group The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 1993;329:1456–1462 [DOI] [PubMed] [Google Scholar]
- 53.Brenner BM, Cooper ME, de Zeeuw D, et al. RENAAL Study Investigators Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861–869 [DOI] [PubMed] [Google Scholar]
- 54.Lewis EJ, Hunsicker LG, Clarke WR, et al. Collaborative Study Group Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345:851–860 [DOI] [PubMed] [Google Scholar]
- 55.Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med 1997;157:1413–1418 [PubMed] [Google Scholar]
- 56.Parving HH, Brenner BM, McMurray JJ, et al. ALTITUDE Investigators Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012;367:2204–2213 [DOI] [PubMed] [Google Scholar]
- 57.Flather MD, Shibata MC, Coats AJS, et al. SENIORS Investigators Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 2005;26:215–225 [DOI] [PubMed] [Google Scholar]
- 58.Packer M, Bristow MR, Cohn JN, et al. U.S. Carvedilol Heart Failure Study Group The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 1996;334:1349–1355 [DOI] [PubMed] [Google Scholar]
- 59.Pitt B, Zannad F, Remme WJ, et al. Randomized Aldactone Evaluation Study Investigators The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:709–717 [DOI] [PubMed] [Google Scholar]
- 60.Biollaz J, Brunner HR, Gavras I, Waeber B, Gavras H. Antihypertensive therapy with MK 421: angiotensin II—renin relationships to evaluate efficacy of converting enzyme blockade. J Cardiovasc Pharmacol 1982;4:966–972 [PubMed] [Google Scholar]
- 61.Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens 2003;16:925–930 [DOI] [PubMed] [Google Scholar]
- 62.Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet 2005;366:1545–1553 [DOI] [PubMed] [Google Scholar]
- 63.Dahlöf B, Sever PS, Poulter NR, et al. ASCOT Investigators Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 2005;366:895–906 [DOI] [PubMed] [Google Scholar]
- 64.Mancia G, Zanchetti A, European Society of Hypertension-European Society of Cardiology Choice of antihypertensive drugs in the European Society of Hypertension-European Society of Cardiology guidelines: specific indications rather than ranking for general usage. J Hypertens 2008;26:164–168 [DOI] [PubMed] [Google Scholar]
- 65.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther 2001;23:1296–1310 [DOI] [PubMed] [Google Scholar]
- 66.Ambrosioni E, Leonetti G, Pessina AC, Rappelli A, Trimarco B, Zanchetti A, Scientific Committee of the Italian Pharmacoepidemiological Survey on Antihypertensive Therapy Patterns of hypertension management in Italy: results of a pharmacoepidemiological survey on antihypertensive therapy. J Hypertens 2000;18:1691–1699 [DOI] [PubMed] [Google Scholar]
- 67.Corrao G, Parodi A, Nicotra F, et al. Better compliance to antihypertensive medications reduces cardiovascular risk. J Hypertens 2011;29:610–618 [DOI] [PubMed] [Google Scholar]