Abstract
The stimulation of insulin secretion by glucose can be modulated by multiple nutritive, hormonal, and pharmacological inputs. Fatty acids potentiate insulin secretion through the generation of intracellular signaling molecules and through the activation of cell surface receptors. The G-protein–coupled receptor 40 (GPR40), also known as free fatty acid receptor 1 (we will use GPR40 in this review), has emerged as an important component in the fatty acid augmentation of insulin secretion. By signaling predominantly through Gαq/11, GPR40 increases intracellular calcium and activates phospholipases to generate diacylglycerols resulting in increased insulin secretion. Synthetic small-molecule agonists of GPR40 enhance insulin secretion in a glucose-dependent manner in vitro and in vivo with a mechanism similar to that found with fatty acids. GPR40 agonists have shown efficacy in increasing insulin secretion and lowering blood glucose in rodent models of type 2 diabetes. Recent phase I and phase II clinical trials in humans have shown that the GPR40 agonist TAK-875 reduces fasting and postprandial blood glucose and lowers HbA1c with efficacy equal to that of the sulfonylurea glimepiride without inducing hypoglycemia or evidence of tachyphylaxis. These data suggest that targeting the GPR40 receptor can be a viable therapeutic option for the treatment of type 2 diabetes.
β-C-Cells in islets of Langerhans secrete insulin in response to increased blood glucose. An acute increase in glucose evokes a rapid release of insulin that is sustained for a short period, designated as the 1st phase, followed by an extended period of lower secretion (2nd phase) that accounts for the majority of insulin secretion. Progressive reduction in β-cell mass or secretory capacity causes abnormalities of glucose metabolism, resulting in diabetes and its complications.
Insulin secretion from β-cells is primarily controlled through the uptake and metabolism of glucose, resulting in a rapid increase in ATP-to-ADP ratio and closure of the KATP channel with membrane depolarization through inhibition of K+ flux. This results in the activation of the voltage-dependent calcium channel with calcium influx and fusion of insulin containing granules and insulin release. This basal mechanism is primarily responsible for the rapid, first phase of insulin secretion. Glucose-derived pyruvate can also enter into the tricarboxylic acid cycle via pyruvate dehydrogenase (PDH) and pyruvate carboxylase, which can impact on insulin secretion by increasing levels of cataplerosis-derived signaling molecules such as oxaloacetate, citrate, glutamate, and NADPH (rev. in 1). Additional nutritive and nonnutritive factors, including cAMP, amino acids, and fatty acids, can directly or indirectly modulate insulin secretion (2).
Fatty acids and modulation of insulin release
Fatty acids play a complex role in the physiology of insulin secretion and also participate in the disruption of β-cell function and mass that leads to type 2 diabetes. Exposure of β-cells to fatty acid in vitro and in vivo has a biphasic effect. Acutely, exposure to fatty acids does not stimulate insulin release; rather, fatty acids are able to dose dependently increase the amount of insulin secreted when exposed to increased glucose concentrations. Moreover, fatty acids are important to maintaining normal insulin secretion. McGarry and colleagues (3) showed that reducing fatty acid levels in fasted rats by inhibiting lipolysis by nicotinic acid significantly reduced subsequent glucose-stimulated insulin secretion. In contrast, elevation of circulating fatty acid levels markedly increased second-phase insulin release (3). In contrast, chronic exposure to elevated fatty acids has a detrimental effect on β-cell function with elevations in basal insulin secretion but reduced glucose-stimulated release. In addition, fatty acids can result in significant decreases in insulin secretion as a result of β-cell death (4) or potentially β-cell dedifferentiation (5), leading to the development of type 2 diabetes.
Oxidation of fatty acids is not required for enhancement of insulin secretion as inhibition of carnitine palmatoyltransferase-1 (CPT-1), responsible for the import of fatty acids (6) into the mitochondria for oxidation, results in enhanced glucose-stimulated insulin secretion in palmitate-treated cells. The long-chain acyl-CoA (LC-CoA) model of glucose-stimulated insulin secretion predicts that cytoplasmic malonyl-CoA, derived from elevated levels of citrate after glucose exposure, inhibits CPT-1, inhibiting LC-CoA uptake and fatty acid oxidation resulting in increases in cytoplasmic LC-CoA levels (7). The importance of LC-CoA in potentiation of insulin secretion is highlighted by studies that show that reducing LC-CoA through increased catabolism or by the inhibition of the formation of LC-CoA by triacsin-C inhibition of Acyl-CoA synthase eliminates the ability of fatty acids to enhance insulin secretion (8).
LC-CoAs have a variety of metabolic fates but by themselves can function as metabolic regulators and signaling molecules. LC-CoAs have been proposed to modulate KATP activity. Some studies have suggested that LC-CoAs activate KATP (9), while others suggest that decreases in LC-CoA concentration enhance the closure of KATP channels (10,11) increasing depolarization of the membrane. Generation of phosphatidic acid and diacylglycerols may also be important end products of LC-CoA metabolism that participate in glucose-stimulated insulin secretion. Diacylglycerol levels increase after glucose exposure (12), which in turn can activate protein kinase C as well as modulate proteins on insulin secretory granules, both of which have been shown to improve insulin secretion (7). A recent study has suggested that diacylglycerol activation of other protein kinase pathways may also be important for augmenting insulin secretion (see below).
G-protein–coupled receptors and fatty acid augmentation of insulin secretion
High-throughput screening assays have allowed identification of endogenous ligands for G-protein–coupled receptors (GPCRs) (13). These screening methodologies have allowed the further identification of GPCRs, which respond to fatty acids with individual GPCRs showing relative selectivity to fatty acid chain length and degree of saturation (14). GPR40 is highly expressed in β-cells and has turned out to be a key protein mediating free fatty acid potentiation of insulin and an attractive target to enhance insulin secretion in patients with type 2 diabetes. GPR40 is a member of a subfamily of homologous, intronless GPCRs residing on chromosome 19q13.1, which include GPR41, GPR42, and GPR43 (15). Subsequent studies from two groups identified free fatty acids as ligands for the GPR40 protein (16,17), and GPR40 shows higher affinity for longer-chain fatty acids with a half-maximal effective concentration (EC50) in the 1–2 μmol/L range (17,18).
GPR40 appears to play an important role in the fatty acid–mediated augmentation in insulin secretion. siRNA or oligonucleotide-mediated reduction of GPR40 in β-cell lines or isolated mouse islets reduced fatty acid augmentation of insulin secretion (19–21). Similarly, disruption of GPR40 in mice (GPR40−/−) reduces fatty acid induction of insulin secretion (22,23) in vivo. Conversely, transgenic overexpression of GPR40 results in mice improved glucose-stimulated insulin secretion in both wild-type and diabetic mice (24). Islets isolated from these animals showed a robust response to palmitic acid in vitro compared with controls. The results from these studies suggest that ~50% of the augmentation of insulin secretion by fatty acids is mediated by GPR40.
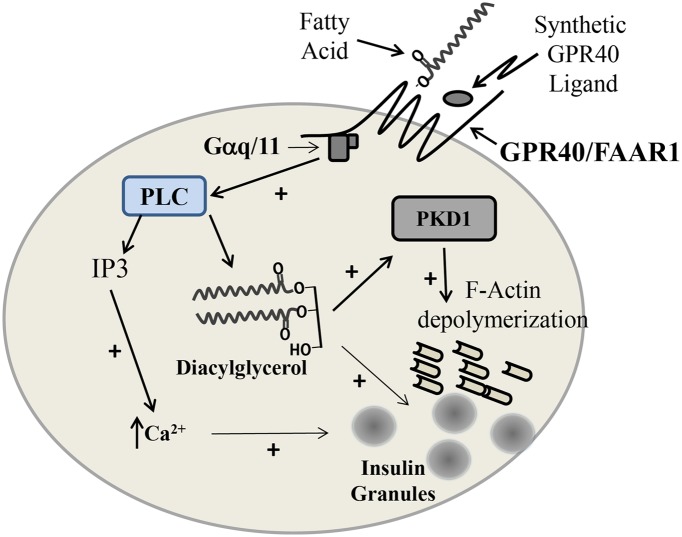
Fatty acid binding to GPR40 appears to activate the Gαq/11-protein complex resulting in activation of phospholipase C (PLC) (16,23). PLC hydrolyses phosphoinositol-containing membrane lipids to generate inositol 1,4,5-trisphosphate (IP3) and diacylglycerol. IP3 in turn appears to mediate the increase in intracellular Ca2+ levels that occurs after GPR40 activation (18). Recent studies by the Poitout group (25) suggest that the generation of diacylglycerol may be the key event after GPR40 activation. They showed that islets derived from mice with genetic disruption of GPR40 (GPR40−/−) responded normally to glucose but showed no increase in insulin secretion after oleic acid exposure. However, treatment with cell-permeable diacylglycerol robustly increased second-phase insulin secretion in both wild-type and GPR40−/− islets. Fatty acids induce the phosphorylation and activation of phospholipase D1 (PKD1), which seems to modulate the reorganization of the cortical actin network that underlies second-phase insulin secretion (25). “Knockdown” of PKD1 protein inhibits the fatty acid augmentation of insulin secretion. What remains uncertain is how activation of GPR40-mediated activation of Gαq/11 results in increases in PLC activity and whether PKD activation is the only way that DAG levels increase in response to glucose stimulation in β-cells.
Is there a potential for adverse effects of prolonged stimulation of GPR40?
Numerous studies in vivo and in vitro have shown that prolonged exposure to fatty acids, with or without elevated glucose, results in β-cell secretory defects and direct toxic effects that result in β-cell death (4). Studies in humans suggest a genetic predisposition in susceptibility to the effects of fatty acids, as first-degree relatives of type 2 diabetic patients without evidence of disease were significantly more susceptible to the impairment of insulin secretion by elevations of plasma fatty acids (26). Given the effect of prolonged exposure of fatty acids, divergent studies in animals with disruption of GPR40 gave rise to a controversy about the potential role of GPR40 to mediate the “toxic” effects of fatty acids. In an initial study, Steneberg et al. (27) showed that disruption of GPR40 in β-cells reduced the ability of fatty acids to potentiate insulin secretion, as would be predicted by the in vitro biology of the receptors. But disruption of GPR40 protected the mice from the adverse effects of prolonged exposure to fatty acids and the detrimental effects of a high-fat diet on insulin secretion. This earlier study suggested that a GPR40 antagonist might be a viable target for diabetes treatment. Subsequent studies by two groups were unable to replicate these findings (28,29) with similar impairment of islet function after in vitro and in vivo exposure to fatty acids. Latour et al. (23) used mice with whole-body disruption of GPR40 and examined glucose homeostasis and insulin secretion in vivo and dynamics of insulin secretion in vitro after short- and long-term exposure to fatty acids. GPR40−/− mice were phenotypically similar to the wild-type animals and showed no changes in glucose or insulin levels at baseline or in response to a glucose tolerance test. Infusion of fatty acids in the form of intralipid augmented insulin secretion in wild-type mice, but this effect was reduced by 50% in GPR40−/− mice. Islets isolated from wild-type and GPR40−/− mice showed identical insulin secretion in response to acute increases in media glucose and to depolarization with KCl. However, fatty acid potentiation of insulin release was reduced in the GPR40−/−-derived islets but showed a similar decrease in insulin secretion after incubation with palmitic acid for 72 h. The reason for these discrepant findings is not clear, though potential reasons have previously been outlined (30).
In vivo pharmacology of GPR40 agonists in rodent models
The development of synthetic agonists to GPR40 with salutary effects on insulin secretion in vitro and in vivo has demonstrated that activation of the receptor appears to be a viable option for diabetes treatment. A large number of synthetic agonists for the GPR40 receptor have been developed, several of which appear to recapitulate the actions of fatty acids to potentiate insulin secretion (19,31–51). Based on the original observation of Kotarsky (52) that some thiazolidinediones could activate surface receptors later identified as GPR40, Tan et al. (45) screened a library of thiazolidinediones and optimized a lead candidate (Cpd-B). These molecules enhanced insulin secretion in islets isolated from wild-type mice but was inactive in islets from GPR40−/− mice. Importantly, exposure of both wild-type and GPR40−/− islets showed reduced insulin secretion after 3 days’ exposure to free fatty acids, while exposure to Cpd-B had no effect on insulin secretion. Similarly, an orally active (2,3-dihydro-1-benzofuran-3-yl)acetic acid derivative showed a dose-dependent reduction in glucose levels in Goto-Kakizaki (GK) rats, a type 2 diabetic model with impaired glucose-dependent insulin secretion. Similar results have been found with other agonists (36,41). Finally, Tsujihata et al. (46) examined the effect of TAK-875, an orally bioactive GPR40 agonist with an EC50 for receptor activation of 72 nmol/L. As with Cpd-B, prolonged exposure of rat islets to TAK-875 did not affect glucose-mediated insulin secretion. Rats rendered glucose intolerant by repeated-dose administration of low doses of streptozotocin showed a dose-dependent improvement in glucose tolerance. Similarly, TAK-875 increased insulin levels and decreased plasma glucose concentrations in Zucker diabetic fatty rats, a model of insulin-resistant type 2 diabetes. In aggregate, these studies provide further evidence that chronic activation of GPR40 does not mediate fatty acid toxicity in β-cells.
Human studies with the GPR40 agonist TAK-875
TAK-875 is the first GPR40 agonist to be tested for efficacy in humans. Initial studies showed the compound to be rapidly absorbed with a half-life of 28–30 h and clearance primarily through glucouronidation in the liver with minimal urinary clearance (40). A phase I, randomized 14-day exposure study in two patients with diabetes showed a dose-dependent reduction in fasting and postglucose challenge glucose levels and increases in post–oral glucose tolerance test C-peptide levels in the serum (53).
Based on these favorable results, a 12-week, double-blind, placebo-controlled phase II study was performed in type 2 diabetic subjects that compared daily administration of TAK-875 and glimepiride with placebo (54). Subjects with an HbA1c between 7.5 and 10.9% who were either drug naïve (24) or on metformin-alone therapy (76%) were randomized to either placebo (n = 61), TAK-875 (6.25, 25, 50, 100, or 200 mg) (n = 303), or glimepiride (4 mg) (n = 62). At the end of treatment, HbA1c levels fell significantly from baseline in the TAK-875 group between −0.65 ± 0.114 and −1.12% ± 0.113%, which was similar to glimepiride, which fell −1.05 ± 111%. Both active treatment groups showed more efficacy than placebo (−0.13 ± 115%). There were consistent, significant changes induced in fasting blood glucose by TAK-875 at doses >50 mg/day, and these were similar to changes seen in glimepiride-treated subjects. The onset of action was rapid, with changes in fasting blood glucose occurring in the first 2 weeks of treatment. Importantly, there does not appear to be a waning of efficacy with time, suggesting that the GPR40 agonist does not induce β-cell dysfunction with prolonged treatment, arguing against a potential adverse effect of the compounds with prolonged exposure.
The data from measurements of fasting and glucose-stimulated insulin levels suggest that the mechanism of action of TAK-875 is due to enhancement of insulin secretion. Insulin sensitivity, as assessed by the Matsuda index (55), did not change from baseline in any treatment group. Higher doses of TAK-875 (25, 100, and 200 mg/day) appeared to enhance β-cell insulin secretion as assessed by a significant increase in the C-peptide–to–glucose ratio at the 30-min time point during the oral glucose tolerance test. Despite glimepiride being an insulin secretagogue, no effect was seen in the glimepiride-treated subjects compared with placebo subjects. This was attributed to the fact that the test was performed 24 h after the last dose of medication where the significantly longer half-life of TAK-875 compared with glimepiride may provide residual effects (40).
The adverse effect profile was similar between the treatment groups with the exception of a significantly higher rate of hypoglycemia and significant weight gain in the glimepiride group compared with placebo. There was no significant weight change in the TAK-875 group compared with baseline, but a small, significant weight gain was seen compared with the placebo group, which demonstrated a small weight loss over the 12-week treatment period. There was not a significant impact of TAK-875 on blood pressure or lipid parameters over the treatment period. The positive results will be further explored in ongoing phase III clinical trials to fully understand the safety and efficacy of GPR40 agonists in the treatment of type 2 diabetes.
Conclusions
The identification of GPR40 has led to a new understanding about the acute effects of fatty acids to increase insulin secretion from β-cells after stimulation with glucose. The data suggest that half of the augmentation of the second phase of insulin secretion by fatty acids is mediated by GPR40 signaling (Fig. 1). Additional studies will be needed to clarify additional details of the mechanisms by which GPR40 signaling increases insulin secretion. Are fatty acids that serve as ligands derived from plasma or could they be mobilized from β-cells and act in an autocrine or paracrine manner? Increases in diacylglycerol seem to play an important role in mediating at least part of the downstream GPR40 signaling. Are the diacylglycerols exclusively generated by PLC, or are there additional pathways? How do fatty acids, independent of GPR40, enhance insulin secretion? Despite these unanswered questions, the evidence from both animal models and in initial human clinical trials suggests that GPR40 agonists effectively increase glucose-dependent insulin secretion with minimal risk of hypoglycemia or evidence of β-cell toxicity.
Figure 1.
Potential mechanism of potentiation of the second phase of insulin secretion by fatty acid and synthetic ligand stimulation of GPR40/free fatty acid receptor 1. Signaling through Gaq/11 results in PLC activation, hydrolyzing phosphoinositols, which generates IP3 and diacylglycerol. IP3 leads to increased Ca2+ release from the endoplasmic reticulum aiding granule movement/fusion. Diacylglycerol may directly assist in granule fusion as well as activating PKD1, resulting in F-actin depolymerazation and also assisting in insulin granule movement, which augments insulin release.
Acknowledgments
This work was supported by the A. Alfred Taubman Research Institute.
No potential conflicts of interest relevant to this article were reported.
C.F.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The author thanks Eckhard Leifke and Brian Sherer, Takeda Global Research & Development, for helpful discussions, and Vincent Poitout, Montreal Diabetes Research Center, for insightful comments on the manuscript.
Footnotes
This publication is based on the presentations from the 4th World Congress on Controversies to Consensus in Diabetes, Obesity and Hypertension (CODHy). The Congress and the publication of this supplement were made possible in part by unrestricted educational grants from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Ethicon Endo-Surgery, Janssen, Medtronic, Novo Nordisk, Sanofi, and Takeda.
References
- 1.Jensen MV, Joseph JW, Ronnebaum SM, Burgess SC, Sherry AD, Newgard CB. Metabolic cycling in control of glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab 2008;295:E1287–E1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newsholme P, Gaudel C, McClenaghan NH. Nutrient regulation of insulin secretion and beta-cell functional integrity. Adv Exp Med Biol 2010;654:91–114 [DOI] [PubMed] [Google Scholar]
- 3.Stein DT, Esser V, Stevenson BE, et al. Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J Clin Invest 1996;97:2728–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang-Chen KJ, Mullur R, Bernal-Mizrachi E. Beta-cell failure as a complication of diabetes. Rev Endocr Metab Disord 2008;9:329–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 2012;150:1223–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem 1997;244:1–14 [DOI] [PubMed] [Google Scholar]
- 7.Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes 2006;55(Suppl. 2):S16–S23 [DOI] [PubMed] [Google Scholar]
- 8.Roduit R, Nolan C, Alarcon C, et al. A role for the malonyl-CoA/long-chain acyl-CoA pathway of lipid signaling in the regulation of insulin secretion in response to both fuel and nonfuel stimuli. Diabetes 2004;53:1007–1019 [DOI] [PubMed] [Google Scholar]
- 9.Larsson O, Deeney JT, Bränström R, Berggren PO, Corkey BE. Activation of the ATP-sensitive K+ channel by long chain acyl-CoA. A role in modulation of pancreatic beta-cell glucose sensitivity. J Biol Chem 1996;271:10623–10626 [DOI] [PubMed] [Google Scholar]
- 10.Tarasov A, Dusonchet J, Ashcroft F. Metabolic regulation of the pancreatic beta-cell ATP-sensitive K+ channel: a pas de deux. Diabetes 2004;53(Suppl. 3):S113–S122 [DOI] [PubMed] [Google Scholar]
- 11.Bränström R, Aspinwall CA, Välimäki S, et al. Long-chain CoA esters activate human pancreatic beta-cell KATP channels: potential role in Type 2 diabetes. Diabetologia 2004;47:277–283 [DOI] [PubMed] [Google Scholar]
- 12.Peter-Riesch B, Fathi M, Schlegel W, Wollheim CB. Glucose and carbachol generate 1,2-diacylglycerols by different mechanisms in pancreatic islets. J Clin Invest 1988;81:1154–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Civelli O. GPCR deorphanizations: the novel, the known and the unexpected transmitters. Trends Pharmacol Sci 2005;26:15–19 [DOI] [PubMed] [Google Scholar]
- 14.Morgan NG, Dhayal S. G-protein coupled receptors mediating long chain fatty acid signalling in the pancreatic beta-cell. Biochem Pharmacol 2009;78:1419–1427 [DOI] [PubMed] [Google Scholar]
- 15.Sawzdargo M, George SR, Nguyen T, Xu S, Kolakowski LF, O’Dowd BF. A cluster of four novel human G protein-coupled receptor genes occurring in close proximity to CD22 gene on chromosome 19q13.1. Biochem Biophys Res Commun 1997;239:543–547 [DOI] [PubMed] [Google Scholar]
- 16.Briscoe CP, Tadayyon M, Andrews JL, et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem 2003;278:11303–11311 [DOI] [PubMed] [Google Scholar]
- 17.Itoh Y, Kawamata Y, Harada M, et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 2003;422:173–176 [DOI] [PubMed] [Google Scholar]
- 18.Fujiwara K, Maekawa F, Yada T. Oleic acid interacts with GPR40 to induce Ca2+ signaling in rat islet beta-cells: mediation by PLC and L-type Ca2+ channel and link to insulin release. Am J Physiol Endocrinol Metab 2005;289:E670–E677 [DOI] [PubMed] [Google Scholar]
- 19.Briscoe CP, Peat AJ, McKeown SC, et al. Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules. Br J Pharmacol 2006;148:619–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salehi A, Flodgren E, Nilsson NE, et al. Free fatty acid receptor 1 (FFA(1)R/GPR40) and its involvement in fatty-acid-stimulated insulin secretion. Cell Tissue Res 2005;322:207–215 [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Xu M, Zhang S, et al. The role of G protein-coupled receptor 40 in lipoapoptosis in mouse beta-cell line NIT-1. J Mol Endocrinol 2007;38:651–661 [DOI] [PubMed] [Google Scholar]
- 22.Alquier T, Peyot ML, Latour MG, et al. Deletion of GPR40 impairs glucose-induced insulin secretion in vivo in mice without affecting intracellular fuel metabolism in islets. Diabetes 2009;58:2607–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Latour MG, Alquier T, Oseid E, et al. GPR40 is necessary but not sufficient for fatty acid stimulation of insulin secretion in vivo. Diabetes 2007;56:1087–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagasumi K, Esaki R, Iwachidow K, et al. Overexpression of GPR40 in pancreatic beta-cells augments glucose-stimulated insulin secretion and improves glucose tolerance in normal and diabetic mice. Diabetes 2009;58:1067–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferdaoussi M, Bergeron V, Zarrouki B, et al. G protein-coupled receptor (GPR)40-dependent potentiation of insulin secretion in mouse islets is mediated by protein kinase D1. Diabetologia 2012;55:2682–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kashyap S, Belfort R, Gastaldelli A, et al. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes 2003;52:2461–2474 [DOI] [PubMed] [Google Scholar]
- 27.Steneberg P, Rubins N, Bartoov-Shifman R, Walker MD, Edlund H. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab 2005;1:245–258 [DOI] [PubMed] [Google Scholar]
- 28.Kebede M, Alquier T, Latour MG, Semache M, Tremblay C, Poitout V. The fatty acid receptor GPR40 plays a role in insulin secretion in vivo after high-fat feeding. Diabetes 2008;57:2432–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lan H, Hoos LM, Liu L, et al. Lack of FFAR1/GPR40 does not protect mice from high-fat diet-induced metabolic disease. Diabetes 2008;57:2999–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alquier T, Poitout V. GPR40: good cop, bad cop? Diabetes 2009;58:1035–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bharate SB, Rodge A, Joshi RK, et al. Discovery of diacylphloroglucinols as a new class of GPR40 (FFAR1) agonists. Bioorg Med Chem Lett 2008;18:6357–6361 [DOI] [PubMed] [Google Scholar]
- 32.Christiansen E, Urban C, Grundmann M, et al. Identification of a potent and selective free fatty acid receptor 1 (FFA1/GPR40) agonist with favorable physicochemical and in vitro ADME properties. J Med Chem 2011;54:6691–6703 [DOI] [PubMed] [Google Scholar]
- 33.Christiansen E, Urban C, Merten N, et al. Discovery of potent and selective agonists for the free fatty acid receptor 1 (FFA(1)/GPR40), a potential target for the treatment of type II diabetes. J Med Chem 2008;51:7061–7064 [DOI] [PubMed] [Google Scholar]
- 34.Garrido DM, Corbett DF, Dwornik KA, et al. Synthesis and activity of small molecule GPR40 agonists. Bioorg Med Chem Lett 2006;16:1840–1845 [DOI] [PubMed] [Google Scholar]
- 35.Hara T, Hirasawa A, Sun Q, et al. Novel selective ligands for free fatty acid receptors GPR120 and GPR40. Naunyn Schmiedebergs Arch Pharmacol 2009;380:247–255 [DOI] [PubMed] [Google Scholar]
- 36.Houze JB, Zhu L, Sun Y, et al. AMG 837: a potent, orally bioavailable GPR40 agonist. Bioorg Med Chem Lett 2012;22:1267–1270 [DOI] [PubMed] [Google Scholar]
- 37.Humphries PS, Benbow JW, Bonin PD, et al. Synthesis and SAR of 1,2,3,4-tetrahydroisoquinolin-1-ones as novel G-protein-coupled receptor 40 (GPR40) antagonists. Bioorg Med Chem Lett 2009;19:2400–2403 [DOI] [PubMed] [Google Scholar]
- 38.McKeown SC, Corbett DF, Goetz AS, et al. Solid phase synthesis and SAR of small molecule agonists for the GPR40 receptor. Bioorg Med Chem Lett 2007;17:1584–1589 [DOI] [PubMed] [Google Scholar]
- 39.Mikami S, Kitamura S, Negoro N, et al. Discovery of phenylpropanoic acid derivatives containing polar functionalities as potent and orally bioavailable G protein-coupled receptor 40 agonists for the treatment of type 2 diabetes. J Med Chem 2012;55:3756–3776 [DOI] [PubMed] [Google Scholar]
- 40.Naik H, Vakilynejad M, Wu J, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamic properties of the GPR40 agonist TAK-875: results from a double-blind, placebo-controlled single oral dose rising study in healthy volunteers. J Clin Pharmacol 2012;52:1007–1016 [DOI] [PubMed] [Google Scholar]
- 41.Negoro N, Sasaki S, Ito M, et al. Identification of fused-ring alkanoic acids with improved pharmacokinetic profiles that act as G protein-coupled receptor 40/free fatty acid receptor 1 agonists. J Med Chem 2012;55:1538–1552 [DOI] [PubMed] [Google Scholar]
- 42.Negoro N, Sasaki S, Mikami S, et al. Optimization of (2,3-dihydro-1-benzofuran-3-yl)acetic acids: discovery of a non-free fatty acid-like, highly bioavailable G protein-coupled receptor 40/free fatty acid receptor 1 agonist as a glucose-dependent insulinotropic agent. J Med Chem 2012;55:3960–3974 [DOI] [PubMed] [Google Scholar]
- 43.Sasaki S, Kitamura S, Negoro N, et al. Design, synthesis, and biological activity of potent and orally available G protein-coupled receptor 40 agonists. J Med Chem 2011;54:1365–1378 [DOI] [PubMed] [Google Scholar]
- 44.Song F, Lu S, Gunnet J, et al. Synthesis and biological evaluation of 3-aryl-3-(4-phenoxy)-propionic acid as a novel series of G protein-coupled receptor 40 agonists. J Med Chem 2007;50:2807–2817 [DOI] [PubMed] [Google Scholar]
- 45.Tan CP, Feng Y, Zhou YP, et al. Selective small-molecule agonists of G protein-coupled receptor 40 promote glucose-dependent insulin secretion and reduce blood glucose in mice. Diabetes 2008;57:2211–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsujihata Y, Ito R, Suzuki M, et al. TAK-875, an orally available G protein-coupled receptor 40/free fatty acid receptor 1 agonist, enhances glucose-dependent insulin secretion and improves both postprandial and fasting hyperglycemia in type 2 diabetic rats. J Pharmacol Exp Ther 2011;339:228–237 [DOI] [PubMed] [Google Scholar]
- 47.Walsh SP, Severino A, Zhou C, et al. 3-Substituted 3-(4-aryloxyaryl)-propanoic acids as GPR40 agonists. Bioorg Med Chem Lett 2011;21:3390–3394 [DOI] [PubMed] [Google Scholar]
- 48.Yazaki R, Kumagai N, Shibasaki M. Cooperative activation of alkyne and thioamide functionalities; direct catalytic asymmetric conjugate addition of terminal alkynes to α,β-unsaturated thioamides. Chem Asian J 2011;6:1778–1790 [DOI] [PubMed] [Google Scholar]
- 49.Yazaki R, Kumagai N, Shibasaki M. Enantioselective synthesis of a GPR40 agonist AMG 837 via catalytic asymmetric conjugate addition of terminal alkyne to α,β-unsaturated thioamide. Org Lett 2011;13:952–955 [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Yan G, Li Y, Zhu W, Wang H. DC260126, a small-molecule antagonist of GPR40, improves insulin tolerance but not glucose tolerance in obese Zucker rats. Biomed Pharmacother 2010;64:647–651 [DOI] [PubMed] [Google Scholar]
- 51.Zhao Y, Song Y, Shen X, Liao J. Feasible Synthesis of Antagonist of GPR40 by Constructing 2-Thiouracil Ring viaAcid Mediated Cyclization. Heterocycles 2011;83:1145–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kotarsky K, Nilsson NE, Flodgren E, Owman C, Olde B. A human cell surface receptor activated by free fatty acids and thiazolidinedione drugs. Biochem Biophys Res Commun 2003;301:406–410 [DOI] [PubMed] [Google Scholar]
- 53.Leifke E, Naik H, Wu J, et al. A multiple-ascending-dose study to evaluate safety, pharmacokinetics, and pharmacodynamics of a novel GPR40 agonist, TAK-875, in subjects with type 2 diabetes. Clin Pharmacol Ther 2012;92:29–39 [DOI] [PubMed] [Google Scholar]
- 54.Burant CF, Viswanathan P, Marcinak J, et al. TAK-875 versus placebo or glimepiride in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2012;379:1403–1411 [DOI] [PubMed] [Google Scholar]
- 55.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]