What is the rationale for adding incretin-based therapies to insulin?
The combination of the incretin-based therapies, i.e., the dipeptidyl peptidase (DPP)-4 inhibitors and glucagon-like peptide (GLP)-1 receptor agonists (GLP-1RAs), with basal insulin has, in theory, logical appeal. While basal insulin primarily improves fasting plasma glucose (FPG) control, the glucose-dependent effect of incretins will additionally benefit postprandial plasma glucose (PPG) control. This should enable improved control of A1C with the expectation of relatively stable blood glucose concentrations, and the combination of incretins with basal insulin might reduce insulin dose requirement and, consequently, weight gain. This also supports the concept that combining the incretin-based therapies with basal insulin should enable tight glycemic control with a low risk of hypoglycemia. Furthermore, a reciprocal benefit of this combination is that the basal insulin will theoretically supplement endogenous insulin production and “rest” the β-cell, enabling greater recovery of the endogenous insulin response when required. The basis of this theory is formed from research showing benefits with incretin therapies for β-cell function (1) and β-cell mass in experimental systems (2–4).
In addition to their antihyperglycemic properties, GLP-1RAs also reduce gastrointestinal motility, which, together with increased satiety, produces a weight-sparing effect (2). This quality could mitigate the weight gain associated with insulin therapy and might be further enhanced through any reductions in insulin dose.
Evidence to date: how do the data from insulin plus incretin clinical studies meet with expectations?
Glycemic control.
Using basal insulin to reduce FPG is an effective way of improving glycemic control; however, the second component of glycemic control, PPG, requires additional consideration. This is one area where incretin-based therapies and basal insulin should have complementary actions.
Adding incretin-based therapies to insulin.
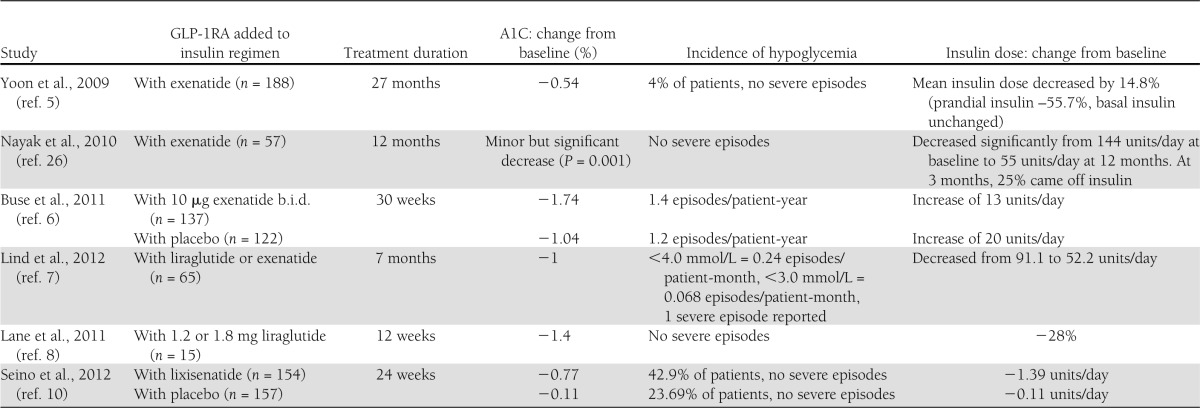
In an uncontrolled, retrospective investigation involving a cohort of 188 patients receiving insulin, the addition of exenatide produced an A1C reduction of –0.66% (P < 0.001) from a baseline value of 8.05% after 6 months of combination therapy—an improvement that was maintained at 27 months (5). Moreover, the patients in this study had a long duration of diabetes, with ~70% having had a diagnosis of type 2 diabetes for >10 years. This demonstrated that improvements in glycemic control can be attained even in the advanced stages of the disease.
A 30-week, prospective, controlled, randomized study, involving 261 participants with type 2 diabetes, found similar improvements in glycemic control when exenatide was added to insulin glargine (with or without oral antidiabetes drugs) (6). Exenatide decreased A1C by –1.74% from baseline values, and this reduction was significantly better (P < 0.001) than in placebo-treated subjects (–1.04%). Furthermore, the placebo group required a seven-unit increase in final insulin dose, highlighting the efficacy of supplementing basal insulin with exenatide. These improvements in A1C were driven exclusively by a greater reduction in PPG with exenatide, lending support to the theory of complementary blood glucose–lowering actions.
The efficacy of an insulin plus GLP-1RA regimen has been further reinforced by a retrospective study (7). Obese patients with type 2 diabetes who added either liraglutide (n = 40) or exenatide (n = 21) to basal insulin exhibited a reduction in mean A1C: from 8.9% at baseline to 7.9% at 7 months (P < 0.001).
A small-scale observational study involving obese patients with type 2 diabetes receiving high doses of basal insulin (mean daily dose 192 ± 77 units/day) (8) revealed the benefit of combination therapy in very insulin-resistant subjects. After 12 weeks of coadministration of liraglutide, A1C decreased by 1.4%. This improvement is remarkable given that basal insulin doses were reduced by 28%.
Incretin-based therapies appear to be particularly effective in Asian patients with type 2 diabetes. This is possibly a result of a pathophysiology of insulin deficiency rather than insulin resistance, and it has been suggested that this is the result of an underlying GLP-1 insufficiency in these patients (9). One recent study, involving an Asian population, has confirmed the advantages of adding GLP-1RAs to basal insulin in patients with poorly controlled A1C (10). Supplementing basal insulin plus or minus sulfonylurea with once-daily lixisenatide significantly improved 2-h PPG, average 7-point self-monitored blood glucose (SMBG), and FPG. A significantly (P < 0.001) greater percentage of patients receiving lixisenatide achieved A1C <7.0% (35.6%) compared with placebo (5.2%). The short-acting profile of lixisenatide has a pronounced effect on postprandial glycemia—reducing glucose excursion by 75% in one recent study (11). This effect likely involves a reduction in gastric emptying rate.
The GetGoal-Duo 1 study assessed the complementary action of lixisenatide and insulin glargine in patients with type 2 diabetes failing on oral antidiabetes medication. After a 12-week run-in phase in which insulin glargine was initiated, patients with A1C ≥7% were randomized to 20 μg lixisenatide (n = 223) or placebo (n = 223) for 24 weeks while continuing on insulin glargine. At study end, A1C was –0.32% lower in lixisenatide-treated patients, P < 0.0001, and there was a significant improvement in 2-h prandial glycemia versus the placebo (12).
In patients with type 2 diabetes already receiving insulin, GetGoal-L compared the addition of 20 μg lixisenatide once daily versus a placebo. After 24 weeks, lixisenatide significantly reduced A1C and improved 2-h prandial glycemia. A greater proportion of patients receiving lixisenatide achieved A1C <7.0% compared with placebo treatment (28 vs. 12%; P < 0.0001) (13). The GetGoal studies lend further support to the theory that short-acting GLP-1RAs, such as lixisenatide, are effective in targeting prandial glycemia.
The combination of long-acting GLP-1RAs with basal insulin is currently being investigated. A recent study paired insulin glargine with either albiglutide or prandial insulin lispro. Basal insulin–treated patients were randomly allocated to receive either once-weekly 30 mg albiglutide (n = 282) or insulin lispro (n = 281) while continuing on metformin or thiazolidinedione. After 26 weeks of treatment, A1C was –0.82 and –0.66% with albiglutide and insulin lispro, respectively. This study demonstrates a potential alternative treatment regimen for patients unable or unwilling to adhere to a prandial insulin injection schedule (14).
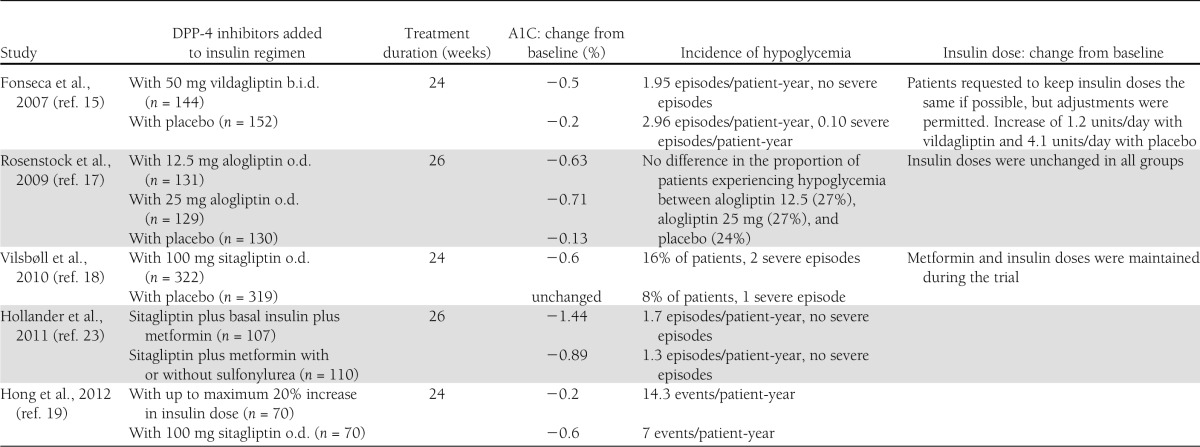
In studies where DPP-4 inhibitors have been added to basal insulin, there have also been improvements in glycemic control. In a study of 296 patients with poorly controlled glycemia (mean A1C 8.4%) requiring high doses of basal insulin (≥30 units/day), either 50 mg vildagliptin twice daily or a placebo was added for a 24-week period (15). At study end, A1C had decreased by –0.5% in the vildagliptin-treated patients and –0.2% in the placebo group (P = 0.01). In a 28-week extension, where placebo-treated subjects were switched to 50 mg vildagliptin once daily (16), patients continuing on 100 mg/day vildagliptin maintained their lower A1C through to 52 weeks, and those switched from placebo to vildagliptin 50 mg once daily benefited from a reduction of –0.4% in A1C.
Another DPP-4 inhibitor study compared the addition of alogliptin (12.5 or 25 mg) or placebo once daily to a basal insulin plus metformin treatment regimen in 390 patients with mean A1C 9.3% (17). During the course of the study, insulin doses were kept constant to allow direct comparisons of A1C to be made between treatment arms. At 26 weeks, alogliptin significantly (P < 0.001) reduced A1C, by –0.63 and –0.71% in the 12.5 and 25 mg/day treated groups, respectively, compared with a –0.13% reduction with placebo (P < 0.001). FPG was largely unchanged during the study (0.1 mmol/L increase with 12.5 mg and –0.6 mmol/L with 25 mg alogliptin), suggesting that the reduction in A1C was largely attributable to lower PPG. Given that the participants in the study had been diagnosed with type 2 diabetes for >12 years, it is possible to theorize that β-cell output was limited and that a significant proportion of the improvement in A1C was the result of enhanced α-cell function. This hypothesis may also be applied to GLP-1RAs; however, further investigation is required to substantiate this.
The effect of combining sitagliptin with basal insulin has been evaluated in a controlled 24-week study (18). As with previous investigations, the participants (n = 641) had a long history of diabetes (diagnosis >12 years) and poorly controlled glycemia (A1C 8.6%). As in the previously described study, insulin and metformin doses were not altered during the course of the study. At end of trial, once-daily 100 mg sitagliptin produced a reduction of –0.6% in A1C, with no change in a placebo-treated group (P < 0.001). Concurring with earlier studies, a reduction in 2-h PPG was the primary factor in improved glycemic control with placebo-corrected reductions of –2.0 mmol/L postmeals and –0.8 mmol/L in FPG.
A 24-week study in patients with poorly controlled A1C (7.5–11%) demonstrated that compared with increasing the basal insulin dose by up to 25%, adding sitagliptin to an insulin-based regimen lowered A1C more effectively (–0.6 vs. –0.2%; P < 0.01) and was associated with lower rates of hypoglycemia (7 vs. 14.3 events per patient-year) and less weight gain (19).
Adding insulin to incretin-based therapies.
Thus far, the studies in this review have only considered the addition of incretin-based therapies to basal insulin; however, there have also been studies examining the reverse situation: initiating insulin therapy in patients already receiving incretin-based therapies.
In one study, 38 patients (duration of diabetes 8.5 years) received exenatide (maximum dose 10 μg twice daily) plus metformin for an 8-week run-in period, after which they were randomly assigned to start either insulin glargine plus continued exenatide injections or insulin glargine with placebo injections (discontinuing exenatide) (20). After a further 24 weeks, A1C was lower in patients continuing on exenatide, falling from baseline values of 7.8–6.45%, compared with 7.3% in patients receiving placebo injections, although the treatment difference was not significant (P = 0.06). A larger proportion of patients continuing with exenatide (76%) reached a target A1C of <7.0% compared with those receiving the placebo (24%, P = 0.003).
One of the largest studies to assess the combination of GLP-1RAs and insulin was a prospective investigation involving 988 insulin-naïve patients with type 2 diabetes (21). Patients failing to control glycemia with metformin plus or minus sulfonylurea underwent a 12-week run-in period, during which they received ≥1,500 mg/day metformin with liraglutide (1.8 mg once daily) and discontinued sulfonylurea: 61% of patients reached a target A1C of <7.0%. After the run-in had completed, patients with A1C >7.0% were randomly allocated to either continue on metformin plus liraglutide or add insulin detemir (titrated to maintain FPG at 4.0–6.0 mmol/L) for an additional 26 weeks. At the end of the study, an A1C reduction of –0.51% was observed in patients treated with insulin detemir plus liraglutide, whereas those continuing on liraglutide plus metformin saw no further decrease in A1C after the run-in period. The results of this study show that combining basal insulin with a GLP-1RA provides an effective treatment regimen for patients failing to meet target A1C concentrations with GLP-1RAs alone. Despite the lack of an insulin-only control group after the run-in period, which prevents the conclusion that the addition of insulin was solely responsible for improvements in A1C, this study, in conjunction with other studies described above, provides compelling evidence in support of this theory.
The benefit of adding insulin to GLP-1RAs was confirmed in a study in which two different insulins, either protaminated insulin lispro or insulin glargine, were added to exenatide (used for >3 months) in patients with type 2 diabetes (duration of diabetes 9.9 years) (22). Reductions in A1C of –1.16 and –1.40% were observed for insulin lispro and insulin glargine, respectively. The design of this investigation did not incorporate insulin-only or exenatide-only treatment arms. As with the insulin detemir study (21), this limits the interpretation of the results but supports the overall conclusion of a benefit from combination therapy.
Research into the effects of adding insulin to DPP-4 inhibitors has been limited. However, one recently published study, involving 217 patients failing to control glycemia with metformin plus or minus sulfonylurea, compared the simultaneous addition of sitagliptin plus insulin detemir once daily (discontinuing sulfonylurea) versus sitagliptin alone over a 26-week period (23). At the end of the trial, A1C had decreased by –1.44% with detemir plus sitagliptin. This was a significant (P < 0.001) improvement over sitagliptin alone where a reduction of –0.89% was observed. Unsurprisingly, patients treated with insulin detemir had a greater reduction in FPG (–3.7%) than patients receiving only sitagliptin: –1.2 mmol/L, P < 0.001. Reductions in SMPG profiles were also significantly greater with sitagliptin plus insulin detemir compared with sitagliptin alone. These results indicate that combining DPP-4 inhibitors with insulin provides a greater benefit to glycemic control than DPP-4 inhibitors alone. The lack of an active comparator (placebo plus detemir) in this study is unfortunate, since the relative contributions of detemir and sitagliptin to A1C reduction cannot be determined.
Body weight.
Increased body weight is a common, undesirable side effect of insulin therapy (24). In contrast, GLP-1RAs have consistently led to reductions in body weight (25). The improvements in glycemic control that result from combining incretin-based therapies with insulin allow reductions in insulin dose to be made—further enhancing the weight loss potential of incretin-based therapies. For example, in the insulin plus exenatide study conducted by Yoon et al. (5), body weight was reduced markedly from baseline values, falling –2.4 kg (P < 0.001) at 6 months and –6.2 kg (P < 0.001) at 12–18 months. An even larger reduction in body weight was recorded in a retrospective study in obese patients with type 2 diabetes (n = 160) (26). Here, 12 months after initiating exenatide in patients already receiving basal insulin, mean weight loss was 12.8 kg, revealing the potential for treating obese patients with type 2 diabetes with insulin plus incretin.
When basal insulin has been added to GLP-1RAs there have been differing results, with some studies showing that increases in body weight have been mitigated and others revealing no difference (20,22,27). Unlike GLP-1RAs, DPP-4 inhibitors in combination with basal insulin have not yielded reductions in body weight, with studies instead reporting a neutral effect (Fig. 1).
Figure 1.
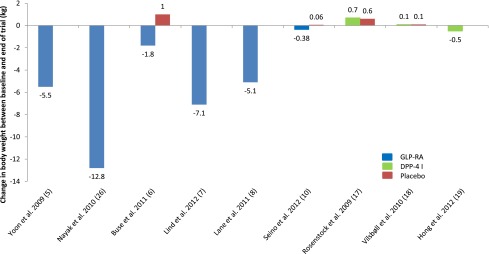
Adding incretin-based therapies to insulin. Change in body weight (kg) between baseline and end of trial for GLP-1RAs and DPP-4 inhibitors (DPP-4 I) compared with placebo treatment where available.
Insulin dose.
The combination of incretin-based therapies and basal insulin offers the prospect of reducing the insulin dose requirement. This is an attractive proposition owing to the likelihood of further decreases in the risk of hypoglycemia and weight gain.
The majority of studies in which GLP-1RAs have been added to basal insulin regimens demonstrated significant reductions in insulin dose (Table 1), and depending on patient characteristics, these have ranged between 15 and 63% (5,6,26). With use of the association between insulin dose and weight gain, GLP-1RAs have been successfully used as an insulin-sparing tactic in obese patients with type 2 diabetes (8,26). For example, one study set out with the aim of reducing the insulin dose in very insulin-resistant patients and achieved a 28% reduction in the total daily insulin dose alongside improved A1C (8). Some patients have completely discontinued insulin after starting GLP-1RAs, but the results of the Association of British Diabetologists audit revealed that complete discontinuation of basal insulin, upon initiation of exenatide treatment, led to a worsening of glycemic control for ∼50% of patients. Therefore, discontinuation of basal insulin therapy is not advised (28). The Association of British Diabetologists audit also revealed evidence of a trade-off between blood glucose control and body weight when insulin dose is reduced. One study has shown that reductions in insulin dose often fail to be sustained after 12 months (5), possibly owing to the progressive nature of type 2 diabetes. In another study where basal insulin was substituted with exenatide, it was concluded that while it is feasible to replace insulin with GLP-1RAs in some patients, those with a longer duration of diabetes, receiving higher doses of insulin (and with less endogenous β-cell function), were at greater risk of failing to maintain glycemic control (29).
Table 1.
Studies where GLP-1RAs have been added to insulin
In most studies in which DPP-4 inhibitors have been added to basal insulin, protocols have specified that insulin dose remains unchanged throughout the investigation; therefore, further research is needed to establish whether DPP-4 inhibitors offer the same advantage as GLP-1RAs in terms of scope for insulin dose reduction.
Practical considerations
As with all new therapies, it takes time for the most effective treatment strategies to emerge; however, the studies to date support the complementary actions of basal insulin and incretins in combination regimens, regardless of the sequence of their introduction, as elaborated below. There are differences between GLP-1RAs and DPP-4 inhibitors that should be taken into account when combining them with basal insulin therapy.
The evidence suggests that GLP-1RAs tend to produce better blood glucose–lowering results than DPP-4 inhibitors (30). The impact of this difference in efficacy might be more important in patients with markedly elevated PPG levels, who need to achieve a greater reduction than those in whom PPG levels are only mildly elevated.
DPP-4 inhibitors have the advantage of being oral agents, but, since administration of basal insulin obliges injections, this should not be an overriding factor in treatment selection. Depending on the products chosen, patients are often able to use identical injection devices for both components of an insulin and GLP-1RA regimen. Moreover, although injections have been traditionally perceived to be a barrier to treatment, the subcutaneous route of administration with GLP-1RAs does not appear to affect patient satisfaction (31).
Gastrointestinal side effects such as nausea are commonly reported after initiating treatment with GLP-1RAs; however, this tends to be transient (32). If the patient has already been established on GLP-1RA treatment, nausea will not be an issue upon starting basal insulin. In the reverse situation, when GLP-1RAs are being added to basal insulin, tolerability can be optimized by dose titration of the incretin. Clinical trials have also shown diarrhea to be a problem in some patients (33–36). Persistent gastrointestinal side effects could have a negative impact on long-term adherence to combination regimens.
The pharmacokinetic profile of incretin-based therapies should be considered when targeting fasting or prandial glycemia. Short-acting GLP-1RAs such as lixisenatide are most effective at lowering prandial glycemia, partly due to slower gastric emptying, whereas long-acting GLP-1RAs and DPP-4 inhibitors additionally benefit fasting glycemia. The long-acting GLP-1RAs (e.g., liraglutide and exenatide once weekly) are most effective at lowering A1C and may be best suited for combination therapy in patients who are well above target A1C levels.
The study data show that basal insulin can be added to incretin therapies and vice versa with the expectation of clinical benefits. Specifically, a large proportion of patients starting on metformin and then sequentially adding GLP-1RA and insulin achieve A1C targets of <7% (20,21). Furthermore, adding basal insulin to incretins obviates the need for downward dose titration of the insulin. Adding an incretin to basal insulin is, however, also worthwhile, since it can reduce the basal insulin dose requirement and thereby reduce hypoglycemia risk and facilitate weight management. Dose adjustment of the insulin is advisable at least when a GLP-1RA is added, but this regimen is nevertheless likely to be a better tolerated intensification step than addition of bolus insulin.
Several studies have reported very low rates of hypoglycemia for insulin plus incretin regimens—in some cases at placebo levels (Tables 1–2). Severe hypoglycemia is rare, with only isolated cases reported across the studies published thus far (6,16,17).
Table 2.
Studies where DPP-4 inhibitors have been added to basal insulin
Sitagliptin, vildagliptin, saxagliptin, and alogliptin are predominantly renally excreted and require dose reductions to be made when prescribed to patients with moderate or severe renal impairment and with end-stage renal disease. Exenatide, also predominantly renally excreted, is not recommended for patients with severe renal impairment (creatinine clearance <30 mL/min) or end-stage renal disease. Conversely, linagliptin has a much lower rate of renal excretion and is better suited for patients with renal impairment—requiring no dose adjustment. Liraglutide also has a lower rate of renal excretion, compared with DPP-4 inhibitors, but should be used with caution (and with dose adjustment), as data on its use in renally impaired patients are limited. Given that renal impairment can occur at any time as a complication of diabetes, it is advisable to monitor renal function regularly in patients who are taking renally excreted drugs.
Regarding patients with hepatic impairment, vildagliptin is not recommended. Sitagliptin, saxagliptin, linagliptin, alogliptin, exenatide, and liraglutide are considered suitable for use in patients with hepatic impairment without dose adjustment.
Exenatide and liraglutide both reduce blood pressure, body weight, and plasma lipid profiles in subjects with type 2 diabetes (37), while DPP-4 inhibitors provide small but significant improvements in blood-lipid profiles. This makes the combination of incretin-based therapies with insulin an attractive prospect for patients with or at risk for cardiovascular disease (38,39). Finally, the weight-sparing effects of GLP-1RAs make them especially appealing for use in obese patients with type 2 diabetes who require insulin (40).
GLP-1RAs and DPP-4 inhibitors are relatively new drugs, and cost remains a limiting factor in their use, particularly in state-funded health care systems such as the National Health Service. The economic advantages of combining incretin-based therapies with basal insulin have not been well explored, but this approach may provide a cost-effective alternative to other treatments. Hypoglycemia places a large financial burden on health care payers (41,42), and the low rate of hypoglycemia demonstrated in insulin plus incretin regimens may help to limit this. If combination therapy can be shown to slow the progression of diabetes, additional cost savings can be anticipated.
Summary
In conclusion, combining incretin-based therapies with basal insulin provides complementary actions, lowering both PPG and FPG, to improve glycemic control in type 2 diabetes. Theoretically, incretin-based therapies are an alternative to bolus insulin for certain groups of patients, such as the elderly, where meeting A1C targets must be balanced against the risk of hypoglycemia; however, studies evaluating any potential benefit in the elderly have not been conducted. Additionally, the weight-sparing effect of GLP-1RAs makes them well suited for use in patients with concerns about insulin-induced weight gain. The improvements in glycemic control may reduce the incidence of diabetes-related complications, and taken together with the reduced risk of hypoglycemia, an incretin plus basal insulin regimen could provide significant health-economic advantages. Further research is needed to establish the long-term benefits of insulin plus incretin therapy.
Acknowledgments
J.V. has received support for research and attendance for national and international educational meetings and honoraria for lecturing and advisory boards from Novo Nordisk, Lilly, Sanofi, MSD, Takeda, Novartis, and Abbott. No other potential conflicts of interest relevant to this article were reported.
J.V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The author thanks Paul Tisdale and Gabrielle Parker of Watermeadow Medical, U.K. (supported by Novo Nordisk, Bagsvaerd, Copenhagen) for assistance in drafting the manuscript.
Footnotes
This publication is based on the presentations from the 4th World Congress on Controversies to Consensus in Diabetes, Obesity and Hypertension (CODHy). The Congress and the publication of this supplement were made possible in part by unrestricted educational grants from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Ethicon Endo-Surgery, Janssen, Medtronic, Novo Nordisk, Sanofi, and Takeda.
References
- 1.Mudaliar S, Henry RR. Effects of incretin hormones on beta-cell mass and function, body weight, and hepatic and myocardial function. Am J Med 2010;123(Suppl.):S19–S27 [DOI] [PubMed] [Google Scholar]
- 2.Drucker DJ. The biology of incretin hormones. Cell Metab 2006;3:153–165 [DOI] [PubMed] [Google Scholar]
- 3.Tews D, Werner U, Eckel J. Enhanced protection against cytokine- and fatty acid-induced apoptosis in pancreatic beta cells by combined treatment with glucagon-like peptide-1 receptor agonists and insulin analogues. Horm Metab Res 2008;40:172–180 [DOI] [PubMed] [Google Scholar]
- 4.Bosi E. Time for testing incretin therapies in early type 1 diabetes? J Clin Endocrinol Metab 2010;95:2607–2609 [DOI] [PubMed] [Google Scholar]
- 5.Yoon NM, Cavaghan MK, Brunelle RL, Roach P. Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther 2009;31:1511–1523 [DOI] [PubMed] [Google Scholar]
- 6.Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in Basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med 2011;154:103–112 [DOI] [PubMed] [Google Scholar]
- 7.Lind M, Jendle J, Torffvit O, Lager I. Glucagon-like peptide 1 (GLP-1) analogue combined with insulin reduces HbA1c and weight with low risk of hypoglycemia and high treatment satisfaction. Prim Care Diabetes 2012;6:41–46 [DOI] [PubMed] [Google Scholar]
- 8.Lane W, Weinrib S, Rappaport J. The effect of liraglutide added to U-500 insulin in patients with type 2 diabetes and high insulin requirements. Diabetes Technol Ther 2011;13:592–595 [DOI] [PubMed] [Google Scholar]
- 9.Yabe D, Watanabe K, Sugawara K, et al. Comparison of incretin immunoassays with or without plasma extraction: incretin secretion in Japanese patients with type 2 diabetes. J Diabetes Invest 2012;3:70–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seino Y, Min KW, Niemoeller E, Takami A, EFC10887 GETGOAL-L Asia Study Investigators Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab 2012;14:910–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fonseca VA, Alvarado-Ruiz R, Raccah D, Boka G, Miossec P, Gerich JE, EFC6018 GetGoal-Mono Study Investigators Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes (GetGoal-Mono). Diabetes Care 2012;35:1225–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenstock J, Forst T, Aronson R, et al. Efficacy and safety of once-daily lixisenatide added on to titrated glargine plus oral agents in type 2 diabetes: GetGoal-Duo 1 Study (Abstract). Diabetes 2012;61(Suppl. 1):A18 [Google Scholar]
- 13.Riddle MC, Home P, Marre M, Niemoeller E, Ping L, Rosenstock J. Efficacy and safety of once-daily lixisenatide in type 2 diabetes insufficiently controlled with basal insulin ± metformin: GetGoal-L Study (Abstract). Diabetes 2012;61(Suppl. 1):A251 [Google Scholar]
- 14.Rosenstock J, Ahrén BO, Chow FC, et al. Once-weekly GLP-1 receptor agonist albiglutide vs. titrated prandial lispro added on to titrated basal insulin glargine in type 2 diabetes (T2D) uncontrolled on glargine plus oral agents: similar glycemic control with weight loss and less hypoglycemia (Abstract). Diabetes 2012;61(Suppl. 1):A15 [Google Scholar]
- 15.Fonseca V, Schweizer A, Albrecht D, Baron MA, Chang I, Dejager S. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia 2007;50:1148–1155 [DOI] [PubMed] [Google Scholar]
- 16.Fonseca V, Baron M, Shao Q, Dejager S. Sustained efficacy and reduced hypoglycemia during one year of treatment with vildagliptin added to insulin in patients with type 2 diabetes mellitus. Horm Metab Res 2008;40:427–430 [DOI] [PubMed] [Google Scholar]
- 17.Rosenstock J, Rendell MS, Gross JL, Fleck PR, Wilson CA, Mekki Q. Alogliptin added to insulin therapy in patients with type 2 diabetes reduces HbA(1C) without causing weight gain or increased hypoglycaemia. Diabetes Obes Metab 2009;11:1145–1152 [DOI] [PubMed] [Google Scholar]
- 18.Vilsbøll T, Rosenstock J, Yki-Järvinen H, et al. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2010;12:167–177 [DOI] [PubMed] [Google Scholar]
- 19.Hong ES, Khang AR, Yoon JW, et al. Comparison between sitagliptin as add-on therapy to insulin and insulin dose-increase therapy in uncontrolled Korean type 2 diabetes: CSI study. Diabetes Obes Metab 2012;14:795–802 [DOI] [PubMed] [Google Scholar]
- 20.Riddle M, Ahmann A, Basu A, Aroda V, Ratner R. Metformin+exenatide+basal insulin vs metformin+placebo+basal insulin: reaching A1c <6.5% without weight-gain or serious hypoglycemia (Abstract). Diabetes 2010;57(Suppl. 1):LB6 [Google Scholar]
- 21.DeVries JH, Bain SC, Rodbard HW, et al. Liraglutide-Detemir Study Group Sequential intensification of metformin treatment in type 2 diabetes with liraglutide followed by randomized addition of basal insulin prompted by A1C targets. Diabetes Care 2012;35:1446–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blevins TC, Arakaki RF, Liljenquist DR. Once-daily basal insulin added to oral antihyperglycemic medications (OAMs) and exenatide (Ex) improves glycemic control in patients (Pts) with type 2 diabetes (T2D) (Abstract). Diabetes 2010;57(Suppl. 1):LB6 [Google Scholar]
- 23.Hollander P, Raslova K, Skjøth TV, Råstam J, Liutkus JF. Efficacy and safety of insulin detemir once daily in combination with sitagliptin and metformin: the TRANSITION randomized controlled trial. Diabetes Obes Metab 2011;13:268–275 [DOI] [PubMed] [Google Scholar]
- 24.Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes—causes, effects and coping strategies. Diabetes Obes Metab 2007;9:799–812 [DOI] [PubMed] [Google Scholar]
- 25.Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 2012;344:d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nayak UA, Govindan J, Baskar V, Kalupahana D, Singh BM. Exenatide therapy in insulin-treated type 2 diabetes and obesity. QJM 2010;103:687–694 [DOI] [PubMed] [Google Scholar]
- 27.Levin PA, Mersey JH, Zhou S, Bromberger LA. Clinical outcomes using long-term combination therapy with insulin glargine and exenatide in patients with type 2 diabetes. Endocr Pract 2012;18:17–25 [DOI] [PubMed] [Google Scholar]
- 28.Thong KY, Jose B, Sukumar N, et al. ABCD Nationwide Exenatide Audit Contributors Safety, efficacy and tolerability of exenatide in combination with insulin in the Association of British Clinical Diabetologists nationwide exenatide audit. Diabetes Obes Metab 2011;13:703–710 [DOI] [PubMed] [Google Scholar]
- 29.Davis SN, Johns D, Maggs D, Xu H, Northrup JH, Brodows RG. Exploring the substitution of exenatide for insulin in patients with type 2 diabetes treated with insulin in combination with oral antidiabetes agents. Diabetes Care 2007;30:2767–2772 [DOI] [PubMed] [Google Scholar]
- 30.Aroda VR, Henry RR, Han J, et al. Efficacy of GLP-1 receptor agonists and DPP-4 inhibitors: meta-analysis and systematic review. Clin Ther 2012;34:1247–1258, e22 [DOI] [PubMed] [Google Scholar]
- 31.Pratley RE, Nauck M, Bailey T, et al. 1860-LIRA-DPP-4 Study Group Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet 2010;375:1447–1456 [DOI] [PubMed] [Google Scholar]
- 32.Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care 2011;34(Suppl. 2):S279–S284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD, Exenatide-113 Clinical Study Group Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004;27:2628–2635 [DOI] [PubMed] [Google Scholar]
- 34.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005;28:1092–1100 [DOI] [PubMed] [Google Scholar]
- 35.Garber A, Henry R, Ratner R, et al. LEAD-3 (Mono) Study Group Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009;373:473–481 [DOI] [PubMed] [Google Scholar]
- 36.Nauck M, Frid A, Hermansen K, et al. LEAD-2 Study Group Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009;32:84–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buse JB, Rosenstock J, Sesti G, et al. LEAD-6 Study Group Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009;374:39–47 [DOI] [PubMed] [Google Scholar]
- 38.Eliasson B, Möller-Goede D, Eeg-Olofsson K, et al. Lowering of postprandial lipids in individuals with type 2 diabetes treated with alogliptin and/or pioglitazone: a randomised double-blind placebo-controlled study. Diabetologia 2012;55:915–925 [DOI] [PubMed] [Google Scholar]
- 39.Monami M, Lamanna C, Desideri CM, Mannucci E. DPP-4 inhibitors and lipids: systematic review and meta-analysis. Adv Ther 2012;29:14–25 [DOI] [PubMed] [Google Scholar]
- 40.Astrup A, Rössner S, Van Gaal L, et al. NN8022-1807 Study Group Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 2009;374:1606–1616 [DOI] [PubMed] [Google Scholar]
- 41.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 2011;365:2002–2012 [DOI] [PubMed] [Google Scholar]
- 42.Quilliam BJ, Simeone JC, Ozbay AB, Kogut SJ. The incidence and costs of hypoglycemia in type 2 diabetes. Am J Manag Care 2011;17:673–680 [PubMed] [Google Scholar]


