Cases of pancreatitis have been described in connection with the use of exenatide (1), liraglutide (2) and other glucagon-like peptide (GLP)-1 receptor agonists. From these findings, the following hypothesis has been generated: stimulating the GLP-1 receptor with respective agonists has a potential to cause pancreatitis, perhaps chronic pancreatitis, and in the long term, potentially even pancreatic cancer (3–5). Furthermore, in rodents like mice and rats, stimulating the GLP-1 receptor raises cAMP in thyroid C cells, initiates the release of calcitonin, and upon longer-term exposure, is accompanied by C-cell proliferation and the formation of C-cell adenomas and (medullary thyroid) carcinomas (6). Based on these findings, it was hypothesized that GLP-1–derived medications have a potential to cause medullary thyroid carcinoma in humans as well (3,7). It is the purpose of the current review to discuss the evidence in favor and against the hypothesis that GLP-1–based therapies increase cancer risk, specifically the risk for pancreatic and thyroid carcinomas in patients with type 2 diabetes treated with exenatide and sitagliptin.
In principle, there could be weak or strong evidence, either in favor of or against, the hypothesis that incretin-based medications can increase the risk for pancreatic, (medullary) thyroid, or other carcinomas. For the purpose of this review, GLP-1–based therapies are GLP-1 receptor agonists such as exenatide, liraglutide, and others or dipeptidyl peptidase-4 (DPP-4) inhibitors such as sitagliptin, vildagliptin, saxagliptin, alogliptin, and linagliptin (8). However, almost all available data and quoted studies have specifically examined exenatide and sitagliptin, which have been available for the longest time.
The questions of whether certain drugs can cause pancreatitis and whether certain drugs can cause pancreatic carcinoma are interrelated, since chronic pancreatitis increases the risk for pancreatic carcinoma approximately 26-fold compared with subjects not suffering from chronic pancreatitis (9). There is an etiological sequence leading from a healthy pancreas to chronic pancreatitis, with the main drivers being genetic susceptibility, alcohol abuse, and certain drugs (10–12). Once chronic pancreatitis has been established, chronic inflammation and enhanced intraductal pressure due to stenosis of the pancreatic duct(s) may lead to the development of pancreatic carcinoma (10–12). While this sequence is established in the case of chronic pancreatitis, it is not as certain whether an episode of acute pancreatitis will have the same consequences. This is of importance because most of the episodes of pancreatitis associated with GLP-1 receptor agonist treatment seem to be episodes of acute pancreatitis (1). In the course of development of chronic pancreatitis, there is an exocrine pancreatic infiltrate of T cells and macrophages, a fibrotic reaction, and a reduction in acinar cells (10–12). The histological hallmarks of developing pancreatic carcinoma after chronic pancreatitis are pancreatic intraepithelial neoplasia, intraductal papillary mucinous neoplasms, and so-called pancreatic duct glands (10–12). These phenomena are characteristic lesions that occur during the transition from chronic pancreatitis to pancreatic cancer. Recent estimations, based on known mutation rates and the accumulation of somatic mutations at different stages of the development of pancreatic cancer, indicate that it is a chronic process. It takes ~12 years for a normal duct cell to get initiated as a tumor cell and to give life to a parental clone, from which a pancreatic carcinoma can grow. It takes another 7 years (approximately) for such cells to develop subclones with metastatic capacity and another 3 years (approximately) before the disease will be diagnosed due to clinical symptoms and a clinically apparent primary tumor accompanied by metastases (13). This time frame is important relative to the duration of exposure to antidiabetes drugs that might have a potential to accelerate the progression of malignant disease. Thus, any database that does not cover at least 5–6 years of observation with any drug cannot contribute reliable information on the risks for malignant disease, since the development of cancer can most likely only be influenced over long periods of time.
GLP-1–based therapies and pancreatitis
In the year 2008, a report of 30 cases of acute pancreatitis associated with the use of exenatide as an antidiabetes treatment for type 2 diabetes was published (1). There was a wide range of time until the onset of symptoms: on average 34 days but ranging from 4 to 300 days. Usually, amylase and lipase were elevated—sometimes substantially—and in the majority of cases, the patients recovered from the apparent episode of acute pancreatitis (1).
With this background, animal experiments were performed exposing transgenic rats who overexpressed human islet amyloid polypeptide in the endocrine pancreas (HIP rats) to treatment with sitagliptin, metformin, the combination of sitagliptin and metformin, or placebo. Matveyenko et al. (14) described an area of pancreatitis in one of the HIP rats treated with sitagliptin and in none of the other groups. Statistical analysis of these numbers does not indicate a significantly increased risk for pancreatitis under sitagliptin treatment. Another remarkable fact was that in the group treated with both sitagliptin and metformin, no pancreatitis was observed. The observation of pancreatitis with sitagliptin treatment in the HIP rats was the reason for a more elaborate analysis of the effects of sitagliptin treatment on the exocrine pancreas. Among other aspects, a significantly enhanced ductal cell proliferation rate based on Ki67 expression (a proliferation marker) was found and again reversed when sitagliptin and metformin were combined (14). Based on these findings, it was reasoned that sitagliptin might cause ductal cell proliferation in these HIP rats, thereby leading to pancreatitis and perhaps, in the long run, to pancreatic carcinoma. In similar experiments in another transgenic animal model, overexpressing human islet amyloid polypeptide did not reveal any ductal abnormalities (histology) or a change in ductal cell proliferation rates with sitagliptin, metformin, sitagliptin with metformin, or placebo treatment (15).
Additional animal studies have also described histological alterations within the exocrine pancreatic tissue—this time with exendin-4 treatment. Exendin-4 is a peptide used for the treatment of type 2 diabetes; the synthetic form is known as exenatide. Nachnani et al. (16) found higher degrees of exocrine pancreatic inflammation after treatment with exendin-4, with a significantly increased number of pyknotic nuclei, but just a trend toward more fibrosis.
Other animal studies tested the hypothesis that the presence of exenatide treatment will affect the outcome of experimentally induced acute pancreatitis in ob/ob and high-fat diet streptozocin diabetic mice. In these studies, the administration of exenatide before the induction of experimental acute pancreatitis using cerulein reduced the increments in amylase or lipase activity (17). Based on these findings, even a protective effect of GLP-1 receptor stimulation could be hypothesized.
In another important study, the expression of cytokines and other molecular markers of the inflammatory state were measured with and without GLP-1 receptor agonist treatment. Koehler et al. (18) described a change toward a less inflammatory state in animals treated with GLP-1 receptor agonists. This certainly does not support GLP-1 receptor stimulation as a mechanism to induce acute pancreatitis. Furthermore, the same authors studied the effects of GLP-1 receptor stimulation on the proliferation of human pancreatic adenomcarcinoma cell lines and found no growth-promoting effects (19). These findings do not support the potential of GLP-1 recpetor stimulation to promote pancreatic tumor proliferation.
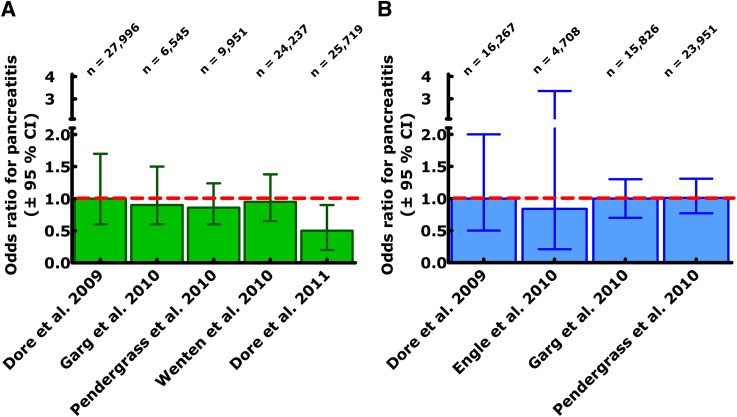
Other studies have examined lipase and amylase activities in serum (20) and the incidence of acute pancreatitis in patients with type 2 diabetes while receiving different forms of antidiabetes treatment (21–26). Small but consistent elevations mostly within the normal range in lipase and amylase, not accompanied by other signs or symptoms of pancreatitis, were observed over time with liraglutide treatment in obese and type 2 diabetic populations. Whether this indicates the induction of low-grade pancreatic inflammation or of membrane leakage leading to more spillover of intracellular (pro)enzymes into the extracellular space or an altered level of expression of digestive enzymes in pancreatic acinar cells will need to be clarified in future studies. Results from pharmacoepidmiological analyses, which have typically been based on claims databases, indicate that the population of obese type 2 diabetic patients is at higher risk of developing acute pancreatitis and that the relative risk is approximately two- to threefold higher than in nonobese control populations (21). However, the rate of diagnosed acute pancreatitis was not different between patients who were treated with exenatide (as an example of a GLP-1 receptor agonist) or with sitagliptin (as an example of a DPP-4 inhibitor) and diabetic patients receiving other forms of antidiabetes treatments (21). The results of this and similar studies, most of which have only been reported as abstracts, are summarized in Fig. 1. Uniformly, such studies described an odds ratio near 1, with, however, 95% CIs that were too wide to exclude a slightly elevated risk (Fig. 1). On the other hand, there was no hint toward such an elevated risk. Based on these findings, a sample size calculation could be performed (nQuery Advisor, version 6.02; Statistical Solutions, Cork, Ireland): this calculation indicates that to rule out a risk for acute pancreatitis elevated by at least 25%, a prospective randomized clinical trial with ~89,000 patient-years of observation would be necessary per group (GLP-1 receptor agonist, DPP-4 inhibitor, or control medication). It is quite obvious that such a large and costly study will never be performed; other methods of surveillance of increased pancreatitis risk need to be developed. The cardiovascular outcome studies under way for incretin-based medications (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results - A Long Term Evaluation [LEADER] with liraglutide [clinicaltrials.gov, NCT01179048] [27], Exenatide Study of Cardiovascular Event Lowering [EXCSEL] with exenatide once weekly [NCT01144338], Trial Evaluating Cardiovascular Outcomes with Sitagliptin [TECOS] with sitagliptin [NCT00790205], Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus [SAVOR-TIMI 53] with saxagliptin [NCT01107886] [28], and Cardiovascular Outcome Study of Linagliptin versus Glimepiride in Patients with Type 2 Diabetes (CAROLINA) with linagliptin [NCT01243424]) will help in that respect, although the number of events from a single trial will probably be too low for definite answers. Meta-analyses might help to draw firmer conclusions. Other helpful initiatives are the Sentinel and Mini-Sentinal programs initiated by the U.S. Food and Drug Administration (FDA), which will allow better postmarketing surveillance regarding rare adverse events of novel drugs (29,30).
Figure 1.
Odds ratio and 95% CIs for chronic pancreatitis in studies examining the risk of pancreatitis in patients with type 2 diabetes receiving treatment with the GLP-1 receptor agonist exenatide (A) or the DPP-4 inhibitor sitagliptin (B) relative to other glucose-lowering medications. Analysis of claims databases capturing both prescriptions of specific medications and (hospitalization due to) acute pancreatitis. Data depicted in this figure have been taken from refs. 21–26.
GLP-1–based therapies and thyroid C-cell carcinomas
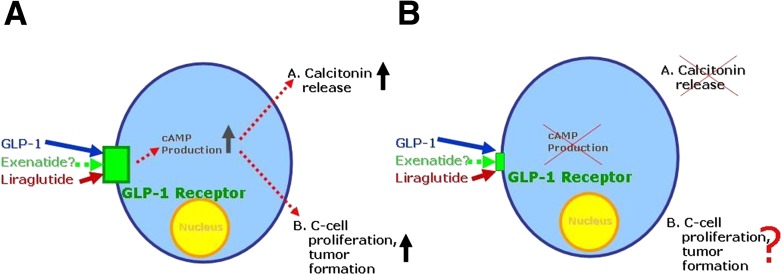
Thyroid cancer, generally speaking, is a rare disease (31). Of all cases of thyroid cancer, medullary thyroid cancer is diagnosed in ~2% of the female patients and <4% of the male patients with an incidence that increases with older age (31). While C-cell abnormalities and especially medullary thyroid carcinoma are a rare disease entity in humans, they occur spontaneously in certain rodent species including mice and rats. C-cell disease ranges from C-cell hyperplasia to C-cell adenoma and medullary carcinoma (6). In mice and rats, a once-daily injection of liraglutide, a GLP-1 receptor agonist that has an approximate half-life of 13 h, leads to permanently elevated circulating drug concentrations (32,33) and increased rate of C-cell abnormalities in both mice and rats, with some animals developing C-cell carcinomas, especially among male rats (6). Experiments with cell lines originating from rodent C cells showed that exposure to GLP-1, exenatide, or liraglutide (GLP-1 receptor agonists) will lead to an elevated production of cAMP and to the immediate stimulation of calcitonin secretion (6). However, similar cell lines originating from human C cells can be exposed to up to a 10−6 molar concentration of GLP-1, exenatide, or liraglutide and not increase in level of cAMP or the secretion of calcitonin (6). Only very high concentrations of forskolin-raised cAMP elicited a calcitonin response, but this occurred independent from the stimulation of GLP-1 receptors. The behavior of human C cells has also been tested in long-term clinical trials, in which liraglutide and other antidiabetes medications were used. In some of these clinical studies, liraglutide treatment had been used for up to 52 weeks at doses ranging up to 3 mg daily (only in nondiabetic obese populations) or up to 1.8 mg daily (patients with type 2 diabetes) with no systematic change in calcitonin concentrations indicating any measurable response to GLP-1 receptor stimulation (34). Thus, the conclusion seems to be justified that chronic exposure to clinically effective doses of GLP-1 receptor agonists does not lead to elevated calcitonin concentrations. From these findings, one can depict the following view (Fig. 2): In rodents, there are sufficient GLP-1 receptors on thyroid C cells to increase cAMP production upon exposure to GLP-1 or other GLP-1 receptor agonists. This will be accompanied by calcitonin release and, in the long term, by C-cell proliferation and the formation of C-cell adenomas or even medullary thyroid carcinomas. In humans, thyroid C cells are equipped with GLP-1 receptors at a much lower degree of expression, and exposure to GLP-1 receptor stimulation does not elevate cAMP or trigger calcitonin release. Since C-cell proliferation in rodents occurs concomitantly with increments in cAMP and the release of calcitonin as immediate markers of GLP-1 receptor stimulation, it is a reasonable assumption that in human C cells, incretin-based medications would not lead to C-cell proliferation, potentially leading to C-cell adenomas or medullary thyroid carcinomas. However, a recent study using immunocytochemistry to detect GLP-1 receptors described such receptors not only on human C cells but also in some, but not all, follicular cells and in some papillary thyroid carcinomas, indicating a potential of GLP-1 receptor stimulation to have an influence on the growth rate of other thyroid cancer types as well (7). Another recent study using an even more specific method to detect (and rule out) GLP-1 receptors in tissues (a radioligand assay) did not confirm GLP-1 receptors in human thyroid tissue outside C cells (35). Therefore, more information is needed to reach a consensus in this respect. The one consequence to be drawn from these considerations is not to treat subjects at high risk for developing medullary thyroid carcinoma based on multiple endocrine neoplasia or familial medullary thyroid carcinoma with liraglutide (according the label), and it is advisable to practice caution with other incretin-based medications as well.
Figure 2.
Schematic representation of thyroid C cells, their equipment with GLP-1 receptors, and physiological responses to stimulation with GLP-1 or GLP-1 receptor agonists like exenatide and liraglutide comparing rodent (A) and human (B) C cells. While rodent C cells respond to GLP-1 receptor stimulation with cAMP production, calcitonin release, and proliferative responses (giving rise to hyperplasia, adenomas, or even medullary carcinomas), human C cells express GLP-1 receptors at much lower levels, do not increase cAMP levels, and do not secrete calcitonin in response to GLP-1 receptors even upon long-term stimulation (exposure to GLP-1 receptor agonists in clinical trials lasting up to 1 year).
Analyses of data reported to the FDA Adverse Event Reporting System
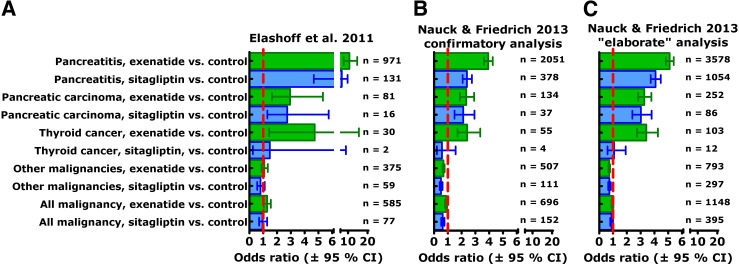
In 2011, Elashoff et al. (3) compared reports of pancreatitis, pancreatic carcinoma, and thyroid carcinoma in patients with type 2 diabetes treated with either exenatide or sitagliptin relative to other antidiabetes treatments (rosiglitazone, repaglinide, nateglinide, and glipizide), also controlling for the reports of “control events” (back pain, chest pain, cough, urinary tract infection, and syncope). Based on this analysis, a significant excess of pancreatitis (11-fold and 7-fold, respectively) was found for exenatide and sitagliptin treatment. Similarly, an approximately threefold, significant excess of pancreatic carcinomas was reported for both treatment groups. Finally, a significant 4.7-fold excess of thyroid carcinomas (histology not specified) was reported for exenatide treatment, but no significantly elevated risk was found with sitagliptin treatment. This report (Fig. 3A) impressed with numbers indicating robust effect sizes but was extremely controversial. It raised the question of whether the FDA Adverse Event Reporting System is a suitable database for reaching solid conclusions regarding the incidence of certain adverse events related to drug treatment (36). Obviously, reporting bias is an important phenomenon to be considered in this respect (Table 1).
Figure 3.
Odds ratios (95% CI) for reporting pancreatitis, pancreatic carcinoma, thyroid carcinoma, and other/all malignancies in patients with type 2 diabetes prescribed exenatide (green bars) or sitagliptin (blue bars) relative to those taking control diabetes medications, taking into account the frequency of reporting control events (back pain, chest pain, cough, syncope, and urinary tract infection) based on data retrieved from the FDA Adverse Event Reporting System (A), as described by Elashoff et al. (3), for the period from the first quarter, 2004, to the third quarter, 2009. In our confirmatory analysis (B), we used broader search terms for pancreatitis (acute and chronic pancreatitis, pancreatic insufficiency, pancreatic pseudocysts, pancreatic duct stenosis, pancreatic calcifications, pancreatolithisasis, and elevated or abnormal lipase and/or amylase) and used all sulfonylureas as control medications. In the elaborate analysis (C), we included data from the second quarter of 2005 to the fourth quarter of 2010, used broader search terms regarding control events, and included pioglitazone, all sulfonylureas, metformin, and any insulin treatment in the control medications. Numbers indicate case subjects with this diagnosis retrieved from the dataset.
Table 1.
Types of reporting bias and examples of how they may have altered reporting rates for adverse events potentially associated with incretin-based medications in analysis based on data from the FDA Adverse Event Reporting System
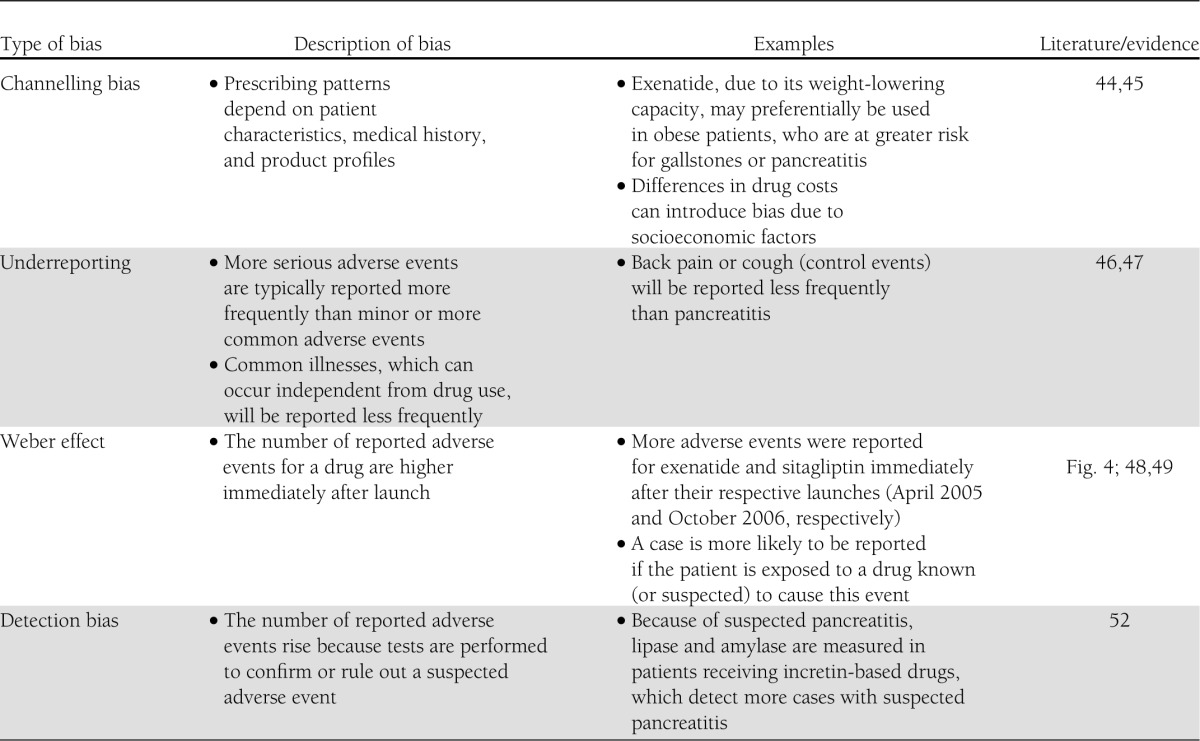
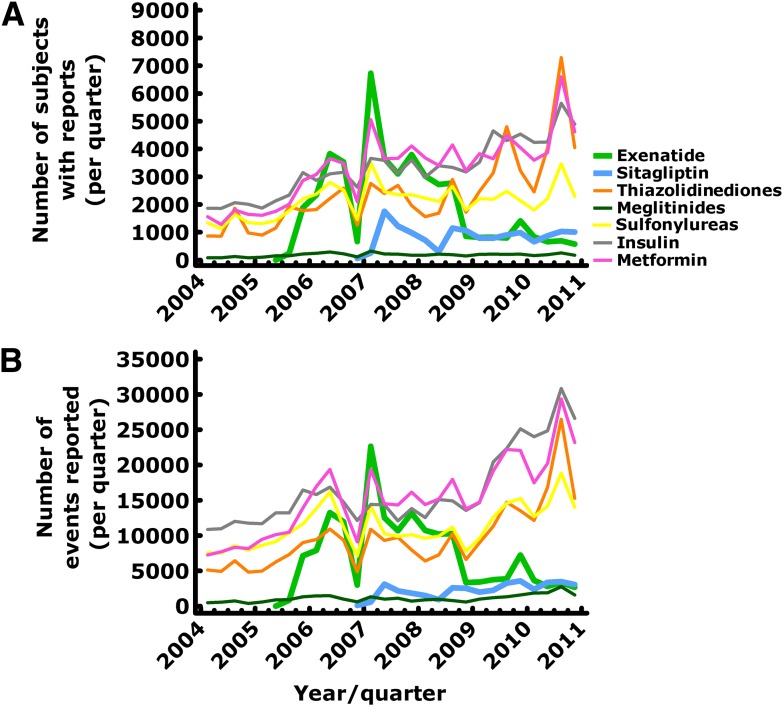
Figure 4.
Number of subjects with reports (A) and number of adverse events (B) reported to the FDA Adverse Event Reporting System between 2004 and 2010 for different glucose-lowering medications including exenatide (green lines) and sitagliptin (blue lines). Obviously, in the case of exenatide there was an initial peak, probably related to the fact that this was the first drug in the new class of GLP-1 receptor agonists, and after a nadir in the last quarter of 2007 there was renewed interest, probably as the consequence of initial reports linking exenatide to cases of pancreatitis (1). With sitagliptin, a similar pattern was observed at a lower level.
One way to capture the value of conclusions derived from analyzing the FDA Adverse Event Reporting System is to pose a question, the answer to which is well-known. As an example, we have looked for reported hypoglycemia in patients treated with different antidiabetes drug classes. The odds ratio was expressed relative to hypoglycemia occurring with metformin treatment (metformin is known not to cause hypoglycemia [37]). Analyzing the FDA Adverse Event Reporting System showed sitagliptin to be the drug reported to cause the most hypoglycemia, whereas the hypoglycemic potential of the meglitinides (short-acting sulfonylurea analogs) was not picked up as significant and the hypoglycemic potential of sulfonylureas and even of insulin was greatly underestimated when these results are compared with well-known epidemiological data (Supplementary Fig. 1) (37,38). The explanation most likely is that the high odds ratio for sitagliptin allegedly causing hypoglycemia is due to concomitant medications, which have not been adequately considered, and that sulfonylurea- or insulin-induced hypoglycemic episodes are such a well-known phenomenon that events falling into this category will be greatly underreported. Overall, such “fake analyses” demonstrate the kind of bias potentially associated with analyzing the FDA Adverse Event Reporting System and shed doubt on simple conclusions derived from such an analysis. Some typical forms of reporting bias are defined and described with their potential impact on the interpretation of data retrieved from the FDA Adverse Event Reporting System in Table 1.
Therefore, it appeared appropriate to reproduce the findings reported by Elashoff et al. (3) and to add some analyses testing the robustness of such an approach. First, we wanted to reproduce the analysis as reported (3). To this end, we analyzed the same period of time (from the 1st quarter, 2004, to the 3rd quarter, 2009), although exenatide and sitagliptin were not approved before 2005 and 2007, respectively. We decided to use a broader set of search terms capturing more pancreatitis-related events, especially those associated with chronic pancreatitis and its complications (Fig. 3), and to allow all sulfonylurea compounds to be analyzed as control medications (not just glipizide as described by Elashoff et al. [3]). Performing this confirmatory, Elashoff-like analysis (Fig. 3B), we also describe a significantly elevated odds ratio for pancreatitis with exenatide and sitagliptin treatment—at, however, a somewhat lower effect size. Likewise, we confirm the significantly elevated odds ratio for pancreatic cancer with exenatide and sitagliptin treatment and the significantly elevated risk for thyroid cancer with exenatide but not sitagliptin treatment. The odds ratios were somewhat lower than in the original publication by Elashoff et al. (3), and the number of captured cases was higher based on the more comprehensive search terms regarding pancreatitis, pancreatic cancer, and thyroid cancer. Basically, however, we confirm that analyzing the FDA Adverse Event Reporting System with a strategy described in detail by Elashoff et al. (3), a greater number of reports of pancreatitis, pancreatic cancer, and thyroid carcinoma is found for type 2 diabetic patients treated with exenatide or sitagliptin compared with other antidiabetes medications.
To further test the robustness of the conclusions, we performed a more elaborate analysis, using as control medications, in addition to rosiglitazone, repaglinide, nateglinide, and glipizide, as originally described: piogitazone, all other sulfonylurea compounds, metformin, and all insulin preparations. This is the least restrictive approach that can be taken, although including all insulin treatment might allow results from type 1 diabetic patients into this analysis, who will always be a minority within the diabetic population. Furthermore, we expanded the search for specific additions to the terms “back pain, chest pain, cough, syncope, and urinary tract infection” by also including urinary tract infections with specific bacterial etiology, noncardiac chest pain, productive cough, and unconsciousness in addition to the narrower search terms used by Elashoff et al. (3). In addition, we extended the period of analysis to the second quarter of 2005 (when exenatide was first approved and used) and to the last quarter of 2010 (the last period available, when the present analysis was performed). This analysis again confirmed a significantly elevated odds ratio for pancreatitis and pancreatic cancer with both exenatide and sitagliptin and for thyroid carcinoma with exenatide but not sitagliptin (Fig. 3C). This analysis included a much greater number of retrieved cases with the index diseases (Fig. 3). In principle, this did not change the overall pattern, attesting to the robustness of findings based on this general strategy. Obviously, the suggested conclusions were not sensitive to minor or even major changes in the methodology. Similar analyses show that with exenatide treatment, not only cases with acute pancreatitis (or all pancreatitis) were reported more frequently but also cases suggestive of chronic pancreatitis (details not shown). This, however, was not the case with sitagliptin treatment. Along the same lines, gallbladder disease was reported more frequently with exenatide but not with sitagliptin treatment (details not shown). More complications related to gallbladder disease, especially gallbladder stones and bile duct stones, could potentially provide clues to a mechanism causing pancreatitis, perhaps related to motility effects of exenatide in the upper gastrointestinal tract (39–41).
In addition to the analysis more or less reproducing the findings by Elashoff et al. (3), we looked at subcategories of malignant disease entities. To our surprise, for several of these subcategories exenatide and sitagliptin apparently significantly reduced the odds ratios, in the case of exenatide, for example, regarding lung cancer, prostate cancer, lymphoma/multiple myeloma, metastasising tumors of unknown origin, and “other tumours not specified.” Sitagliptin significantly reduced the odds ratios for colon cancer, prostate cancer, and “other tumours not specified.” On the other hand, with exenatide treatment the rate of reporting carcinoid and stromal tumors and tumors of the female genital tract was apparently significantly elevated, and with sitagliptin treatment leukemias appeared to be reported significantly more often. This analysis was not based on any specific hypothesis, and the results are based on relatively small numbers of tumors and likely are subject to the same reporting bias as those originally reported by Elashoff et al. (3).
Summing up the odds ratios for “all other malignant disease” (except for pancreatic carcinoma and thyroid cancer) resulted in significantly reduced odds ratios regardless of whether insulin-treated patients were used as control subjects. This additional sensitivity analysis was performed because of the well-known tumor-promoting potential of insulin (42,43).
When taking into consideration all malignant disease categories together (including pancreatic carcinoma and thyroid cancer), the analysis demonstrated not an overall increase in the odds ratio for “all malignant disease” but, rather, a trend toward a reduction, especially with sitagliptin treatment (P = 0.068; not significant [details not shown]). Thus, even if there were an increased risk for the development of pancreatic cancer and thyroid carcinomas with exenatide and sitagliptin treatment or perhaps with incretin-based medications in general, this would not indicate any tumor-promoting potential in more general terms. If there might be an apparently increased risk for specific malignant disease entities, this seems to be counterbalanced by a potentially protective effect for other specific malignant tumors. However, the same reservations regarding conclusions to be drawn from such an analysis of the FDA Adverse Event Reporting System apply as discussed above regarding the findings of an increased reporting rate for pancreatitis, pancreatic carcinoma, and thyroid cancer with exenatide and sitagliptin.
Conclusions
Regarding the controversy of whether GLP-1–based therapy can increase the risk for specific malignant disease like pancreatic carcinoma and thyroid cancer, our conclusion is that apparently there is neither firm evidence in favor of this hypothesis nor evidence strong enough to rule out any such increased risk based on results available at present. We may learn answers to some of the questions from ongoing randomized controlled trials (e.g., cardiovascular safety trials underway for most approved compounds within the classes of GLP-1 receptor agonists or DPP-4 inhibitors) by analyzing databases or registries better suited for an unbiased postmarketing surveillance of adverse events associated with novel antidiabetes drugs. However, as of today the evidence in favor of the hypothesis that incretin-based medications cause specific types of malignant disease (e.g., pancreatic or [medullary] thyroid cancer) or increase the risk for cancer in a more general sense is not convincing enough to be seriously considered when making treatment decisions regarding the choice of antidiabetes medications.
Acknowledgments
M.A.N. has received research grants (to his institution, Diabeteszentrum Bad Lauterberg) from AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., Merck Sharp & Dohme, Novartis Pharma, GlaxoSmithKline, Novo Nordisk, Roche, and Tolerx. He has received consulting fees or honoraria for speaking from AstraZeneca, Berlin Chemie, Boehringer Ingelheim, Bristol-Myers Squibb, Diartis, Eli Lilly & Co., F. Hoffmann-La Roche, Intarcia Therapeutics, Merck Sharp & Dohme, Novo Nordisk, Sanofi, and Versartis, including reimbursement for travel expenses in connection with the above-mentioned activities. No other potential conflicts of interest relevant to this article were reported.
M.A.N. designed the analysis, researched data, and wrote the manuscript. N.F. retrieved data from the FDA Adverse Event Reporting System, helped with analyses, and reviewed the manuscript for critical content. M.A.N. and N.F. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Dr. C.J. Girman, Executive Director, Epidemiology, Merck Research Laboratories (North Wales, PA), for helpful input, especially regarding the table listing different forms of reporting bias.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dcS13-2004/-/DC1.
This publication is based on the presentations from the 4th World Congress on Controversies to Consensus in Diabetes, Obesity and Hypertension (CODHy). The Congress and the publication of this supplement were made possible in part by unrestricted educational grants from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Ethicon Endo-Surgery, Janssen, Medtronic, Novo Nordisk, Sanofi, and Takeda.
References
- 1.Ahmad SR, Swann J. Exenatide and rare adverse events. N Engl J Med 2008;358:1970–1971 [PubMed]
- 2.Nyborg NC, Mølck AM, Madsen LW, Knudsen LB. The human GLP-1 analog liraglutide and the pancreas: evidence for the absence of structural pancreatic changes in three species. Diabetes 2012;61:1243–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011;141:150–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler PC, Matveyenko AV, Dry S, Bhushan A, Elashoff R. Glucagon-like peptide-1 therapy and the exocrine pancreas: innocent bystander or friendly fire? Diabetologia 2010;53:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gier B, Matveyenko AV, Kirakossian D, Dawson D, Dry SM, Butler PC. Chronic GLP-1 receptor activation by exendin-4 induces expansion of pancreatic duct glands in rats and accelerates formation of dysplastic lesions and chronic pancreatitis in the Kras(G12D) mouse model. Diabetes 2012;61:1250–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjerre Knudsen L, Madsen LW, Andersen S, et al. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 2010;151:1473–1486 [DOI] [PubMed] [Google Scholar]
- 7.Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab 2012;97:121–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006;368:1696–1705 [DOI] [PubMed] [Google Scholar]
- 9.Lowenfels AB, Maisonneuve P, Cavallini G, et al. International Pancreatitis Study Group Pancreatitis and the risk of pancreatic cancer. N Engl J Med 1993;328:1433–1437 [DOI] [PubMed] [Google Scholar]
- 10.Bhanot UK, Möller P. Mechanisms of parenchymal injury and signaling pathways in ectatic ducts of chronic pancreatitis: implications for pancreatic carcinogenesis. Lab Invest 2009;89:489–497 [DOI] [PubMed]
- 11.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet 2011;378:607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strobel O, Rosow DE, Rakhlin EY, et al. Pancreatic duct glands are distinct ductal compartments that react to chronic injury and mediate Shh-induced metaplasia. Gastroenterology 2010;138:1166–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010;467:1114–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matveyenko AV, Dry S, Cox HI, et al. Beneficial endocrine but adverse exocrine effects of sitagliptin in the human islet amyloid polypeptide transgenic rat model of type 2 diabetes: interactions with metformin. Diabetes 2009;58:1604–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hull R, Aston-Mourney K, Subramanian SL, Goldstein L. One year of sitagliprin treatment does not result in exocrine pancreatic pathology in mice (Abstract). Diabetes 2011;60(Suppl. 1):A267
- 16.Nachnani JS, Bulchandani DG, Nookala A, et al. Biochemical and histological effects of exendin-4 (exenatide) on the rat pancreas. Diabetologia 2010;53:153–159 [DOI] [PubMed] [Google Scholar]
- 17.Tatarkiewicz K, Smith PA, Sablan EJ, et al. Exenatide does not evoke pancreatitis and attenuates chemically induced pancreatitis in normal and diabetic rodents. Am J Physiol Endocrinol Metab 2010;299:E1076–E1086 [DOI] [PMC free article] [PubMed]
- 18.Koehler JA, Baggio LL, Lamont BJ, Ali S, Drucker DJ. Glucagon-like peptide-1 receptor activation modulates pancreatitis-associated gene expression but does not modify the susceptibility to experimental pancreatitis in mice. Diabetes 2009;58:2148–2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koehler JA, Drucker DJ. Activation of glucagon-like peptide-1 receptor signaling does not modify the growth or apoptosis of human pancreatic cancer cells. Diabetes 2006;55:1369–1379 [DOI] [PubMed] [Google Scholar]
- 20.Steinberg W, DeVries JH, Wadden T, et al. Longitudinal monitoring of lipase and amylase in adults with type 2 diabetes and obesity: evidence from two phase 3 randomized clinical trials with the once-daily GLP-1 analog lirgalutide (Abstract). Gastroenterol 2012;142(Suppl. 1):S850–S851
- 21.Dore DD, Seeger JD, Arnold Chan K. Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr Med Res Opin 2009;25:1019–1027 [DOI] [PubMed] [Google Scholar]
- 22.Garg R, Chen W, Pendergrass M. Acute pancreatitis in type 2 diabetes treated with exenatide or sitagliptin: a retrospective observational pharmacy claims analysis. Diabetes Care 2010;33:2349–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pendergrass M, Chen W. Association between diabetes, exenatide, sitagliptin and acute pancreatitis (Abstract). Diabetes 2010;59(Suppl. 1):A160
- 24.Wenten M, Gaebler JA, Hussein M, et al. Relative risk of acute pancreatitis in initiators of exenatide twice daily compared with other anti-diabetic medication: a follow-up study. Diabet Med 2012;29:1412–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dore DD, Bloomgren GL, Wenten M, et al. A cohort study of acute pancreatitis in relation to exenatide use. Diabetes Obes Metab 2011;13:559–566 [DOI] [PubMed] [Google Scholar]
- 26.Engel SS, Williams-Herman DE, Golm GT, et al. Sitagliptin: review of preclinical and clinical data regarding incidence of pancreatitis. Int J Clin Pract 2010;64:984–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weng C, Bigger JT, Busacca L, Wilcox A, Getaneh A. Comparing the effectiveness of a clinical registry and a clinical data warehouse for supporting clinical trial recruitment: a case study. AMIA Annu Symp Proc 2010;2010:867–871 [PMC free article] [PubMed] [Google Scholar]
- 28.Scirica BM, Bhatt DL, Braunwald E, et al. The design and rationale of the saxagliptin assessment of vascular outcomes recorded in patients with diabetes mellitus-thrombolysis in myocardial infarction (SAVOR-TIMI) 53 study. Am Heart J 2011;162:818.e6–825.e6 [DOI] [PubMed]
- 29.Mullard A. Unleashing the mini-sentinel. Nat Rev Drug Discov 2012;11:255–257 [DOI] [PubMed] [Google Scholar]
- 30.Forrow S, Campion DM, Herrinton LJ, et al. The organizational structure and governing principles of the Food and Drug Administration’s Mini-Sentinel pilot program. Pharmacoepidemiol Drug Saf 2012;21(Suppl 1):12–17 [DOI] [PubMed] [Google Scholar]
- 31.Aschebrook-Kilfoy B, Ward MH, Sabra MM, Devesa SS. Thyroid cancer incidence patterns in the United States by histologic type, 1992-2006. Thyroid 2011;21:125–134 [DOI] [PMC free article] [PubMed]
- 32.Elbrønd B, Jakobsen G, Larsen S, et al. Pharmacokinetics, pharmacodynamics, safety, and tolerability of a single-dose of NN2211, a long-acting glucagon-like peptide 1 derivative, in healthy male subjects. Diabetes Care 2002;25:1398–1404 [DOI] [PubMed] [Google Scholar]
- 33.Agersø H, Jensen LB, Elbrønd B, Rolan P, Zdravkovic M. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia 2002;45:195–202 [DOI] [PubMed] [Google Scholar]
- 34.Hegedüs L, Moses AC, Zdravkovic M, Le Thi T, Daniels GH. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J Clin Endocrinol Metab 2011;96:853–860 [DOI] [PubMed] [Google Scholar]
- 35.Waser B, Beetschen K, Pellegata NS, Reubi JC. Incretin receptors in non-neoplastic and neoplastic thyroid C cells in rodents and humans: relevance for incretin-based diabetes therapy. Neuroendocrinology 2011;94:291–301 [DOI] [PubMed] [Google Scholar]
- 36.Spranger J, Gundert-Remy U, Stammschulte T. GLP-1-based therapies: the dilemma of uncertainty. Gastroenterology 2011;141:20–23 [DOI] [PubMed] [Google Scholar]
- 37.Miller CD, Phillips LS, Ziemer DC, Gallina DL, Cook CB, El-Kebbi IM. Hypoglycemia in patients with type 2 diabetes mellitus. Arch Intern Med 2001;161:1653–1659 [DOI] [PubMed] [Google Scholar]
- 38.Simeone JC, Quilliam BJ. Predictors of emergency department and outpatient visits for hypoglycemia in type 2 diabetes: an analysis of a large US administrative claims database. Ann Pharmacother 2012;46:157–168 [DOI] [PubMed] [Google Scholar]
- 39.Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes 2011;60:1561–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hellström PM. GLP-1: broadening the incretin concept to involve gut motility. Regul Pept 2009;156:9–12 [DOI] [PubMed] [Google Scholar]
- 41.Edholm T, Cejvan K, Abdel-Halim SM, Efendic S, Schmidt PT, Hellström PM. The incretin hormones GIP and GLP-1 in diabetic rats: effects on insulin secretion and small bowel motility. Neurogastroenterol Motil 2009;21:313–321 [DOI] [PubMed]
- 42.Janghorbani M, Dehghani M, Salehi-Marzijarani M. Systematic review and meta-analysis of insulin therapy and risk of cancer. Horm cancer 2012;3:137–146 [DOI] [PMC free article] [PubMed]
- 43.Platts J. Insulin therapy and cancer risk in diabetes mellitus. Clin Med 2010;10:509–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lobo FS, Wagner S, Gross CR, Schommer JC. Addressing the issue of channeling bias in observational studies with propensity scores analysis. Res Social Adm Pharm 2006;2:143–151 [DOI] [PubMed] [Google Scholar]
- 45.Wolfe F, Flowers N, Burke TA, Arguelles LM, Pettitt D. Increase in lifetime adverse drug reactions, service utilization, and disease severity among patients who will start COX-2 specific inhibitors: quantitative assessment of channeling bias and confounding by indication in 6689 patients with rheumatoid arthritis and osteoarthritis. J Rheumatol 2002;29:1015–1022 [PubMed] [Google Scholar]
- 46.Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Determinants of under-reporting of adverse drug reactions: a systematic review. Drug Saf 2009;32:19–31 [DOI] [PubMed]
- 47.van der Heijden PG, van Puijenbroek EP, van Buuren S, van der Hofstede JW. On the assessment of adverse drug reactions from spontaneous reporting systems: the influence of under-reporting on odds ratios. Stat Med 2002;21:2027–2044 [DOI] [PubMed] [Google Scholar]
- 48.McAdams MA, Governale LA, Swartz L, Hammad TA, Dal Pan GJ. Identifying patterns of adverse event reporting for four members of the angiotensin II receptor blockers class of drugs: revisiting the Weber effect. Pharmacoepidemiol Drug Saf 2008;17:882–889 [DOI] [PubMed] [Google Scholar]
- 49.Hartnell NR, Wilson JP. Replication of the Weber effect using postmarketing adverse event reports voluntarily submitted to the United States Food and Drug Administration. Pharmacotherapy 2004;24:743–749 [DOI] [PubMed] [Google Scholar]
- 50.Pariente A, Didailler M, Avillach P, et al. A potential competition bias in the detection of safety signals from spontaneous reporting databases. Pharmacoepidemiol Drug Saf 2010;19:1166–1171 [DOI] [PubMed] [Google Scholar]
- 51.Goodman MJ, Nordin J. Vaccine adverse event reporting system reporting source: a possible source of bias in longitudinal studies. Pediatrics 2006;117:387–390 [DOI] [PubMed] [Google Scholar]
- 52.Riddle MS, Porter CK. Detection bias and the association between inflammatory bowel disease and Salmonella and Campylobacter infection. Gut 2012;61:635. [DOI] [PubMed] [Google Scholar]