Abstract
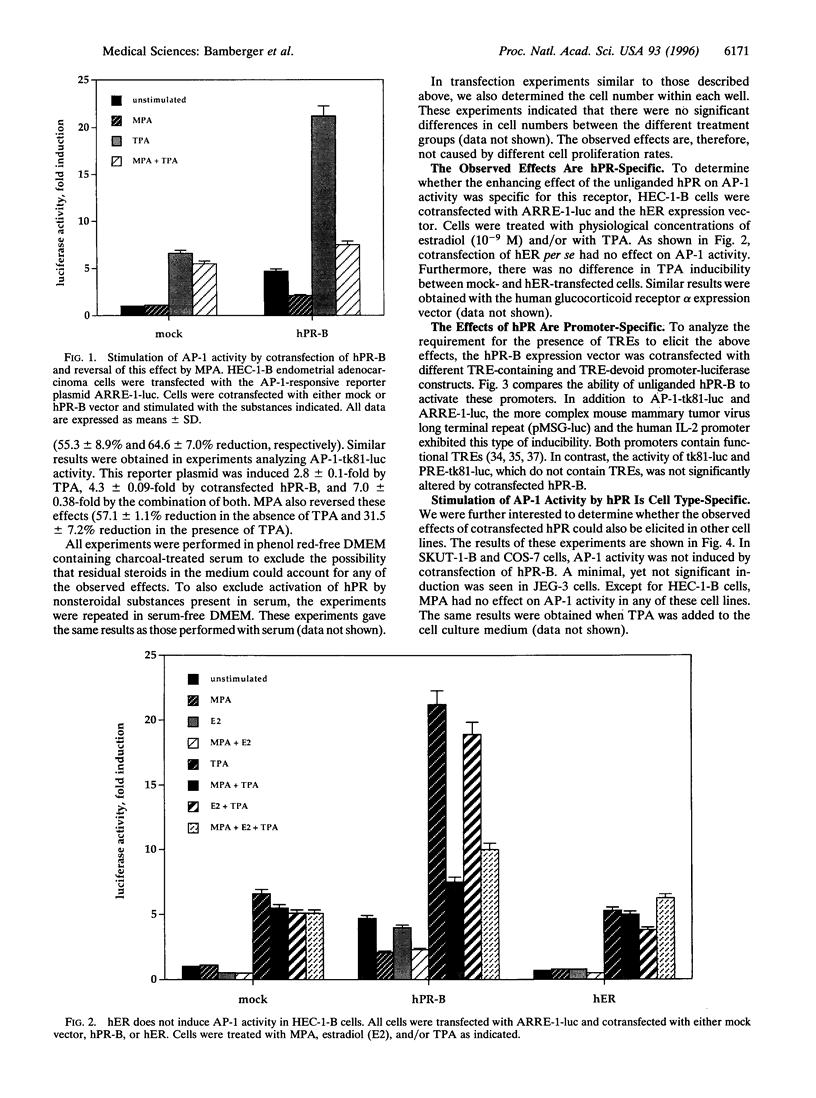
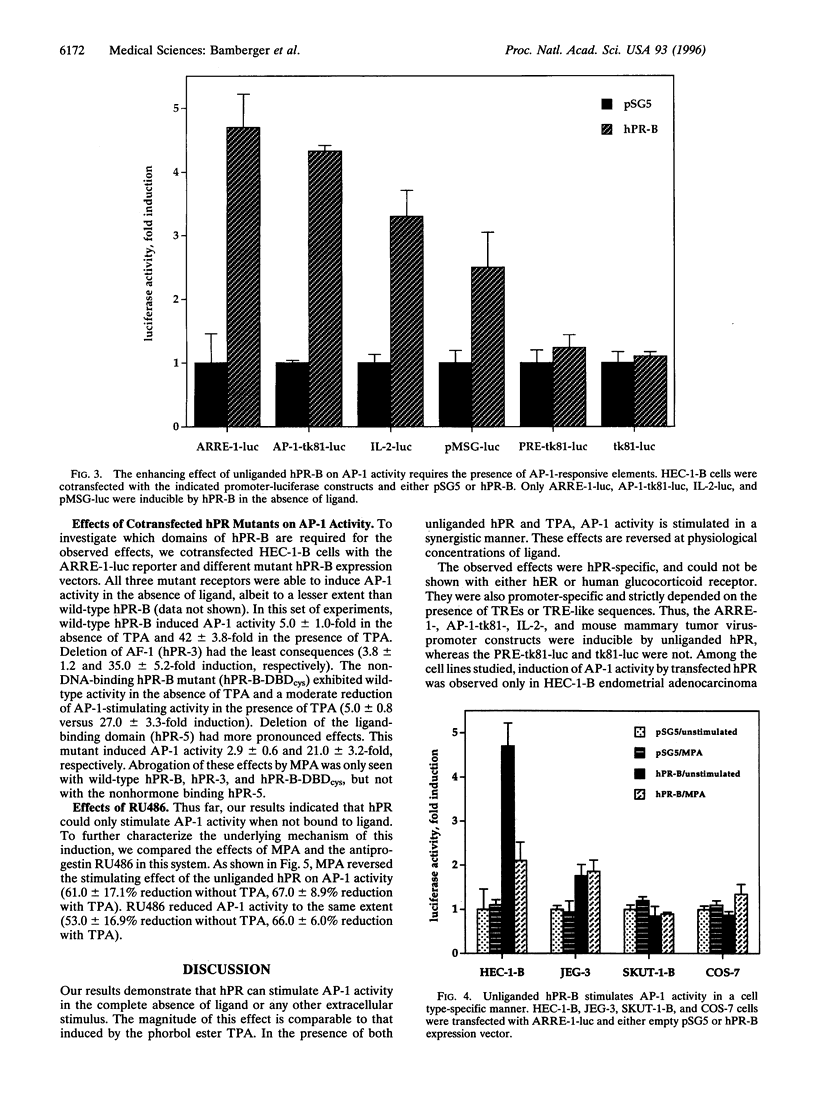
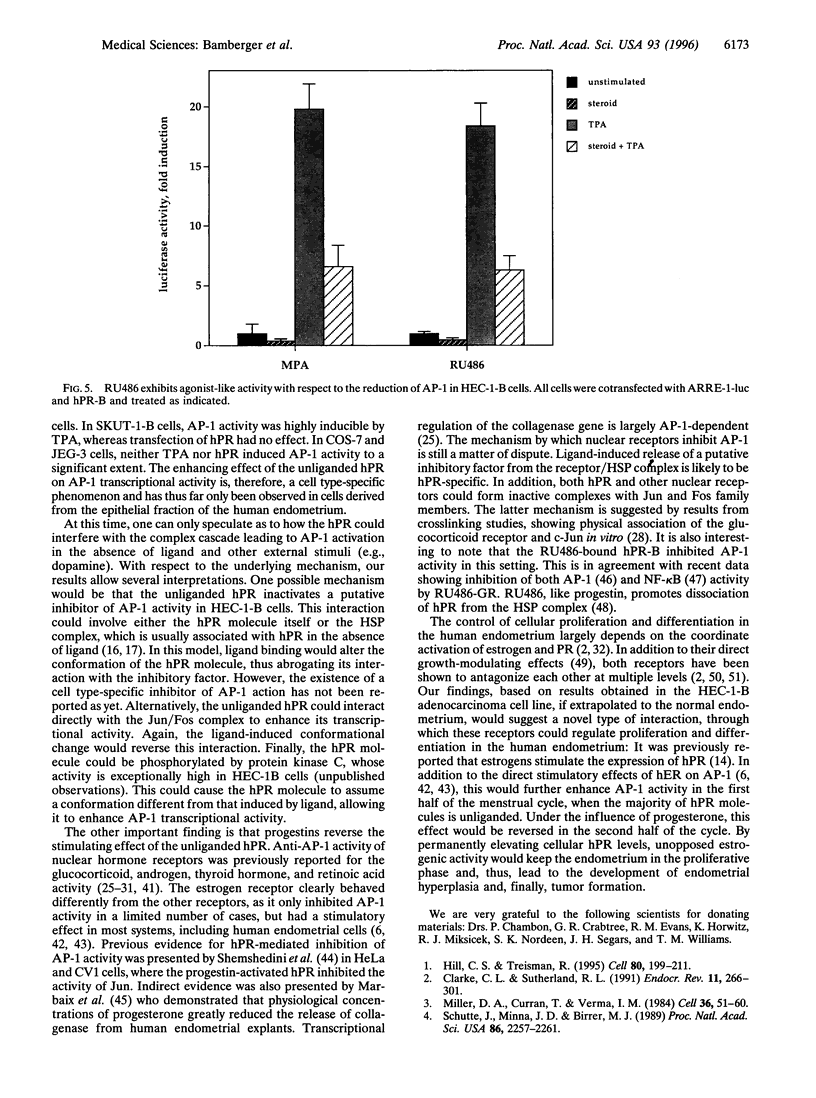
The composite transcription factor activating protein 1 (AP-1) integrates various mitogenic signals in a large number of cell types, and is therefore a major regulator of cell proliferation. In the normal human endometrium, proliferation and differentiation alternate in a cyclic fashion, with progesterone being largely implicated in the latter process. However, the effects of progesterone and the progesterone receptor (hPR) on AP-1 activity in the human endometrium are not known. To address this issue, HEC-1-B endometrial adenocarcinoma cells, which are devoid of hPR, were transfected with luciferase reporter constructs driven by two different AP-1-dependent promoters. Unexpectedly, cotransfection of hPR caused a marked induction of luciferase activity in the absence of ligand on both promoters. The magnitude of this induction was similar to that observed in response to the phorbol ester TPA. Addition of ligand reversed the stimulating effect of the unliganded hPR on AM activity in these cells. These effects were specific for hPR, and were not observed with either human estrogen receptor or human glucocorticoid receptor. Furthermore, they strictly depended on the presence of AP-1-responsive sequences within target promoters. Finally, the described effects of hPR on AP-1 activity were shown to be cell-type specific, because they could not be demonstrated in SKUT-1-B, JEG-3, and COS-7 cells. To our knowledge this is the first report of an unliganded steroid receptor stimulating AP-1 activity. This effect and its reversal in the presence of ligand suggest a novel mechanism, through which hPR can act as a key regulator of both proliferation and differentiation in the human endometrium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991 Dec 10;1072(2-3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Chalbos D., Galtier F. Differential effect of forms A and B of human progesterone receptor on estradiol-dependent transcription. J Biol Chem. 1994 Sep 16;269(37):23007–23012. [PubMed] [Google Scholar]
- Clarke C. L., Sutherland R. L. Progestin regulation of cellular proliferation. Endocr Rev. 1990 May;11(2):266–301. doi: 10.1210/edrv-11-2-266. [DOI] [PubMed] [Google Scholar]
- DeMarzo A. M., Beck C. A., Onate S. A., Edwards D. P. Dimerization of mammalian progesterone receptors occurs in the absence of DNA and is related to the release of the 90-kDa heat shock protein. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):72–76. doi: 10.1073/pnas.88.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J. G., Mazella J., Powell D. R., Tseng L. Identification of a distal regulatory sequence of the human IGFBP-1 gene promoter and regulation by the progesterone receptor in a human endometrial adenocarcinoma cell line. DNA Cell Biol. 1994 Aug;13(8):829–837. doi: 10.1089/dna.1994.13.829. [DOI] [PubMed] [Google Scholar]
- Giudice L. C. Growth factors and growth modulators in human uterine endometrium: their potential relevance to reproductive medicine. Fertil Steril. 1994 Jan;61(1):1–17. doi: 10.1016/s0015-0282(16)56447-4. [DOI] [PubMed] [Google Scholar]
- Hill C. S., Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995 Jan 27;80(2):199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B. The molecular biology of RU486. Is there a role for antiprogestins in the treatment of breast cancer? Endocr Rev. 1992 May;13(2):146–163. doi: 10.1210/edrv-13-2-146. [DOI] [PubMed] [Google Scholar]
- Hsu T. C., Melchiorre L. P., Jr, Maksymowych A. B., Kmiec E., Litwack G. Assembly of glucocorticoid receptor and c-JUN homodimer on the promoter of mouse mammary tumor virus-long terminal repeat is influenced by order of addition. Biochem Biophys Res Commun. 1993 Dec 30;197(3):1260–1266. doi: 10.1006/bbrc.1993.2613. [DOI] [PubMed] [Google Scholar]
- Hyder S. M., Nawaz Z., Chiappetta C., Yokoyama K., Stancel G. M. The protooncogene c-jun contains an unusual estrogen-inducible enhancer within the coding sequence. J Biol Chem. 1995 Apr 14;270(15):8506–8513. doi: 10.1074/jbc.270.15.8506. [DOI] [PubMed] [Google Scholar]
- Jonat C., Rahmsdorf H. J., Park K. K., Cato A. C., Gebel S., Ponta H., Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990 Sep 21;62(6):1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Kallio P. J., Poukka H., Moilanen A., Jänne O. A., Palvimo J. J. Androgen receptor-mediated transcriptional regulation in the absence of direct interaction with a specific DNA element. Mol Endocrinol. 1995 Aug;9(8):1017–1028. doi: 10.1210/mend.9.8.7476976. [DOI] [PubMed] [Google Scholar]
- Kastner P., Krust A., Turcotte B., Stropp U., Tora L., Gronemeyer H., Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990 May;9(5):1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus W. L., Weis K. E., Katzenellenbogen B. S. Inhibitory cross-talk between steroid hormone receptors: differential targeting of estrogen receptor in the repression of its transcriptional activity by agonist- and antagonist-occupied progestin receptors. Mol Cell Biol. 1995 Apr;15(4):1847–1857. doi: 10.1128/mcb.15.4.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Liu W., Hillmann A. G., Harmon J. M. Hormone-independent repression of AP-1-inducible collagenase promoter activity by glucocorticoid receptors. Mol Cell Biol. 1995 Feb;15(2):1005–1013. doi: 10.1128/mcb.15.2.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucibello F. C., Slater E. P., Jooss K. U., Beato M., Müller R. Mutual transrepression of Fos and the glucocorticoid receptor: involvement of a functional domain in Fos which is absent in FosB. EMBO J. 1990 Sep;9(9):2827–2834. doi: 10.1002/j.1460-2075.1990.tb07471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani S. K., Allen J. M., Clark J. H., Blaustein J. D., O'Malley B. W. Convergent pathways for steroid hormone- and neurotransmitter-induced rat sexual behavior. Science. 1994 Aug 26;265(5176):1246–1249. doi: 10.1126/science.7915049. [DOI] [PubMed] [Google Scholar]
- Marbaix E., Donnez J., Courtoy P. J., Eeckhout Y. Progesterone regulates the activity of collagenase and related gelatinases A and B in human endometrial explants. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11789–11793. doi: 10.1073/pnas.89.24.11789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matkovits T., Christakos S. Ligand occupancy is not required for vitamin D receptor and retinoid receptor-mediated transcriptional activation. Mol Endocrinol. 1995 Feb;9(2):232–242. doi: 10.1210/mend.9.2.7776973. [DOI] [PubMed] [Google Scholar]
- Meyer M. E., Pornon A., Ji J. W., Bocquel M. T., Chambon P., Gronemeyer H. Agonistic and antagonistic activities of RU486 on the functions of the human progesterone receptor. EMBO J. 1990 Dec;9(12):3923–3932. doi: 10.1002/j.1460-2075.1990.tb07613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Curran T., Verma I. M. c-fos protein can induce cellular transformation: a novel mechanism of activation of a cellular oncogene. Cell. 1984 Jan;36(1):51–60. doi: 10.1016/0092-8674(84)90073-4. [DOI] [PubMed] [Google Scholar]
- Nordeen S. K. Luciferase reporter gene vectors for analysis of promoters and enhancers. Biotechniques. 1988 May;6(5):454–458. [PubMed] [Google Scholar]
- Pfahl M. Nuclear receptor/AP-1 interaction. Endocr Rev. 1993 Oct;14(5):651–658. doi: 10.1210/edrv-14-5-651. [DOI] [PubMed] [Google Scholar]
- Power R. F., Mani S. K., Codina J., Conneely O. M., O'Malley B. W. Dopaminergic and ligand-independent activation of steroid hormone receptors. Science. 1991 Dec 13;254(5038):1636–1639. doi: 10.1126/science.1749936. [DOI] [PubMed] [Google Scholar]
- Ransone L. J., Verma I. M. Nuclear proto-oncogenes fos and jun. Annu Rev Cell Biol. 1990;6:539–557. doi: 10.1146/annurev.cb.06.110190.002543. [DOI] [PubMed] [Google Scholar]
- Renoir J. M., Radanyi C., Jung-Testas I., Faber L. E., Baulieu E. E. The nonactivated progesterone receptor is a nuclear heterooligomer. J Biol Chem. 1990 Aug 25;265(24):14402–14406. [PubMed] [Google Scholar]
- Sartorius C. A., Melville M. Y., Hovland A. R., Tung L., Takimoto G. S., Horwitz K. B. A third transactivation function (AF3) of human progesterone receptors located in the unique N-terminal segment of the B-isoform. Mol Endocrinol. 1994 Oct;8(10):1347–1360. doi: 10.1210/mend.8.10.7854352. [DOI] [PubMed] [Google Scholar]
- Scheinman R. I., Gualberto A., Jewell C. M., Cidlowski J. A., Baldwin A. S., Jr Characterization of mechanisms involved in transrepression of NF-kappa B by activated glucocorticoid receptors. Mol Cell Biol. 1995 Feb;15(2):943–953. doi: 10.1128/mcb.15.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Kliewer S., Ransone L. J., Bolado J., Yang N., Verma I. M., Evans R. M. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990 Sep 21;62(6):1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Yang N., Kliewer S., Ransone L. J., Bolado J., Verma I. M., Evans R. M. Retinoic acid is a negative regulator of AP-1-responsive genes. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6092–6096. doi: 10.1073/pnas.88.14.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütte J., Minna J. D., Birrer M. J. Deregulated expression of human c-jun transforms primary rat embryo cells in cooperation with an activated c-Ha-ras gene and transforms rat-1a cells as a single gene. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2257–2261. doi: 10.1073/pnas.86.7.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segars J. H., Marks M. S., Hirschfeld S., Driggers P. H., Martinez E., Grippo J. F., Brown M., Wahli W., Ozato K. Inhibition of estrogen-responsive gene activation by the retinoid X receptor beta: evidence for multiple inhibitory pathways. Mol Cell Biol. 1993 Apr;13(4):2258–2268. doi: 10.1128/mcb.13.4.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemshedini L., Knauthe R., Sassone-Corsi P., Pornon A., Gronemeyer H. Cell-specific inhibitory and stimulatory effects of Fos and Jun on transcription activation by nuclear receptors. EMBO J. 1991 Dec;10(12):3839–3849. doi: 10.1002/j.1460-2075.1991.tb04953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truss M., Beato M. Steroid hormone receptors: interaction with deoxyribonucleic acid and transcription factors. Endocr Rev. 1993 Aug;14(4):459–479. doi: 10.1210/edrv-14-4-459. [DOI] [PubMed] [Google Scholar]
- Tsai M. J., O'Malley B. W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Tung L., Mohamed M. K., Hoeffler J. P., Takimoto G. S., Horwitz K. B. Antagonist-occupied human progesterone B-receptors activate transcription without binding to progesterone response elements and are dominantly inhibited by A-receptors. Mol Endocrinol. 1993 Oct;7(10):1256–1265. doi: 10.1210/mend.7.10.8123133. [DOI] [PubMed] [Google Scholar]
- Ullman K. S., Northrop J. P., Admon A., Crabtree G. R. Jun family members are controlled by a calcium-regulated, cyclosporin A-sensitive signaling pathway in activated T lymphocytes. Genes Dev. 1993 Feb;7(2):188–196. doi: 10.1101/gad.7.2.188. [DOI] [PubMed] [Google Scholar]
- Vegeto E., Shahbaz M. M., Wen D. X., Goldman M. E., O'Malley B. W., McDonnell D. P. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993 Oct;7(10):1244–1255. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- Webb P., Lopez G. N., Uht R. M., Kushner P. J. Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol Endocrinol. 1995 Apr;9(4):443–456. doi: 10.1210/mend.9.4.7659088. [DOI] [PubMed] [Google Scholar]
- Williams T. M., Moolten D. M., Makni H., Kim H. W., Kant J. A., Kamoun M. CD28-stimulated IL-2 gene expression in Jurkat T cells occurs in part transcriptionally and is cyclosporine-A sensitive. J Immunol. 1992 Apr 15;148(8):2609–2616. [PubMed] [Google Scholar]
- Yang-Yen H. F., Chambard J. C., Sun Y. L., Smeal T., Schmidt T. J., Drouin J., Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990 Sep 21;62(6):1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- Yang-Yen H. F., Zhang X. K., Graupner G., Tzukerman M., Sakamoto B., Karin M., Pfahl M. Antagonism between retinoic acid receptors and AP-1: implications for tumor promotion and inflammation. New Biol. 1991 Dec;3(12):1206–1219. [PubMed] [Google Scholar]
- Zhang X. K., Wills K. N., Husmann M., Hermann T., Pfahl M. Novel pathway for thyroid hormone receptor action through interaction with jun and fos oncogene activities. Mol Cell Biol. 1991 Dec;11(12):6016–6025. doi: 10.1128/mcb.11.12.6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Bai W., Allgood V. E., Weigel N. L. Multiple signaling pathways activate the chicken progesterone receptor. Mol Endocrinol. 1994 May;8(5):577–584. doi: 10.1210/mend.8.5.8058067. [DOI] [PubMed] [Google Scholar]



