Elevated blood pressure (BP) is a major risk factor for cardiovascular (CV) events and mortality (1) and a leading contributor to the global disease burden (2). Overwhelming evidence is now available showing that BP measured in the office shows a linear relationship with a number of CV and renal outcomes as well as with overall mortality and that lowering of office BP (OBP) with treatment is effective in reducing morbidity and mortality (3,4). However, application over the last 40 years of techniques for out-of-office BP monitoring including home BP monitoring (HBPM) and 24-h ambulatory BP monitoring (ABPM) has led to further important findings. In particular, 1) average BP measured in everyday life conditions may be an even better predictor of CV outcomes than isolated OBP readings and 2) the extent of fluctuations of BP over time may provide additional, independent prognostic information compared with both isolated office readings and average ambulatory BP (ABP) levels, respectively. These findings are of upmost relevance in the case of diabetic patients who are characterized by a significantly higher risk of CV events compared with nondiabetic individuals, with diabetes itself currently considered a CV disease equivalent (5,6). The aim of the present article is to review the available evidence on the prognostic importance of BP mean levels and of BP variability (BPV) estimates and to critically evaluate whether antihypertensive treatment strategies should be targeted at reducing not only average BP levels but also the degree of BPV in order to optimize CV protection in diabetic patients.
Prognostic value of OBP values
Consistent evidence from observational studies has indicated that the risk of CV morbidity and mortality has a strong and continuous relationship with OBP levels (3), without any evidence of a threshold down to at least 115/75 mmHg (4). Furthermore, large meta-analyses of major interventional trials in hypertensive subjects have shown that lowering OBP levels confers significant CV protection regardless of the drug class used with a direct relationship between the degree of OBP lowering and the magnitude of risk reduction for most of the outcomes considered (7).
Patients with diabetes and elevation in clinic BP levels are at higher CV risk compared with nondiabetic individuals (8–10). Evidence on this has been provided from several studies, showing a continuous direct and synergic relationship of elevated BP levels and type 2 diabetes with risk of target-organ damage and CV events without any evidence of BP threshold level (8–11). In line with these findings, evidence has also been provided that in hypertensive patients with coexisting type 2 diabetes, tighter control of clinic BP is particularly important in order to improve CV protection (12–23). Notwithstanding the current recommendation that OBP measurement remains the cornerstone of hypertension diagnosis and management, there is now a general consensus that isolated OBP readings alone are no longer sufficient. This is because of several well-acknowledged limitations that characterize OBP measurements, including their inability to track the dynamic behavior of BP, the inherent inaccuracy of the technique, the operator dependence of the auscultatory technique (observer bias, digit preference), variable interference by the “white coat effect,” and the inability of this approach to collect information on BP during subjects’ usual activities and over a long period of time.
Are out-of-office mean BP levels superior to OBP in predicting CV outcomes?
The introduction of out-of-office BP measurement techniques (i.e., HBPM and 24-h ABPM) over the past four decades has significantly improved the management of hypertension, overcoming some limitations of OBP. Noninvasive ABPM techniques, based on automated oscillometric arm cuff BP readings, are nowadays widely available and provide BP measurements throughout the 24 h, not only during daytime, but also at night, when BP levels are known to bear the strongest prognostic value (24–28). Although, compared with ABPM, self-measurements of BP by patients at home cannot provide the extensive information on daily life BP values yielded by 24-h ambulatory recordings, they do give accurate and frequent out-of-office BP measurements over a single day, several days, weeks, or months in a usual life setting, representing an ideal tool not only for a population-wide diagnostic approach to hypertension but also for BP monitoring over long-term follow-up (29). Both HBPM and ABPM are thus able to provide BP measurements in the patient’s natural environment—on one hand, detecting BP changes in real-life conditions, and on the other hand preventing the alarm reaction associated with OBP measurement, responsible for the white coat effect (30). Because of these features, disagreement between OBP and out-of-office BP measurements (either with ABPM or HBPM) is frequently reported, leading to the identification of two conditions where BP status is differently classified by these methods: white-coat hypertension, also termed isolated office hypertension (elevated OBP and normal ABP or HBP), and masked hypertension (normal OBP and elevated ambulatory or home BP levels) (31,32). Identification of these two conditions, the latter in particular, is of relevance since several studies have demonstrated a worse CV prognosis among subjects with masked hypertension compared with those with isolated office hypertension (31,32). In support of the prognostic value of out-of-office mean BP levels, several observational studies either in diabetic or nondiabetic populations have provided consistent evidence on the superiority of ambulatory and home BP levels over conventional OBP in predicting CV events (see below), leading major international hypertension management guidelines to recommend the use of ABPM and HBPM in clinical practice as a complement to conventional OBP measurements (1,29,33,34).
Superior prognostic value of ABP over OBP.
As mentioned above, a major advantage of ABPM over OBP is its ability to provide more accurate measurements of BP and to better track BP changes induced by antihypertensive treatment as well as the actual coverage of a given treatment over the 24-h period. It is thus not surprising that both cross-sectional and longitudinal studies have shown a superior prognostic value for 24-h, daytime, and nighttime average ABP values compared with OBP. In these studies, compared with OBP measures ABP values were more closely associated with hypertensive subclinical organ damage and with its changes after antihypertensive treatment (35–39), and more importantly, it was also more effective in predicting the development of CV events (24,25,40–42) as well as CV and non-CV mortality (24–26,41–45) both in a general population (25–28,43,45) and in hypertensive patients alone (24,28,40,42,44). Of relevance, compared with awake or 24-h BP means, nocturnal BP levels have been shown to be superior in predicting CV morbidity and mortality (24–26,28,46,47), the development of CV events (24,25,40–42), and overall mortality (24–26,41,44,45). This is not surprising given that a patient’s nocturnal BP level, without the pressor effects of physical activity, emotional stress, and other environmental factors that usually occur during the day, may be more reproducible and representative of a patient’s actual BP levels and organ damage status. Also, in the case of diabetic subjects out-of-office BP values obtained through ABPM may be a powerful tool for a better stratification of CV risk related to elevated BP. In support of this, several studies have shown ABP values to be better correlated with target-organ damage (48,49) and to be better predictors of CV events (50) than OBP in diabetic patients.
While all the above evidence highlights the superiority of ABPM in stratifying BP-related risk of hypertensive patients, in particular of those with diabetes, the evidence on the prognostic relevance of treatment-induced reductions in ABP compared with that of OBP reductions is limited. Nevertheless, it appears that compared with OBP, ABP reductions are more closely related to regression of subclinical cardiac organ damage (36) and to reductions in the risk of fatal and nonfatal CV events and in general mortality (51) in particular when nighttime BP levels are considered (52).
Superior prognostic value of home BP over OBP.
In general, the prognostic value of HBPM has also been found to be superior to that of OBP measurements in hypertensive patients with or without diabetes (25,27,36,41,53–59). When averaged over a few days, home BP measurements have been shown to significantly predict the development of major CV events (myocardial infarction, stroke, and CV death), all-cause mortality, progression of chronic kidney disease (60), and functional decline in the elderly, with HBPM being a better predictor of CV mortality (fatal CV events) than OBP in most (25,53–55,59–62) although not in all (63) studies. As a general remark, however, there is less evidence supporting the prognostic value of HBPM than for ABPM, notably due to the smaller number of HBPM-related outcome studies available so far (29).
BPV: a complex phenomenon
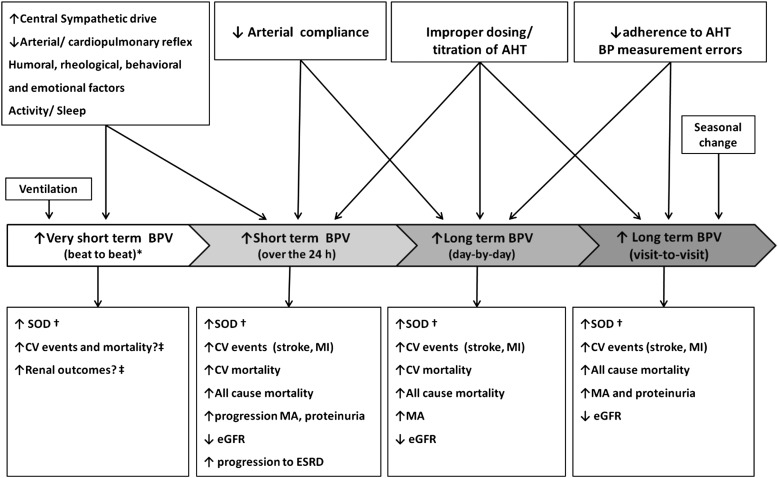
Although the adverse CV consequences of hypertension are largely considered dependent on average BP values, evidence provided by observational studies and clinical trials has also indicated a possible role of increased BPV in this regard. BP is characterized by marked short-term fluctuations within the 24 h (including those occurring in an apparently random fashion over seconds or minutes and those following the circadian rhythm of activity). Significant BP variations have also been shown to occur over more prolonged periods of time (i.e., between days, weeks, months, and seasons and even years). BPV is the result of complex interactions between extrinsic environmental and behavioral factors with intrinsic CV regulatory mechanisms (humoral and neural central or reflex influences) not yet completely understood. Measures of BPV can be obtained through different methods, i.e., continuous beat-to-beat BP recordings, repeated conventional OBP measures, 24-h ABPM, or HBPM over longer time windows. Depending on the method and time interval considered for its assessment, the clinical significance and prognostic implications of a given measure of BPV may indeed substantially differ (Fig. 1).
Figure 1.
Different types of BPV, their determinants, and prognostic relevance for CV and renal outcomes. AHT, antihypertensive treatment; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; MA, microalbuminuria; MI, myocardial infarction; SOD, subclinical organ damage. *Assessed in laboratory conditions. †Cardiac, vascular, and renal SOD. ‡BPV on a beat-by-beat basis has not been routinely measured in population studies. Reprinted with permission from Parati, Ochoa, and Bilo (64).
Short-term BPV: measurement, mechanisms, and prognostic relevance
Assessment of short-term BPV.
The dynamic behavior of BP values over the 24-h period was first shown through use of intra-arterial BP monitoring in ambulant subjects (65–67). Although an accurate assessment of short-term BPV within the 24 h requires continuous BP monitoring, its evaluation is also possible (although less precisely) through use of intermittent, noninvasive 24-h ABPM. From these recordings, it is possible to calculate the SD of average systolic (SBP), diastolic (DBP), and mean BP values over the 24 h, with the daytime and the nighttime periods separately considered (68). Given the opposite clinical significance of short-term BPV within daytime or nighttime on one side, and of slower changes between day and night on the other side, several new methods for the separate assessment of slower circadian BP fluctuations and of the residual faster BP changes occurring during daytime or nighttime have been proposed. These methods, which allow exclusion of the contribution of circadian BP changes from the estimate of overall 24-h BPV, include assessment of 1) “weighted” SD of the 24-h mean value (i.e., the average of daytime and nighttime BP SD, each weighted for the duration of the day and night periods, respectively) (69), 2) so-called “average real variability” (average of absolute differences between consecutive readings) (70), or 3) residual spectral components of BPV (obtained by removing main slower cyclic components through Fourier analysis) (71). These parameters, which focus on short-term BP changes and are not affected by the dipping phenomenon, have been shown to be better predictors of organ damage and CV risk than the conventional 24-h SD (69,70,72).
Mechanisms of short-term BPV.
The results of several studies trying to disentangle the precise contribution of humoral, neural, and environmental factors to BPV have indicated that these factors are often inextricably intertwined. BP variations in the very short term (i.e., beat to beat) and in the short term (i.e., within 24 h) mainly reflect the influences of central neural factors either in response to behavioral challenges or as a result of rhythmic influences originating in the central nervous system, as well as the influences of reflex autonomic modulation (i.e., an increased central sympathetic drive and reduced sensitivity of arterial and cardiopulmonary reflexes may both lead to an increased BPV) (73–76). In this context, also changes in elastic properties of large arteries (i.e., an increased arterial stiffness) (77) and the effects of humoral (insulin, angiotensin II, bradykinin, endothelin-1, and nitric oxide) and rheological (i.e., blood viscosity) factors (78) may play a role. In addition, BP fluctuations also occur in response to the mechanical forces generated by ventilation.
In diabetic patients with hypertension, overall BPV (assessed through SD of average 24-h, daytime, or nighttime BP values) has been found to be frequently increased compared with that in hypertensive subjects without diabetes (79). Although fasting blood glucose levels have been found to be a major determinant for this increase in BPV, other factors such as diabetic autonomic dysfunction (which may induce significant impairment in baroreflex sensitivity) and increased arterial stiffness might also play an important role (79–81). Interestingly, some studies in the diabetic population have shown plasma norepinephrine levels to be directly correlated to nighttime systolic BPV, suggesting a potential role of sympathetic activation for the increase in nighttime BPV observed in the diabetic population (82). Several studies using 24-h ABPM have also indicated an increased prevalence of alterations in day-to-night BP changes in diabetic patients. In particular, an impaired nocturnal BP fall (i.e., nondipping) or even increase in nighttime BP (i.e., rising pattern) has been shown to be common in this population, with a prevalence that may be as high as 30 and 31%, respectively (83). Proposed mechanisms for these abnormalities in circadian BP rhythms include alterations in autonomic CV modulation (which are frequently associated with diabetes) (84,85) but also other conditions such as obstructive sleep apnea, commonly observed in obese diabetic subjects (86).
Prognostic significance of short-term BPV.
The degree of BPV is in general roughly proportional to mean BP values, and this dependence of BPV on average BP has led to methods for assessing BPV in normalized units aimed at accounting for average BP level. This can be done by computing BP coefficient of variation, while in the research setting this issue is overcome by adjusting in multivariate models the impact of BPV on clinical outcome for the concomitant influence of mean BP levels. Early studies using 24-h intra-arterial ABPM for the first time showed that BPV (quantified as the SD of the 24-h, day, and night mean values) increases from normotensive to hypertensive subjects, the increase in BP SD being proportional to the increase in mean BP, with no change in the coefficient of variation (65). Interestingly, an increase in BPV was also shown within individuals, as mean BP levels of different subperiods within the 24 h increased (65).
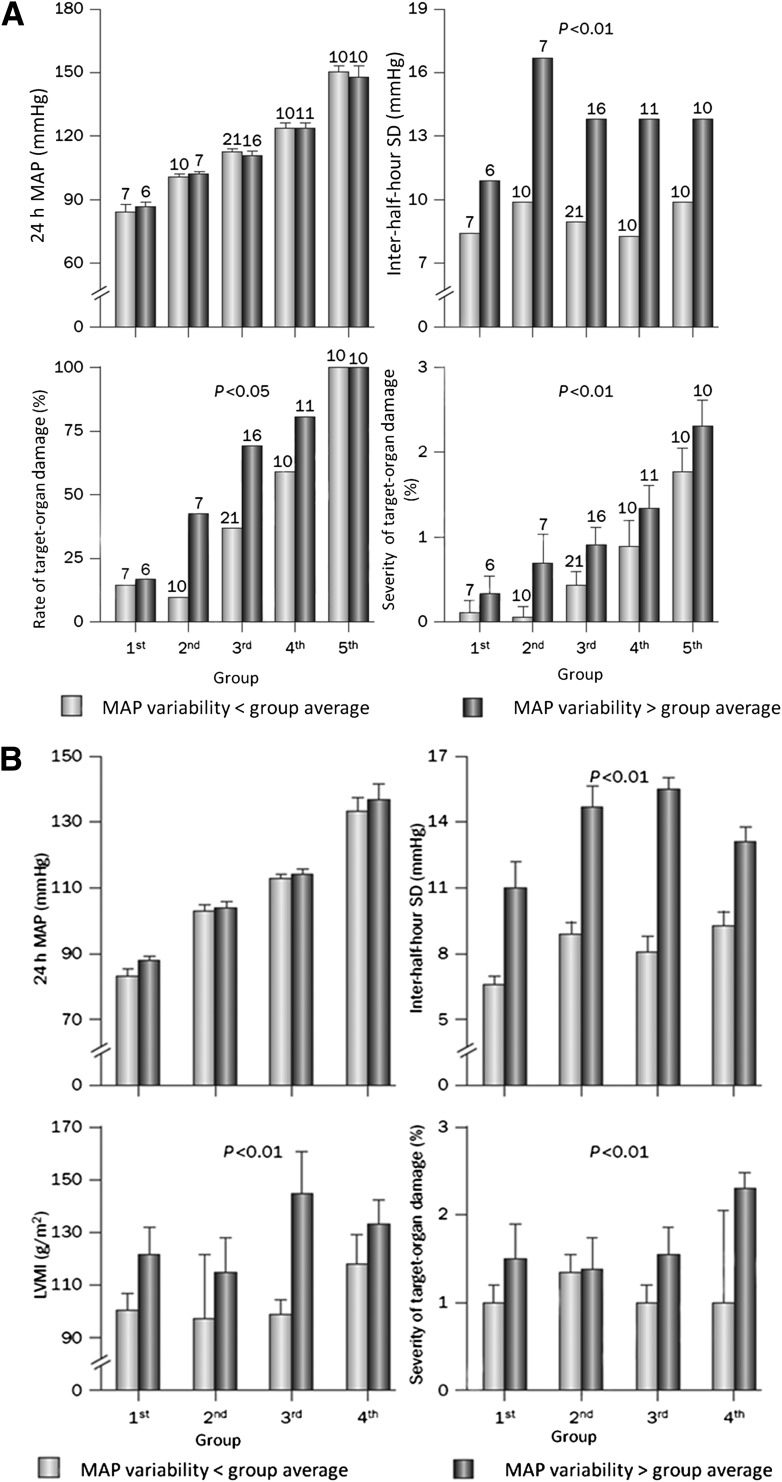
Evidence from longitudinal and observational studies has indicated that short-term BPV within the 24 h may have a nonmarginal contribution to CV risk. By using either intra-arterial or noninvasive BP monitoring, several studies have indeed shown that cardiac, vascular, and renal organ damage for a given 24-h BP mean value is more prevalent and severe as 24-h BPV increases (66,87–92) (Fig. 2).
Figure 2.
A: Short-term BPV and subclinical organ damage (cross-sectional study). Rate and severity of target-organ damage in patients with essential hypertension divided into quintiles of increasing 24-h mean intra-arterial BP (MAP). Patients in each group were further classified into two categories according to whether the between-half-hour SD of mean intra-arterial BP was below or above the average variability of the group. Within each group, the two classes had similar 24-h mean intra-arterial BP values. For each class, the severity of target-organ damage was expressed as the average score accounting for both the presence and extent of target-organ damage. The score ranged in each patient from 0 (either no clinical events or electrocardiogram, chest radiograph, fundus, or renal function alterations) to 3 (major alterations on the electrocardiogram, chest radiograph, or fundus plus a clinical event, renal abnormality, or both). For any level of 24-h mean intra-arterial BP, patients in whom 24-h BPV was low had a lower prevalence and severity of target-organ damage than those in whom 24-h BPV was high. B: Rate and severity of target-organ damage and left ventricular mass index (LVMI) in the same patients as in A after 7.4 years of follow-up. Patients were divided into quartiles of increasing 24-h mean intra-arterial BP. Patients in each group were further classified into two categories according to whether the between-half-hour SD of mean intra-arterial BP was below or above the average variability of the group. Within each group, the two classes had similar 24-h mean intra-arterial BP values. For each class, the severity of target-organ damage was expressed as the average score, as assessed in A with the addition of echocardiographic data. BPV (between-half-hour SD of 24-h mean intra-arterial BP) at the initial evaluation was a significant predictor of target-organ damage at follow-up, indicating that the cardiovascular complications of hypertension might depend on the degree of 24-h BPV. Modified with permission from Parati et al. (66) (A) and Frattola et al. (92) (B).
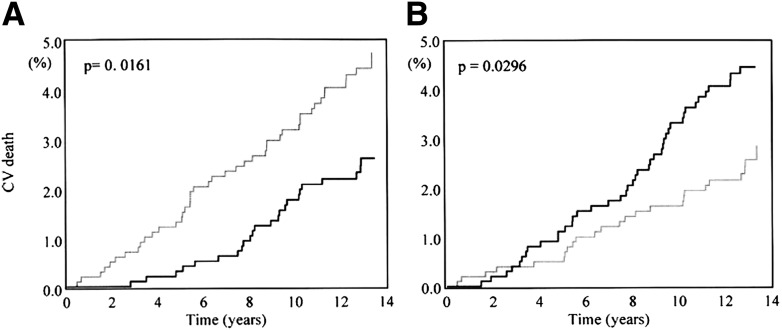
Most importantly, prospective studies have provided evidence that an initial increase in BPV within the 24 h is an independent predictor of progression of subclinical organ damage (i.e., increased left ventricular mass index or carotid intima-media thickness) (92,93), CV events (71,72,94–97), and CV mortality (71,72,93–99). In particular, data from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study found the risk of CV death not only to be inversely related to day-night DBP fall (Fig. 3) but also to bear a significant positive relationship with residual short-term diastolic BPV (71).
Figure 3.
Kaplan-Meier curves for CV mortality in subjects with day-night DBP difference (A) and residual variability (B) above (black lines) and below (gray lines) the median value of the population. Modified with permission from Mancia et al. (71).
Overall, this evidence supports the concept that the adverse CV consequences of high BP depend not only on mean BP values but also on BPV, which may independently add to CV risk over and above the contribution of elevated mean BP levels. Recent reports of the International Database on Ambulatory BP in relation to Cardiovascular Outcome have confirmed that indices of BPV such as the weighted 24-h BP SD and the average real variability (70) may predict outcome, but they improve prediction of the composite CV events provided by mean BP levels by only a small percentage (100). Such analysis, however, is limited by the lack of standardization of BP recordings performed in different countries.
In diabetic patients, the incidence of coronary artery disease has been shown to be significantly greater in patients with increased 24-h systolic BPV (67 vs. 11%; P < 0.0005). Interestingly, nighttime systolic BPV was an independent risk factor for coronary artery disease (odds ratio 3.13 [82]; P < 0.05) (82).
Day-to-night BP profiles: assessment and prognostic relevance
Assessment of day-to-night BP profiles with ABPM.
In addition to providing information on mean BP levels and on relatively fast BP changes during selected subperiods over the 24 h, ABPM also makes possible the identification of slower BP fluctuations occurring between day and night, which are significantly influenced both by the subject’s level of activity during daytime and by the sleep/wakefulness cycle. By considering the degree of BP change from awake to sleep, different patterns of circadian BP variation may be identified in relation to nocturnal BP fall and morning BP surge. In the general population, BP falls on average by 10–20% of daytime values during sleep, a phenomenon referred to as dipping. Dippers exhibiting nighttime BP fall >20% are known as extreme dippers. In some individuals, nocturnal decrease in BP is blunted (“non-dippers,” with a fall in nighttime SBP and DBP <10% of daytime BP but still presenting some reduction compared with awake BP) or even increases (so-called “risers” or “inverted dippers”) compared with daytime values (101).
Prognostic relevance of alterations in day-to-night BP changes.
The relevance of alterations in circadian BP profiles for CV prognosis has been explored in several studies. Subjects in whom nocturnal BP reduction is blunted have been reported to have a greater prevalence of subclinical organ damage (93,102) and increased risk of CV events (103) and mortality (28,104). The risk of CV events is even higher for patients in whom BP increases rather than decreases at night (104,105) while the predictive relevance of extreme dipping remains controversial (104). Remarkably, nondipping profile of BP is frequently accompanied by increased nocturnal mean BP levels (i.e., nighttime BP >125/75 mmHg) (101). However, whether it is the reduction in nocturnal BP dipping or the increase in absolute level of average BP at night that really matters is an issue still under investigation (28,47). In addition, evidence is also available that an increased morning BP surge is associated with a higher incidence of CV events and mortality (94,95,103,106). However, the actual prognostic value of morning BP surge is still a matter of debate due, on one hand, to the difficulties in identifying the most reliable method for defining and assessing this parameter and on the other hand, to the significant correlation between two features of the 24-h BP profile with contradictory prognostic significance: the degree of morning BP surge (a potentially risky phenomenon) and the degree of BP fall at night (a potentially protective phenomenon)—a correlation that makes the clinical interpretation of the degree of morning BP surge more difficult. Indeed, the adverse prognostic impact of a blunted or inverted nighttime BP fall (which is associated with a blunted morning BP surge as well) seems difficult to reconcile with the hypothesis that an excessive morning BP surge is also predictive of a worse outcome. A recent large cohort study in hypertensive patients showed that a blunted day-night BP dip but not an increased morning BP surge was the key predictor of CV outcomes, probably because of the superior reproducibility of nocturnal BP compared with early-morning BP surge (107). When focusing on a diabetic population, several studies have indicated that a nondipping profile and in particular a rising night BP pattern may be of relevance for CV prediction. In either type 1 (102,108) or type 2 (109–112) diabetic subjects, several studies have indicated that elevated SBP during nighttime sleep may precede development of renal damage (assessed trough microalbuminuria) and contribute to progression of renal damage when nephropathy is already present. Evidence has also been provided that a nondipping pattern of BP is associated with an increased mortality rate, regardless of diabetes type (113). Of note, the combination of nondipping and subsequent renal impairment was shown to be associated with the highest mortality rate (113). However, other studies in type 2 diabetic subjects have found mean nighttime SBP to be a stronger predictor of organ damage and fatal and nonfatal vascular events than the nondipping pattern in nocturnal SBP (114).
Long-term BPV
BP has been shown to exhibit important variations not only in the short term but also over more prolonged periods of time (i.e., day-by-day, visit-to-visit, or seasonal BP variations). Although these long-term BP variations have been shown to be a reproducible and not a random phenomenon (115), evidence is limited about the factors responsible for BP changes between visits spaced by weeks, months, or years in observational studies and clinical trials (116–118). Long-term BPV might not entirely consist of spontaneous BP variations or reflect the same physiological CV control mechanisms of short-term BP fluctuations, but it may also be the result of imperfect stability of BP control in treated subjects (in particular, visit-to-visit BP variations during follow-up), or additionally, it might reflect the inconstant accuracy of OBP readings (Fig. 1) (119). Thus, factors influencing the degree of BP control (i.e., patient’s adherence to treatment and proper dosing/titration of antihypertensive treatment) or errors in BP measurement may in due course influence day-by-day but especially visit-to-visit BPV. In particular, patients’ poor compliance with antihypertensive treatment may influence long-term BPV, as dose omission or delay in drug intake during the follow-up period may also contribute to an increased day-by-day and visit-to-visit BPV.
Assessment of day-by-day BPV.
In the time line of BPV, day-by-day BPV finds its position somewhere between short-term BPV (i.e., within the 24 h) and visit-to-visit BPV (i.e., within weeks, months, or years). Measures of this particular type of BPV can be obtained by means of ABPM performed over consecutive days (i.e., over 48 h) or, more easily, by means of home BP measurements performed by patients over subsequent days in fairly standardized conditions. Although HBPM may not provide the same extensive information on 24-h BP patterns as ABPM does, it may provide information on BP changes over a longer but still relatively short time (several days), when both subjects’ physiological characteristics and treatment regimen remain stable. Thus, HBPM appears more appropriate for the long-term assessment of BPV and BP control than repeated office or ABP measurements.
Prognostic significance of day-by-day BPV.
Recent studies exploring the prognostic role of BPV identified by HBPM have indicated that an increased day-by-day variability in home BP values is associated with a higher prevalence and severity of cardiac, vascular, and renal organ damage (120) and with an increased risk of fatal and nonfatal CV events (121,122). A recent cross-sectional analysis in a population of never-treated hypertensive patients showed an increased day-by-day home systolic BPV to be associated with the severity of cardiac (i.e., left ventricular mass index), macrovascular (i.e., increased carotid intima-media thickness), and microvascular (i.e., urinary albumin-to-creatinine ratio) organ damage regardless of the mean home BP level, suggesting that home systolic BPV may add to prediction of hypertensive subclinical organ damage over and above mean home SBP (120). When it comes to prediction of major CV events, the Ohasama study provided the first evidence that an increased day-by-day systolic home BPV is associated with an increased risk of a composite of cardiac and stroke mortality, but only with a significant risk of stroke mortality, when the components of the main end point were independently considered (121). More recently, evidence on the prognostic value of day-by-day home BPV was provided in a cohort of adults from the general population in the frame of the Finn-Home Study (122) showing increasing levels of day-by-day home BPV to be associated with a higher risk of CV events even after adjustments for age and mean home BP level. The prognostic role of day-by-day BPV is also supported by the demonstration by Matsui et al. (120) that the maximum value of HBP over a given monitoring time is a significant predictor of events in a population of elderly hypertensive patients.
The prognostic role of day-by-day home-measured BPV in predicting development/progression of nephropathy in type 2 diabetic patients has also been explored in several studies (123,124). In a large cohort of Japanese subjects with type 2 diabetes, increasing values of day-by-day variability in home systolic BP (assessed through the coefficient of variation of BP measures performed over 14 consecutive days) were positively correlated with urinary albumin excretion (UAE) and associated with an increased risk of macroalbuminuria (UAE ≥300 mg ⋅ g−1 creatinine). Remarkably, after adjustment for common confounders and mean BP levels, CVs of morning SBP, morning DBP, and evening SBP remained independent predictors for UAE and were associated with a significant risk for macroalbuminuria of 1.35 (P < 0.05), 1.29 (P < 0.05), and 1.44 (P < 0.05), respectively.
Assessment of visit-to-visit BPV.
In clinical practice, obtaining BP measurements over a consistent number of visits to achieve a meaningful estimate of visit-to-visit BPV is usually difficult. Moreover, OBP readings obtained in the clinic may not provide information on BP during subjects’ usual activities and over a long period of time, being thus unable to offer a representative evaluation of patients’ actual BP burden. OBP is thus an imperfect indicator of BP control and is far from being an ideal means to assess visit-to-visit BPV. On the other hand, it has been shown that in treated hypertensive patients the reproducibility of 24-h ABP is clearly higher than that of isolated OBP readings (125). This is in part due to the fact that OBP can be altered by several environmental stimuli and by the alert reaction generated by the doctor’s or nurse’s visit, which have no effect on 24-h mean BP values. However, despite the relevant and extensive information on BP levels within the 24 h provided by ABPM, it can neither be repeated frequently nor routinely applied to assess visit-to-visit BPV. Although measures performed by patients at home using HBPM may not provide information on 24-h BP profiles, they seem to be an appropriate alternative approach for the assessment of long-term BPV (126).
Prognostic relevance of visit-to-visit BPV.
Recent studies have shown increasing values of visit-to-visit BPV to be associated with a higher prevalence and incidence of cardiac (i.e., diastolic dysfunction) (127), macrovascular (i.e., increased intima-media thickness and stiffness) (128), microvascular (i.e., development of micro- and macroalbuminuria and renal vascular atherosclerosis) (129,130), and cerebral (i.e., white matter hyperintensity volume and presence of brain infarctions) (131) organ damage as well as with endothelial dysfunction (132).
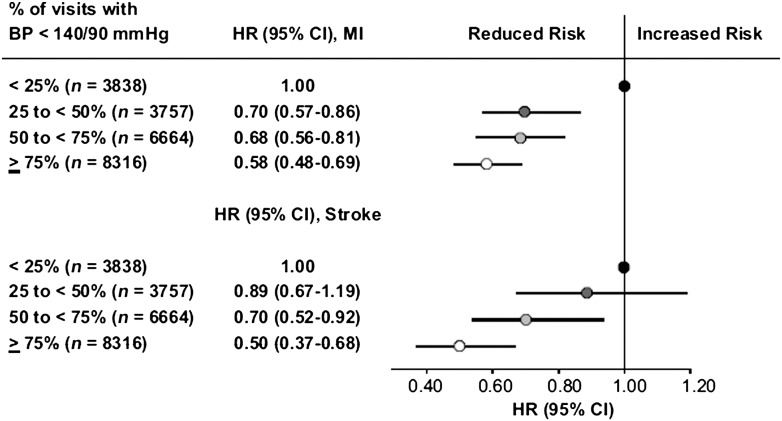
Longitudinal studies and post hoc analyses of clinical trials in hypertension have found increasing values of intraindividual visit-to-visit variability in conventional office or ABP (as assessed by the SD or the coefficient of variation of the average in-treatment BP) to be predictive of cerebrovascular (116,133–135) and coronary fatal and nonfatal events (134–137) and of all-cause mortality (117) independently of mean office or ABP values. In some analyses, the predictive value of intraindividual visit-to-visit BPV has been shown to be even higher than that of average BP during treatment, suggesting that the protective effect of antihypertensive treatment depends not only on the magnitude of the achieved mean BP reduction but also on the consistency of BP reduction (i.e., the stability of the on-treatment BP control over the long term). This has been supported by results of the International Verapamil-Trandolapril (INVEST) Study conducted in a hypertensive population at high CV risk (i.e., all of whom had a history of coronary disease). In this study, the incidence of fatal and nonfatal CV events, but in particular of stroke, showed a steep reduction as the percentage of on-treatment visits with BP controlled (i.e., BP <140/90 mmHg) increased throughout the treatment period independently of the achieved control of mean clinic BP (118) (Fig. 4).
Figure 4.
Hazard ratio (HR) of myocardial infarction (MI) or stroke according to the percentage of visits with BP <140/90 mmHg. The group in which this occurred in <25% of the visits was taken as reference. Data were adjusted for differences in baseline demographic data, BP, and CV risk factors as well as for in-treatment average BP. Reprinted with permission from Mancia et al. (118).
The prognostic relevance of visit-to-visit BPV shown in these studies supports the recommendation of avoiding inconsistent BP control and large BP differences from one visit to another, not only by a proper dosing/titration of antihypertensive treatment, but also by improving patients’ adherence to treatment. However, while in treated hypertensive patients at high CV risk an increased visit-to-visit BPV may be prognostically relevant (as shown in the INVEST study), in mildly to moderately treated hypertensive patients, BPV between visits has been shown to make little or no contribution to CV risk prediction over mean BP levels (125).
A post hoc analysis of the Diabetes Control and Complications Trial (DCCT) investigated whether mean BP values and annual visit-to-visit BPV (as assessed through SD of SBP and DBP) might influence the development of microvascular complications in initially normotensive type 1 diabetic patients. Overall, mean SBP and SD of SBP were related to an increased risk of development/progression of nephropathy with an odds ratio for albuminuria of 1.005 (95% CI 1.002–1.008, P < 0.001) and 1.093 (1.069–1.117, P < 0.001), respectively, for each 1 mmHg change. Visit-to-visit variability in SBP remained a significant predictor of albuminuria even after adjustment for mean BP levels, independently adding to mean BP in predicting the risk of nephropathy (129).
Evidence has also been provided in type 2 diabetic patients that an increase in BPV may add to mean BP values in predicting outcome as shown in a large cohort of type 2 diabetic patients in whom BP levels were measured at every visit over a year. Increasing values of visit-to-visit systolic BPV (as assessed through coefficient of variation) were directly correlated with UAE and with arterial stiffness (assessed through pulse wave velocity) and inversely correlated with ankle-brachial index (an indirect marker of atherosclerosis) (138).
Potential implications of short-term BPV for hypertension management
Although it has been suggested that in order to optimize CV protection in hypertensive patients, antihypertensive treatment should be targeted at stabilizing BPV in addition to reducing absolute BP values, most controlled trials using different antihypertensive drug classes as an active regimen have strongly supported the preponderant role of mean BP reduction in achieving CV protection (7). At present, the only evidence that modulation of 24-h BPV with antihypertensive treatment may be beneficial in terms of CV protection comes from studies making use of the smoothness index (i.e., the ratio between the average of the 24-h BP changes induced by a given medication and its SD) in assessing the distribution of BP reduction by treatment (67,139). This index has been shown to be related to drug-induced regression of target-organ damage at cardiac level (i.e., left ventricular mass index) (67) as well as to drug-induced reduction in the progression of changes in carotid artery wall thickness, partially independent of basal mean BP values (139).
Evidence is available that some classes of oral antidiabetes drugs (i.e., thiazolidinediones), may not only have a beneficial effect on 24-h BP levels but also improve day-night BP profile in diabetic subjects (140,141). Although several studies in diabetic patients have shown the efficacy of different antihypertensive drug classes in controlling BP levels over the 24 h (142,143), no significant impact on day-night BP profiles has been evident in these studies. However, some studies in type 2 diabetic subjects have shown that treatment with long-acting calcium antagonist (i.e., lacidipine) is effective in reducing not only mean ABP values during the 24 h, daytime, and nighttime but also the respective BPVs expressed as SD and coefficient of variation. Interestingly, reductions in BPV in these studies were accompanied by an improvement of baroreflex sensitivity, suggesting a plausible mechanism for the effects of antihypertensive treatment in stabilizing BP variation (144).
More recently, a study in type 2 diabetic patients explored whether treatment with an angiotensin II type 1 receptor blocker could improve ambulatory short-term BPV in hypertensive patients with diabetic nephropathy. After 12 weeks of treatment, 24-h, daytime, and nighttime short-term BPV (as assessed through calculation of the coefficient of variation) was significantly decreased by treatment. Interestingly, these reductions in BPV were accompanied by significant reductions in urinary protein excretion, brachial-ankle pulse wave velocity, and indices of autonomic CV modulation (i.e., low frequency–to–high frequency ratio, an index of sympathovagal balance) suggesting potential mechanisms for the beneficial effects of angiotensin receptor blockade on diabetic nephropathy (145).
Potential implications of long-term BPV for hypertension management
When it comes to reducing BPV in the long term, recent post hoc analyses performed on the basis of the data of the Anglo-Scandinavian Cardiac Outcome Trial (ASCOT) and the Medical Research Council Trial of Treatment of Hypertension in Older Adults (MRC-elderly) have reported that intraindividual visit-to-visit BPV (i.e., variability of an individual’s BP from visit to visit) may be differentially affected by antihypertensive drug classes and that these differences might explain the variable effects of BP-lowering drugs in preventing outcome, with calcium channel blocker scoring best (134). However, contrasting results were observed in a post hoc analysis of the European Lacidipine Study on Atherosclerosis (ELSA), in mild-to-moderate hypertensive patients, where no substantial differences between a β-blocker and a calcium antagonist in relation to intraindividual visit-to-visit BPV were observed (125). Indeed, when the pooled data of this study were analyzed, carotid intima-media thickness and CV outcomes were related to the mean clinic or ambulatory SBP achieved by treatment but not to on-treatment visit-to-visit clinic or 24-h BPV (146). Meta-analyses extended to a larger number of trials using the post hoc approach showed that differences between classes of antihypertensive drugs in their effectiveness in preventing stroke despite no or little difference in mean BP might be due to differential class effects on interindividual BPV (i.e., between-patient dispersion of mean BP values during treatment) (147). However, a major limitation of interindividual BPV (or group BPV) is that it represents the effect of BP treatment in a group of patients and cannot accurately reflect variations in BP values from visit to visit in individual subjects. Based on the prognostic relevance of visit-to-visit BPV, one could infer that consistency of BP control represents an additional important goal of antihypertensive treatment (118). However, it should be considered that most evidence on visit-to-visit BPV in relation to progression of organ damage or incidence of CV events has been obtained from post hoc analyses of trial data and based on comparisons between nonrandomized groups, which may have introduced a large number of potential confounders, thus undermining the study conclusions.
Conclusions
Evidence from observational and interventional studies has indicated that the risk of CV morbidity and mortality has a strong and continuous relationship with mean BP levels (3,4) and that lowering BP levels confers significant CV protection regardless of the drug class used (7,148). Out-of-office BP levels measured either with ABPM or with HBPM have been shown to be even better predictors of outcomes compared with OBP and may also allow identification of masked hypertension, a condition that needs to be properly treated, as it bears an adverse CV prognosis similar to that of sustained hypertension. ABPM in diabetic subjects may be a powerful tool for a better stratification of the CV risk related to elevated BP, which in turn is a substantial contributor to CV morbidity and mortality in diabetes. Moreover, ABPM may also be helpful in detecting alterations in autonomic control of the CV system, reflected by the absence of nocturnal BP fall or by a reduced 24-h heart rate variability and an increased 24-h BPV. Despite the large body of evidence supporting the preponderant role of average BP values in determining the CV risk associated with high BP levels, the extent of fluctuations of BP over time may provide additional, independent prognostic information. Evidence supporting this concept has been provided by several studies showing that not only elevation in average BP levels but also an increased BPV (either in the short term or in the long term) may independently add to CV risk prediction over and above the contribution of elevated mean BP levels. Although it has been suggested that in order to achieve the highest CV protection in hypertensive patients antihypertensive treatment should be targeted at normalizing 24-h BPV in addition to reducing absolute 24-h BP levels, evidence is still limited regarding the targets of BPV to achieve with antihypertensive treatment. Because 24-h ABPM has not been routinely used in large-scale trials on antihypertensive treatment, the protective effect of treatment-induced changes in 24-h BPV with respect to the concomitant changes in mean BP levels still needs to be properly documented. When it comes to long-term BPV, recent meta-analyses of clinical trials on hypertension have shown an increased visit-to-visit BPV or lack of BP control at any given visit to be associated with an adverse CV prognosis. These data suggest that smoothing control of BP levels not only throughout the 24 h but also in the long-term follow-up of hypertensive patients may be important for optimizing CV protection. However, before being recommended as a target for antihypertensive treatment in daily clinical practice, further prospective outcome studies should be conducted to support that a treatment-induced reduction in BPV is accompanied by a corresponding reduction in CV risk over and above that already achieved by reducing mean BP levels.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
G.P., J.E.O., P.S., C.L., and G.B. performed research and wrote, reviewed, and edited the manuscript. G.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This publication is based on the presentations from the 4th World Congress on Controversies to Consensus in Diabetes, Obesity and Hypertension (CODHy). The Congress and the publication of this supplement were made possible in part by unrestricted educational grants from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Ethicon Endo-Surgery, Janssen, Medtronic, Novo Nordisk, Sanofi, and Takeda.
References
- 1.Mancia G, De Backer G, Dominiczak A, et al. Management of Arterial Hypertension of the European Society of Hypertension. European Society of Cardiology 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2007;25:1105–1187 [DOI] [PubMed] [Google Scholar]
- 2.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ, Comparative Risk Assessment Collaborating Group Selected major risk factors and global and regional burden of disease. Lancet 2002;360:1347–1360 [DOI] [PubMed] [Google Scholar]
- 3.MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 1990;335:765–774 [DOI] [PubMed] [Google Scholar]
- 4.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903–1913 [DOI] [PubMed] [Google Scholar]
- 5.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–234 [DOI] [PubMed] [Google Scholar]
- 6.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979;241:2035–2038 [DOI] [PubMed] [Google Scholar]
- 7.Turnbull F, Blood Pressure Lowering Treatment Trialists’ Collaboration Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003;362:1527–1535 [DOI] [PubMed] [Google Scholar]
- 8.Hypertension in Diabetes Study (HDS) Hypertension in Diabetes Study (HDS): II. Increased risk of cardiovascular complications in hypertensive type 2 diabetic patients. J Hypertens 1993;11:319–325 [DOI] [PubMed] [Google Scholar]
- 9.Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 1998;316:823–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hypertension in Diabetes Study (HDS) Hypertension in Diabetes Study (HDS): I. Prevalence of hypertension in newly presenting type 2 diabetic patients and the association with risk factors for cardiovascular and diabetic complications. J Hypertens 1993;11:309–317 [DOI] [PubMed] [Google Scholar]
- 11.Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ 2000;321:412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins R, MacMahon S. Blood pressure, antihypertensive drug treatment and the risks of stroke and of coronary heart disease. Br Med Bull 1994;50:272–298 [DOI] [PubMed] [Google Scholar]
- 13.Holman RR, Paul SK, Bethel MA, Neil HA, Matthews DR. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med 2008;359:1565–1576 [DOI] [PubMed] [Google Scholar]
- 14.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 15.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580–591 [DOI] [PubMed] [Google Scholar]
- 16.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 17.Hansson L, Zanchetti A, Carruthers SG, et al. HOT Study Group Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet 1998;351:1755–1762 [DOI] [PubMed] [Google Scholar]
- 18.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317:703–713 [PMC free article] [PubMed] [Google Scholar]
- 19.Estacio RO, Jeffers BW, Hiatt WR, Biggerstaff SL, Gifford N, Schrier RW. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non-insulin-dependent diabetes and hypertension. N Engl J Med 1998;338:645–652 [DOI] [PubMed] [Google Scholar]
- 20.Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007;370:829–840 [DOI] [PubMed] [Google Scholar]
- 21.Heart Outcomes Prevention Evaluation Study Investigators Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 2000;355:253–259 [PubMed] [Google Scholar]
- 22.Tuomilehto J, Rastenyte D, Birkenhäger WH, et al. Systolic Hypertension in Europe Trial Investigators Effects of calcium-channel blockade in older patients with diabetes and systolic hypertension. N Engl J Med 1999;340:677–684 [DOI] [PubMed] [Google Scholar]
- 23.Bakris GL, Gaxiola E, Messerli FH, et al. INVEST Investigators Clinical outcomes in the diabetes cohort of the INternational VErapamil SR-Trandolapril study. Hypertension 2004;44:637–642 [DOI] [PubMed] [Google Scholar]
- 24.Staessen JA, Thijs L, Fagard R, et al. Systolic Hypertension in Europe Trial Investigators Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. JAMA 1999;282:539–546 [DOI] [PubMed] [Google Scholar]
- 25.Sega R, Facchetti R, Bombelli M, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation 2005;111:1777–1783 [DOI] [PubMed] [Google Scholar]
- 26.Kikuya M, Ohkubo T, Asayama K, et al. Ambulatory blood pressure and 10-year risk of cardiovascular and noncardiovascular mortality: the Ohasama study. Hypertension 2005;45:240–245 [DOI] [PubMed] [Google Scholar]
- 27.Fagard RH, Celis H. Prognostic significance of various characteristics of out-of-the-office blood pressure. J Hypertens 2004;22:1663–1666 [DOI] [PubMed] [Google Scholar]
- 28.Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension 2011;57:3–10 [DOI] [PubMed] [Google Scholar]
- 29.Parati G, Stergiou GS, Asmar R, et al. ESH Working Group on Blood Pressure Monitoring European Society of Hypertension guidelines for blood pressure monitoring at home: a summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens 2008;26:1505–1526 [DOI] [PubMed] [Google Scholar]
- 30.Parati G, Mancia G. White coat effect: semantics, assessment and pathophysiological implications. J Hypertens 2003;21:481–486 [DOI] [PubMed] [Google Scholar]
- 31.Pickering TG, Davidson K, Gerin W, Schwartz JE. Masked hypertension. Hypertension 2002;40:795–796 [DOI] [PubMed] [Google Scholar]
- 32.Parati G, Ulian L, Santucciu C, Omboni S, Mancia G. Difference between clinic and daytime blood pressure is not a measure of the white coat effect. Hypertension 1998;31:1185–1189 [DOI] [PubMed] [Google Scholar]
- 33.Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute. National High Blood Pressure Education Program Coordinating Committee Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206–1252 [DOI] [PubMed] [Google Scholar]
- 34.Parati G, Pickering TG. Home blood-pressure monitoring: US and European consensus. Lancet 2009;373:876–878 [DOI] [PubMed] [Google Scholar]
- 35.Fagard RH, Staessen JA, Thijs L. Relationships between changes in left ventricular mass and in clinic and ambulatory blood pressure in response to antihypertensive therapy. J Hypertens 1997;15:1493–1502 [DOI] [PubMed] [Google Scholar]
- 36.Mancia G, Zanchetti A, Agabiti-Rosei E, et al. SAMPLE Study Group. Study on Ambulatory Monitoring of Blood Pressure and Lisinopril Evaluation Ambulatory blood pressure is superior to clinic blood pressure in predicting treatment-induced regression of left ventricular hypertrophy. Circulation 1997;95:1464–1470 [DOI] [PubMed] [Google Scholar]
- 37.Fagard RH, Staessen JA, Thijs L. Prediction of cardiac structure and function by repeated clinic and ambulatory blood pressure. Hypertension 1997;29:22–29 [DOI] [PubMed] [Google Scholar]
- 38.Verdecchia P, Schillaci G, Guerrieri M, et al. Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation 1990;81:528–536 [DOI] [PubMed] [Google Scholar]
- 39.Mancia G, Omboni S, Parati G, et al. Twenty-four hour ambulatory blood pressure in the Hypertension Optimal Treatment (HOT) study. J Hypertens 2001;19:1755–1763 [DOI] [PubMed] [Google Scholar]
- 40.Clement DL, De Buyzere ML, De Bacquer DA, et al. Office versus Ambulatory Pressure Study Investigators Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med 2003;348:2407–2415 [DOI] [PubMed] [Google Scholar]
- 41.Fagard RH, Van Den Broeke C, De Cort P. Prognostic significance of blood pressure measured in the office, at home and during ambulatory monitoring in older patients in general practice. J Hum Hypertens 2005;19:801–807 [DOI] [PubMed] [Google Scholar]
- 42.Redon J, Campos C, Narciso ML, Rodicio JL, Pascual JM, Ruilope LM. Prognostic value of ambulatory blood pressure monitoring in refractory hypertension: a prospective study. Hypertension 1998;31:712–718 [DOI] [PubMed] [Google Scholar]
- 43.Imai Y, Ohkubo T, Sakuma M, et al. Predictive power of screening blood pressure, ambulatory blood pressure and blood pressure measured at home for overall and cardiovascular mortality: a prospective observation in a cohort from Ohasama, northern Japan. Blood Press Monit 1996;1:251–254 [PubMed] [Google Scholar]
- 44.Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension 2005;46:156–161 [DOI] [PubMed] [Google Scholar]
- 45.Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C. Ambulatory blood pressure and mortality: a population-based study. Hypertension 2005;45:499–504 [DOI] [PubMed] [Google Scholar]
- 46.Fagard RH, Celis H, Thijs L, et al. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension 2008;51:55–61 [DOI] [PubMed] [Google Scholar]
- 47.Boggia J, Li Y, Thijs L, et al. International Database on Ambulatory blood pressure monitoring in relation to Cardiovascular Outcomes (IDACO) investigators Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 2007;370:1219–1229 [DOI] [PubMed] [Google Scholar]
- 48.Bauduceau B, Genès N, Chamontin B, et al. Ambulatory blood pressure and urinary albumin excretion in diabetic (non-insulin-dependent and insulin-dependent) hypertensive patients: relationships at baseline and after treatment by the angiotensin converting enzyme inhibitor trandolapril. Am J Hypertens 1998;11:1065–1073 [DOI] [PubMed] [Google Scholar]
- 49.da Costa Rodrigues T, Pecis M, Azevedo MJ, Esteves JF, Gross JL. Ambulatory blood pressure monitoring and progression of retinopathy in normotensive, normoalbuminuric type 1 diabetic patients: a 6-year follow-up study. Diabetes Res Clin Pract 2006;74:135–140 [DOI] [PubMed] [Google Scholar]
- 50.Eguchi K, Pickering TG, Hoshide S, et al. Ambulatory blood pressure is a better marker than clinic blood pressure in predicting cardiovascular events in patients with/without type 2 diabetes. Am J Hypertens 2008;21:443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verdecchia P, Reboldi G, Porcellati C, et al. Risk of cardiovascular disease in relation to achieved office and ambulatory blood pressure control in treated hypertensive subjects. J Am Coll Cardiol 2002;39:878–885 [DOI] [PubMed] [Google Scholar]
- 52.Dolan E, Stanton AV, Thom S, et al. ASCOT Investigators Ambulatory blood pressure monitoring predicts cardiovascular events in treated hypertensive patients—an Anglo-Scandinavian cardiac outcomes trial substudy. J Hypertens 2009;27:876–885 [DOI] [PubMed] [Google Scholar]
- 53.Mancia G, Facchetti R, Bombelli M, Grassi G, Sega R. Long-term risk of mortality associated with selective and combined elevation in office, home, and ambulatory blood pressure. Hypertension 2006;47:846–853 [DOI] [PubMed] [Google Scholar]
- 54.Ohkubo T, Imai Y, Tsuji I, et al. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population-based observation in Ohasama, Japan. J Hypertens 1998;16:971–975 [DOI] [PubMed] [Google Scholar]
- 55.Hozawa A, Ohkubo T, Nagai K, et al. Prognosis of isolated systolic and isolated diastolic hypertension as assessed by self-measurement of blood pressure at home: the Ohasama study. Arch Intern Med 2000;160:3301–3306 [DOI] [PubMed] [Google Scholar]
- 56.Ohkubo T, Asayama K, Kikuya M, et al. Ohasama Study How many times should blood pressure be measured at home for better prediction of stroke risk? Ten-year follow-up results from the Ohasama study. J Hypertens 2004;22:1099–1104 [DOI] [PubMed] [Google Scholar]
- 57.Asayama K, Ohkubo T, Kikuya M, et al. Use of 2003 European Society of Hypertension-European Society of Cardiology guidelines for predicting stroke using self-measured blood pressure at home: the Ohasama study. Eur Heart J 2005;26:2026–2031 [DOI] [PubMed] [Google Scholar]
- 58.Nishinaga M, Takata J, Okumiya K, Matsubayashi K, Ozawa T, Doi Y. High morning home blood pressure is associated with a loss of functional independence in the community-dwelling elderly aged 75 years or older. Hypertens Res 2005;28:657–66 [DOI] [PubMed]
- 59.Okumiya K, Matsubayashi K, Wada T, et al. A U-shaped association between home systolic blood pressure and four-year mortality in community-dwelling older men. J Am Geriatr Soc 1999;47:1415–1421 [DOI] [PubMed] [Google Scholar]
- 60.Agarwal R, Andersen MJ. Prognostic importance of clinic and home blood pressure recordings in patients with chronic kidney disease. Kidney Int 2006;69:406–411 [DOI] [PubMed] [Google Scholar]
- 61.Agarwal R, Andersen MJ. Blood pressure recordings within and outside the clinic and cardiovascular events in chronic kidney disease. Am J Nephrol 2006;26:503–510 [DOI] [PubMed] [Google Scholar]
- 62.Stergiou GS, Baibas NM, Kalogeropoulos PG. Cardiovascular risk prediction based on home blood pressure measurement: the Didima study. J Hypertens 2007;25:1590–1596 [DOI] [PubMed] [Google Scholar]
- 63.Bobrie G, Chatellier G, Genes N, et al. Cardiovascular prognosis of “masked hypertension” detected by blood pressure self-measurement in elderly treated hypertensive patients. JAMA 2004;291:1342–1349 [DOI] [PubMed] [Google Scholar]
- 64.Parati G, Ochoa JE, Bilo G. Blood pressure variability, cardiovascular risk, and risk for renal disease progression. Curr Hypertens Rep 2012;14:421–431 [DOI] [PubMed] [Google Scholar]
- 65.Mancia G, Ferrari A, Gregorini L, et al. Blood pressure and heart rate variabilities in normotensive and hypertensive human beings. Circ Res 1983;53:96–104 [DOI] [PubMed] [Google Scholar]
- 66.Parati G, Pomidossi G, Albini F, Malaspina D, Mancia G. Relationship of 24-hour blood pressure mean and variability to severity of target-organ damage in hypertension. J Hypertens 1987;5:93–98 [DOI] [PubMed] [Google Scholar]
- 67.Parati G, Omboni S, Rizzoni D, Agabiti-Rosei E, Mancia G. The smoothness index: a new, reproducible and clinically relevant measure of the homogeneity of the blood pressure reduction with treatment for hypertension. J Hypertens 1998;16:1685–1691 [DOI] [PubMed] [Google Scholar]
- 68.Mancia G, Di Rienzo M, Parati G. Ambulatory blood pressure monitoring use in hypertension research and clinical practice. Hypertension 1993;21:510–524 [DOI] [PubMed] [Google Scholar]
- 69.Bilo G, Giglio A, Styczkiewicz K, et al. A new method for assessing 24-h blood pressure variability after excluding the contribution of nocturnal blood pressure fall. J Hypertens 2007;25:2058–2066 [DOI] [PubMed] [Google Scholar]
- 70.Mena L, Pintos S, Queipo NV, Aizpúrua JA, Maestre G, Sulbarán T. A reliable index for the prognostic significance of blood pressure variability. J Hypertens 2005;23:505–511 [DOI] [PubMed] [Google Scholar]
- 71.Mancia G, Bombelli M, Facchetti R, et al. Long-term prognostic value of blood pressure variability in the general population: results of the Pressioni Arteriose Monitorate e Loro Associazioni Study. Hypertension 2007;49:1265–1270 [DOI] [PubMed] [Google Scholar]
- 72.Stolarz-Skrzypek K, Thijs L, Richart T, et al. Blood pressure variability in relation to outcome in the International Database of Ambulatory Blood Pressure in relation to cardiovascular outcome. Hypertens Res 2010;33:757–766 [DOI] [PubMed]
- 73.Mancia G, Parati G, Pomidossi G, Casadei R, Di Rienzo M, Zanchetti A. Arterial baroreflexes and blood pressure and heart rate variabilities in humans. Hypertension 1986;8:147–153 [DOI] [PubMed] [Google Scholar]
- 74.Parati G, Saul JP, Di Rienzo M, Mancia G. Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation. A critical appraisal. Hypertension 1995;25:1276–1286 [DOI] [PubMed] [Google Scholar]
- 75.Conway J, Boon N, Davies C, Jones JV, Sleight P. Neural and humoral mechanisms involved in blood pressure variability. J Hypertens 1984;2:203–208 [DOI] [PubMed] [Google Scholar]
- 76.Parati G, Castiglioni P, Di Rienzo M, Omboni S, Pedotti A, Mancia G. Sequential spectral analysis of 24-hour blood pressure and pulse interval in humans. Hypertension 1990;16:414–421 [DOI] [PubMed] [Google Scholar]
- 77.Schillaci G, Bilo G, Pucci G, et al. Relationship between short-term blood pressure variability and large-artery stiffness in human hypertension: findings from 2 large databases. Hypertension 2012;60:369–377 [DOI] [PubMed] [Google Scholar]
- 78.Bertinieri G, Parati G, Ulian L, et al. Hemodilution reduces clinic and ambulatory blood pressure in polycythemic patients. Hypertension 1998;31:848–853 [DOI] [PubMed] [Google Scholar]
- 79.Ozawa M, Tamura K, Iwatsubo K, et al. Ambulatory blood pressure variability is increased in diabetic hypertensives. Clin Exp Hypertens 2008;30:213–224 [DOI] [PubMed] [Google Scholar]
- 80.Ruiz J, Monbaron D, Parati G, et al. Diabetic neuropathy is a more important determinant of baroreflex sensitivity than carotid elasticity in type 2 diabetes. Hypertension 2005;46:162–167 [DOI] [PubMed] [Google Scholar]
- 81.Frattola A, Parati G, Gamba P, et al. Time and frequency domain estimates of spontaneous baroreflex sensitivity provide early detection of autonomic dysfunction in diabetes mellitus. Diabetologia 1997;40:1470–1475 [DOI] [PubMed] [Google Scholar]
- 82.Tamura K, Tsurumi Y, Sakai M, et al. A possible relationship of nocturnal blood pressure variability with coronary artery disease in diabetic nephropathy. Clin Exp Hypertens 2007;29:31–42 [DOI] [PubMed] [Google Scholar]
- 83.Fogari R, Zoppi A, Malamani GD, Lazzari P, Destro M, Corradi L. Ambulatory blood pressure monitoring in normotensive and hypertensive type 2 diabetes. Prevalence of impaired diurnal blood pressure patterns. Am J Hypertens 1993;6:1–7 [DOI] [PubMed] [Google Scholar]
- 84.Ueda Y, Aoi W, Yamachika S, Nagataki S. Loss of nocturnal decline in blood pressure in diabetic patients with autonomic neuropathy. Jpn Heart J 1992;33:801–815 [DOI] [PubMed] [Google Scholar]
- 85.Spallone V, Maiello MR, Morganti R, Mandica S, Frajese G. Usefulness of ambulatory blood pressure monitoring in predicting the presence of autonomic neuropathy in type I diabetic patients. J Hum Hypertens 2007;21:381–386 [DOI] [PubMed] [Google Scholar]
- 86.Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest 2008;133:496–506 [DOI] [PubMed] [Google Scholar]
- 87.Mancia G, Parati G, Hennig M, et al. ELSA Investigators Relation between blood pressure variability and carotid artery damage in hypertension: baseline data from the European Lacidipine Study on Atherosclerosis (ELSA). J Hypertens 2001;19:1981–1989 [DOI] [PubMed] [Google Scholar]
- 88.Mancia G, Parati G: The role of blood pressure variability in end-organ damage. J Hypertens Suppl 2003;21:S17–S23 [DOI] [PubMed]
- 89.Sega R, Corrao G, Bombelli M, et al. Blood pressure variability and organ damage in a general population: results from the PAMELA study (Pressioni Arteriose Monitorate E Loro Associazioni). Hypertension 2002;39:710–714 [DOI] [PubMed] [Google Scholar]
- 90.Tatasciore A, Renda G, Zimarino M, et al. Awake systolic blood pressure variability correlates with target-organ damage in hypertensive subjects. Hypertension 2007;50:325–332 [DOI] [PubMed] [Google Scholar]
- 91.Manios E, Tsagalis G, Tsivgoulis G, et al. Time rate of blood pressure variation is associated with impaired renal function in hypertensive patients. J Hypertens 2009;27:2244–2248 [DOI] [PubMed] [Google Scholar]
- 92.Frattola A, Parati G, Cuspidi C, Albini F, Mancia G. Prognostic value of 24-hour blood pressure variability. J Hypertens 1993;11:1133–1137 [DOI] [PubMed] [Google Scholar]
- 93.Sander D, Kukla C, Klingelhöfer J, Winbeck K, Conrad B. Relationship between circadian blood pressure patterns and progression of early carotid atherosclerosis: A 3-year follow-up study. Circulation 2000;102:1536–1541 [DOI] [PubMed] [Google Scholar]
- 94.Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation 2003;107:1401–1406 [DOI] [PubMed] [Google Scholar]
- 95.Kario K, Ishikawa J, Pickering TG, et al. Morning hypertension: the strongest independent risk factor for stroke in elderly hypertensive patients. Hypertens Res 2006;29:581–587 [DOI] [PubMed]
- 96.Verdecchia P, Angeli F, Gattobigio R, Rapicetta C, Reboldi G. Impact of blood pressure variability on cardiac and cerebrovascular complications in hypertension. Am J Hypertens 2007;20:154–161 [DOI] [PubMed] [Google Scholar]
- 97.Hansen TW, Thijs L, Li Y, et al. International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes Investigators Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension 2010;55:1049–1057 [DOI] [PubMed] [Google Scholar]
- 98.Dawson SL, Manktelow BN, Robinson TG, Panerai RB, Potter JF. Which parameters of beat-to-beat blood pressure and variability best predict early outcome after acute ischemic stroke? Stroke 2000;31:463–468 [DOI] [PubMed] [Google Scholar]
- 99.Pringle E, Phillips C, Thijs L, et al. Syst-Eur investigators Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. J Hypertens 2003;21:2251–2257 [DOI] [PubMed] [Google Scholar]
- 100.Stolarz-Skrzypek K, Thijs L, Li Y, et al. Short-term blood pressure variability in relation to outcome in the International Database of Ambulatory blood pressure in relation to Cardiovascular Outcome (IDACO). Acta Cardiol 2011;66:701–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pickering TG, Hall JE, Appel LJ, et al. Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 2005;45:142–161 [DOI] [PubMed] [Google Scholar]
- 102.Lurbe E, Redon J, Kesani A, et al. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med 2002;347:797–805 [DOI] [PubMed] [Google Scholar]
- 103.Metoki H, Ohkubo T, Kikuya M, et al. Prognostic significance for stroke of a morning pressor surge and a nocturnal blood pressure decline: the Ohasama study. Hypertension 2006;47:149–154 [DOI] [PubMed] [Google Scholar]
- 104.Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens 2002;20:2183–2189 [DOI] [PubMed] [Google Scholar]
- 105.Verdecchia P. Prognostic value of ambulatory blood pressure : current evidence and clinical implications. Hypertension 2000;35:844–851 [DOI] [PubMed] [Google Scholar]
- 106.Amici A, Cicconetti P, Sagrafoli C, et al. Exaggerated morning blood pressure surge and cardiovascular events. A 5-year longitudinal study in normotensive and well-controlled hypertensive elderly. Arch Gerontol Geriatr 2009;49:e105–e109 [DOI] [PubMed] [Google Scholar]
- 107.Verdecchia P, Angeli F, Mazzotta G, et al. Day-night dip and early-morning surge in blood pressure in hypertension: prognostic implications. Hypertension 2012;60:34–42 [DOI] [PubMed] [Google Scholar]
- 108.Lurbe E, Redon J, Pascual JM, Tacons J, Alvarez V. The spectrum of circadian blood pressure changes in type I diabetic patients. J Hypertens 2001;19:1421–1428 [DOI] [PubMed] [Google Scholar]
- 109.Palmas W, Pickering T, Teresi J, et al. Nocturnal blood pressure elevation predicts progression of albuminuria in elderly people with type 2 diabetes. J Clin Hypertens (Greenwich) 2008;10:12–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nakano S, Uchida K, Kigoshi T, et al. Circadian rhythm of blood pressure in normotensive NIDDM subjects. Its relationship to microvascular complications. Diabetes Care 1991;14:707–711 [DOI] [PubMed] [Google Scholar]
- 111.Felício JS, de Souza AC, Kohlmann N, Kohlmann O, Jr, Ribeiro AB, Zanella MT. Nocturnal blood pressure fall as predictor of diabetic nephropathy in hypertensive patients with type 2 diabetes. Cardiovasc Diabetol 2010;9:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Farmer CK, Goldsmith DJ, Quin JD, et al. Progression of diabetic nephropathy–is diurnal blood pressure rhythm as important as absolute blood pressure level? Nephrol Dial Transplant 1998;13:635–639 [DOI] [PubMed]
- 113.Sturrock ND, George E, Pound N, Stevenson J, Peck GM, Sowter H. Non-dipping circadian blood pressure and renal impairment are associated with increased mortality in diabetes mellitus. Diabet Med 2000;17:360–364 [DOI] [PubMed]
- 114.Nakano S, Ito T, Furuya K, et al. Ambulatory blood pressure level rather than dipper/nondipper status predicts vascular events in type 2 diabetic subjects. Hypertens Res 2004;27:647–656 [DOI] [PubMed]
- 115.Muntner P, Joyce C, Levitan EB, et al. Reproducibility of visit-to-visit variability of blood pressure measured as part of routine clinical care. J Hypertens 2011;29:2332–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 2010;375:895–905 [DOI] [PubMed] [Google Scholar]
- 117.Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension 2011;57:160–166 [DOI] [PubMed] [Google Scholar]
- 118.Mancia G, Messerli F, Bakris G, Zhou Q, Champion A, Pepine CJ. Blood pressure control and improved cardiovascular outcomes in the International Verapamil SR-Trandolapril Study. Hypertension 2007;50:299–305 [DOI] [PubMed] [Google Scholar]
- 119.Parati G, Bilo G. Calcium antagonist added to angiotensin receptor blocker: a recipe for reducing blood pressure variability? Evidence from day-by-day home blood pressure monitoring. Hypertension 2012;59:1091–1093 [DOI] [PubMed] [Google Scholar]
- 120.Matsui Y, Ishikawa J, Eguchi K, Shibasaki S, Shimada K, Kario K. Maximum value of home blood pressure: a novel indicator of target organ damage in hypertension. Hypertension 2011;57:1087–1093 [DOI] [PubMed] [Google Scholar]
- 121.Kikuya M, Ohkubo T, Metoki H, et al. Day-by-day variability of blood pressure and heart rate at home as a novel predictor of prognosis: the Ohasama study. Hypertension 2008;52:1045–1050 [DOI] [PubMed] [Google Scholar]
- 122.Johansson JK, Niiranen TJ, Puukka PJ, Jula AM. Prognostic value of the variability in home-measured blood pressure and heart rate: the Finn-Home Study. Hypertension 2012;59:212–218 [DOI] [PubMed] [Google Scholar]
- 123.Tamura K, Azushima K, Umemura S. Day-by-day home-measured blood pressure variability: another important factor in hypertension with diabetic nephropathy? Hypertens Res 2011;34:1249–1250 [DOI] [PubMed]
- 124.Ushigome E, Fukui M, Hamaguchi M, et al. The coefficient variation of home blood pressure is a novel factor associated with macroalbuminuria in type 2 diabetes mellitus. Hypertens Res 2011;34:1271–1275 [DOI] [PubMed]
- 125.Mancia G, Facchetti R, Parati G, Zanchetti A. Visit-to-visit blood pressure variability in the European Lacidipine Study on Atherosclerosis: methodological aspects and effects of antihypertensive treatment. J Hypertens 2012;30:1241–1251 [DOI] [PubMed] [Google Scholar]
- 126.Stergiou GS, Nasothimiou EG. Home monitoring is the optimal method for assessing blood pressure variability. Hypertens Res 2011;34:1246–1248 [DOI] [PubMed]
- 127.Masugata H, Senda S, Murao K, et al. Visit-to-visit variability in blood pressure over a 1-year period is a marker of left ventricular diastolic dysfunction in treated hypertensive patients. Hypertens Res 2011;34:846–850 [DOI] [PubMed]
- 128.Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Visit-to-visit blood pressure variations: new independent determinants for carotid artery measures in the elderly at high risk of cardiovascular disease. J Am Soc Hypertens 2011;5:184–192 [DOI] [PubMed] [Google Scholar]
- 129.Kilpatrick ES, Rigby AS, Atkin SL. The role of blood pressure variability in the development of nephropathy in type 1 diabetes. Diabetes Care 2010;33:2442–2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kawai T, Ohishi M, Kamide K, et al. The impact of visit-to-visit variability in blood pressure on renal function. Hypertens Res 2012;35:239–243 [DOI] [PubMed]
- 131.Brickman AM, Reitz C, Luchsinger JA, et al. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol 2010;67:564–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Diaz KM, Veerabhadrappa P, Kashem MA, et al. Relationship of visit-to-visit and ambulatory blood pressure variability to vascular function in African Americans. Hypertens Res 2012;35:55–61 [DOI] [PMC free article] [PubMed]
- 133.Hata Y, Kimura Y, Muratani H, Fukiyama K, Kawano Y, Ashida T, Yokouchi M, Imai Y, Ozawa T, Fujii J et al: Office blood pressure variability as a predictor of brain infarction in elderly hypertensive patients. Hypertens Res 2000;23:553–560 [DOI] [PubMed]
- 134.Rothwell PM, Howard SC, Dolan E, et al. ASCOT-BPLA and MRC Trial Investigators Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol 2010;9:469–480 [DOI] [PubMed] [Google Scholar]
- 135.Eguchi K, Hoshide S, Schwartz JE, Shimada K, Kario K. Visit-to-visit and ambulatory blood pressure variability as predictors of incident cardiovascular events in patients with hypertension. Am J Hypertens 2012;25:962–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hata Y, Muratani H, Kimura Y, et al. Office blood pressure variability as a predictor of acute myocardial infarction in elderly patients receiving antihypertensive therapy. J Hum Hypertens 2002;16:141–146 [DOI] [PubMed] [Google Scholar]
- 137.Grove JS, Reed DM, Yano K, Hwang LJ. Variability in systolic blood pressure—a risk factor for coronary heart disease? Am J Epidemiol 1997;145:771–776 [DOI] [PubMed] [Google Scholar]
- 138.Okada H, Fukui M, Tanaka M, et al. Visit-to-visit variability in systolic blood pressure is correlated with diabetic nephropathy and atherosclerosis in patients with type 2 diabetes. Atherosclerosis 2012;220:155–159 [DOI] [PubMed] [Google Scholar]
- 139.Rizzoni D, Muiesan ML, Salvetti M, et al. The smoothness index, but not the trough-to-peak ratio predicts changes in carotid artery wall thickness during antihypertensive treatment. J Hypertens 2001;19:703–711 [DOI] [PubMed] [Google Scholar]
- 140.Anan F, Masaki T, Fukunaga N, et al. Pioglitazone shift circadian rhythm of blood pressure from non-dipper to dipper type in type 2 diabetes mellitus. Eur J Clin Invest 2007;37:709–714 [DOI] [PubMed] [Google Scholar]
- 141.Komajda M, Curtis P, Hanefeld M, et al. RECORD Study Group Effect of the addition of rosiglitazone to metformin or sulfonylureas versus metformin/sulfonylurea combination therapy on ambulatory blood pressure in people with type 2 diabetes: a randomized controlled trial (the RECORD study). Cardiovasc Diabetol 2008;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rossing K, Christensen PK, Andersen S, Hovind P, Hansen HP, Parving HH, Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Comparative effects of Irbesartan on ambulatory and office blood pressure: a substudy of ambulatory blood pressure from the Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria study. Diabetes Care 2003;26:569–574 [DOI] [PubMed] [Google Scholar]
- 143.Poulsen PL, Ebbehøj E, Nosadini R, et al. Early ACE-i intervention in microalbuminuric patients with type 1 diabetes: effects on albumin excretion, 24 h ambulatory blood pressure, and renal function. Diabetes Metab 2001;27:123–128 [PubMed] [Google Scholar]
- 144.Frattola A, Parati G, Castiglioni P, et al. Lacidipine and blood pressure variability in diabetic hypertensive patients. Hypertension 2000;36:622–628 [DOI] [PubMed] [Google Scholar]
- 145.Masuda S, Tamura K, Wakui H, et al. Effects of angiotensin II type 1 receptor blocker on ambulatory blood pressure variability in hypertensive patients with overt diabetic nephropathy. Hypertens Res 2009;32:950–955 [DOI] [PubMed]
- 146.Mancia G, Facchetti R, Parati G, Zanchetti A. Visit-to-visit blood pressure variability, carotid atherosclerosis, and cardiovascular events in the European Lacidipine Study on Atherosclerosis. Circulation 2012;126:569–578 [DOI] [PubMed] [Google Scholar]
- 147.Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet 2010;375:906–915 [DOI] [PubMed] [Google Scholar]
- 148.Turnbull F, Neal B, Algert C, et al. Blood Pressure Lowering Treatment Trialists’ Collaboration Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med 2005;165:1410–1419 [DOI] [PubMed] [Google Scholar]