Abstract
Background & Aims
Treatment of inflammatory bowel disease (IBD) would benefit from specific targeting of therapeutics to the intestine. We developed a strategy for localized delivery of the immunosuppressive cytokine IL27, which is actively synthesized in situ by the food-grade bacterium Lactococcuslactis (LL-IL-27), and tested its ability to reduce colitis in mice.
Methods
The 2 genes encoding mouse IL27 were synthesized with optimal codon usage for L lactis and joined with a linker; a signal sequence was added to allow for secretion of the product. The construct was introduced into L lactis. Colitis was induced via transfer of CD4+CD45RBhi T cells into Rag−/− mice to induce colitis; 7.5 weeks later, LL-IL-27 was administered to mice via gavage. Intestinal tissues were collected and analyzed.
Results
LL-IL-27 administration protected mice from T-cell transfer-induced enterocolitis and death. LL-IL-27 reduced disease activity scores, pathology features of large and small bowel, and levels of inflammatory cytokines in colonic tissue. LL-IL-27 also reduced numbers of CD4+ and IL17+ T cells in gut-associated lymphoid tissue. The effects of LL-IL-27 required production of IL10 by the transferred T cells. LL-IL-27 was more effective than either LL-IL-10 or systemic administration of recombinant IL27 in reducing colitis in mice. LL-IL-27 also reduced colitis in mice following administration of dextran sodium sulfate.
Conclusions
L lactis engineered to express IL27 (LL-IL-27) reduces colitis in mice, by increasing production of IL10. Mucosal delivery of LL-IL-27 could be a more effective and safer therapy for IBD.
Keywords: mouse model, Crohn’s Disease, ulcerative colitis, immune regulation
Introduction
Inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis, is a significant public health problem in Western societies, affecting 1 in 1000 individuals, and is characterized by chronic, nonspecific inflammation in the large and/or small intestine1. IBD greatly predisposes to colorectal cancer, in that twenty percent of ulcerative colitis patients will develop it unless the colon is surgically removed2. It is currently thought that IBD represents an atypical inflammatory immune response to normal gut flora3, 4.
The existing treatments for IBD include anti-inflammatory drugs, immunosuppressive drugs, and, in severe cases, partial or complete resection of the bowel. Use of therapeutics resulting in total immunosuppression risks compromising protection against pathogens such as viruses and bacteria. Selective delivery to the target organ would be desirable. IL-10, for example, is an anti-inflammatory cytokine that has a protective role in both mouse5 and human6 IBD; however, systemic IL-10 treatment has yielded rather disappointing results in multicenter trials7, 8 most likely due to low final concentrations of IL-10 in the intestine.
IL-27, a pleiotropic cytokine belonging to the IL-12 family, is composed of IL-27p28 and Epstein Barr virus–induced protein 3 (Ebi3)9. It is primarily expressed by antigen presenting cells and signals through a heterodimeric receptor (IL-27R) that contains a unique IL-27Rα (WSX-1, TCCR) subunit and a gp130 subunit, which is shared by several cytokine receptors in the IL-6 family10.
IL-27 was initially described as an immune stimulator of TH1 responses9; however, recent studies have identified mechanisms in which IL-27 has an immunosuppressive role11, 12 including its ability to antagonize TH17 development13–16, induce IL-10 production12, 16–18, suppress IL-6–induced T cell proliferation13, and promote Treg generation19. Furthermore, a therapeutic effect in experimental allergic encephalomyelitis15, collagen-induced arthritis20, and colitis21 was observed following IL-27 administration, and in a genome-wide association study, low expressing variants of the IL-27 gene were found to be associated specifically with human early onset IBD22.
In this study, we investigated mucosal delivery of IL-27 using a well-described delivery system that enables oral delivery of biopharmaceuticals to the gastrointestinal tract by genetically engineered Lactococcus lactis (L. lactis)23–25. We show that LL-IL-27 has a therapeutic benefit in T cell-dependent chronic enterocolitis suggesting it may offer a safer, more effective treatment option for IBD patients.
Results
Genetically engineered L lactis express bioactive IL-27
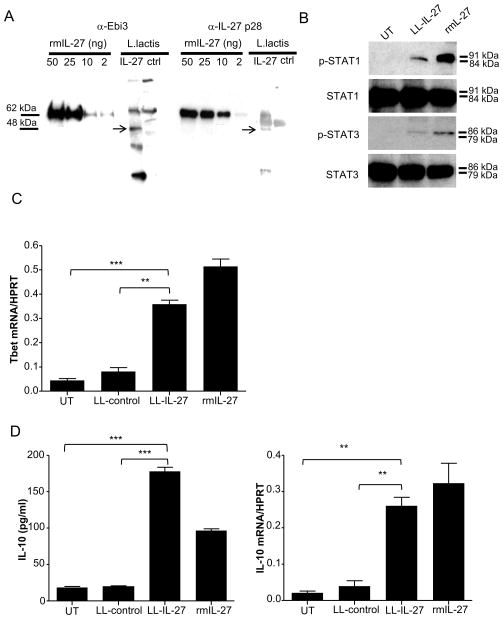
Murine IL-27 was synthesized in L lactis by incorporating a linker between its two chains, and using codons and a secretory signal sequence preferred by L lactis (LL-IL-27) (Supplementary Fig. 1). Culture supernatants of LL-IL-27 were analyzed by western blot, showing that LL-IL-27 expressed the Ebi3 (Fig. 1A, left) and p28 (Fig. 1A, right) subunits of IL-27 at the predicted molecular weight of the IL-27 hyperkine (48.2 kDa).
Figure 1.
Genetically engineered L. lactis express active IL-27. (A) Ebi3 (left) and IL-27 p28 (right) protein were detected in supernatants of LL-IL-27 by western blot. Murine CD4+ T cells were stimulated with anti-CD3/CD28 in the presence of LL-IL-27 or rmIL-27. Bioactivity of LL-IL-27 was confirmed through (B) phosphorylation of STAT1/3, (C) induction of Tbet mRNA expression, and (D) increased IL-10 protein and gene expression measured by ELISA and RT-PCR, respectively. Data representative of three independent experiments. Data represents mean ± s.e.m. **P ≤ .01, *** P ≤ .005, determined by two-tailed Student’s t test.
LL-IL-27 induced phosphorylation of STAT1 and STAT3 albeit to a lesser degree than rmIL-27 at comparable concentrations (Fig. 1B). TH1 transcription regulator Tbet was upregulated by LL-IL-27 stimulation of naïve CD4+ T cells (Fig. 1C). LL-IL-27 stimulated both IL-10 protein secretion (Fig. 1D, left) and gene expression (Fig. 1D, right) to comparable levels as rmIL-27 in CD4+ cells. Neutralizing rmIL-27 and LL-IL-27 with IL-27 antibodies resulted in similar inhibition levels in all functional assays (Supplementary Fig. 2), confirming that LL-IL-27’s bioactivity is mediated by IL-27.
We investigated LL-IL-27’s localization and ability to induce IL-10 in vivo. Healthy C57BL/6 mice were administered serial gavages of LL-IL-27 and GI tract sections were assayed. The majority of L lactis was found in the intestinal lumen (Supplementary Fig. 3A), more than 80% of gavaged L lactis was recovered (Supplementary Fig. 3B), and increased IL-10 levels were found in intestinal luminal contents of LL-IL-27-treated mice compared to LL-control-treated mice (Supplementary Fig. 3C).
LL-IL-27 treatment improves survival in murine enterocolitis
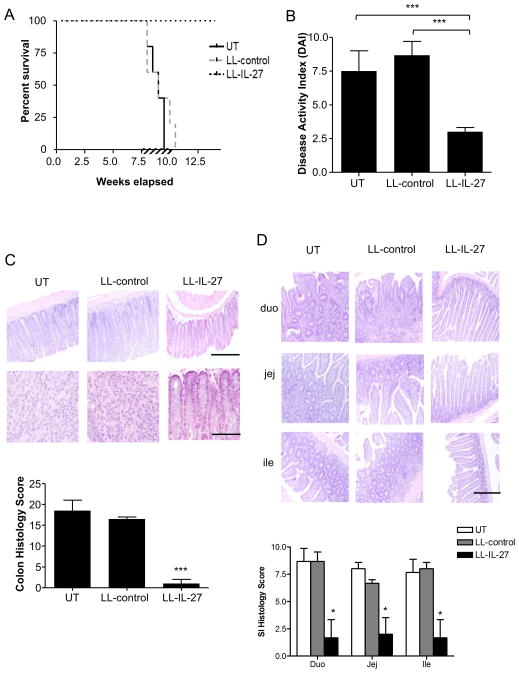
Transferring CD4+CD45RBhi T cells from healthy wildtype mice into Rag−/− mice induces a diffuse enterocolitis at 5–8 weeks following T cell transfer26. Gavages of BM9 media23 (untreated), LL-control or LL-IL-27 were begun 7.5 weeks following naïve T cell transfer and continued for 2 weeks. By week 8 post-transfer, untreated and LL-control-treated mice began to die or had to be euthanized due to extent of disease, and by 10.5 weeks, all had succumbed to disease. In contrast, LL-IL-27-treated mice were protected from death (Fig. 2A). A disease activity index (DAI) was used that reflects several parameters of IBD27. LL-IL-27-treated mice did not show occult/gross blood in stool, stool consistency was nearly normal, whereas weight loss was partially relieved, thus contributing to a decreased DAI (Fig. 2B). Histopathological analysis of distal colons demonstrated that LL-IL-27-treated mice had normal morphology, while untreated and LL-control-treated mice had extensive inflammatory infiltration and goblet cell loss (Fig. 2C). LL-IL-27-treated mice also had less pathology in the small intestine compared to untreated and LL-control-treated mice (Fig. 2D).
Figure 2.
LL-IL-27 improves survival in T cell transfer induced enterocolitis. CD4+CD45Rbhi T cells from C57BL/6 mice were transferred to Rag−/− mice. Following onset of enterocolitis around 7.5 weeks, mice were gavaged with BM9 media (untreated, UT), LL-control, or LL-IL-27 for 14 days (shown by hatched bar). (A) Percent survival following T cell transfer (n=5, data representative of three independent experiments), (B) Clinical disease activity index (DAI) was determined at time of death (UT, LL-control) or 7 days after the last gavage (LL-IL-27). (C) Representative H&E-stained sections of distal colons (top). Scale bar (top row) = 100 μm, (bottom row) = 25 μm. Histopathological scores were determined for the distal colon (bottom). (D) Representative H&E-stained sections of small intestines (SI) (duodenum=duo, jejunum=jej, ileum=ile) (top). Scale bar = 100 μm. Histopathological scores were determined for each section of the SI (bottom). Data represents mean ± s.e.m. *P ≤ .05, ***P ≤ .005, determined by two-tailed Student’s t test.
To verify whether treatment with LL-IL-27 had a negative consequence on intestinal barrier function, we used the limulus amoebocyte lysate (LAL) assay to measure LPS in the plasma. Our analysis showed comparable LPS levels among healthy, untreated, LL-control-, and LL-IL-27-treated mice indicating an intact intestinal barrier (Supplementary Fig. 4). We also tested whether LL-IL-27 increased susceptibility to the intestinal pathogen Citrobacter rodentium. LL-control- and LL-IL-27-treated mice had similar body weights (Supplementary Fig. 5A) as untreated mice, but had lower CFU in fecal material, colon, spleen (Supplementary Fig. 5B), and liver (Supplementary Fig. 5B), demonstrating that LL-IL-27 does not exacerbate infection by an enteric pathogen.
To determine if LL-IL-27 was effective in a different mouse model of colitis, independent of T cells, acute colitis induced by dextran sulfate sodium (DSS) was evaluated. Although LL-IL-27 treatment did not protect from weight loss (Supplementary Fig. 6A), stool consistency was normal (Supplementary Fig. 6B) and there was no occult/gross blood in the stool (Supplementary Fig. 6C), resulting in a lower DAI (Supplementary Fig. 6D).
LL-IL-27 is more effective than systemic IL-27 treatment in T cell induced colitis
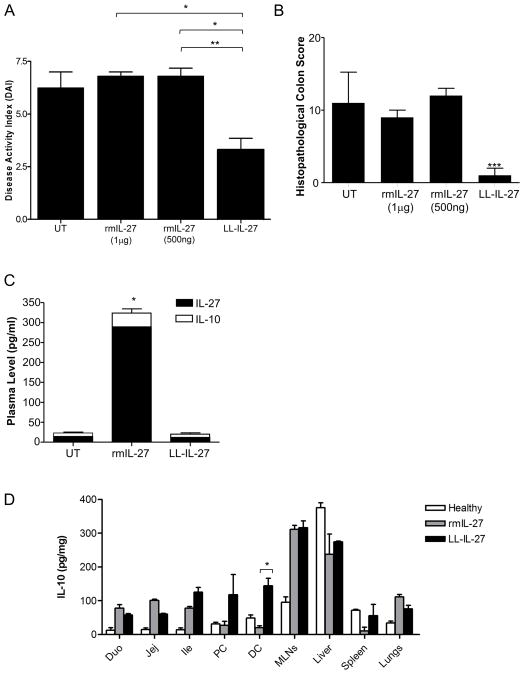
To compare LL-IL-27 with systemically administered IL-27 protein, recombinant mouse (rm) IL-27 was injected intraperitoneally for five days and compared with LL-IL-27 by gavage. Although LL-IL-27 treatment over five days was somewhat less effective than a two week treatment, it reduced the DAI by about half (Fig. 3A) and eliminated microscopic lesions (Fig. 3B). By comparison, systemic rmIL-27 had no therapeutic effect (Fig. 3A and B). Following rmIL-27 treatment, IL-27 was readily detectable in plasma and induced circulating IL-10 (Fig. 3C); however, IL-10 levels in the distal colon were lower compared to mice receiving LL-IL-27 (Fig. 3D). In healthy mice, LL-IL-27 did not induce higher IL-10 levels than rmIL-27 in any tissue analyzed(Supplementary Fig. 7).
Figure 3.
Systemic rmIL-27 treatment does not improve disease activity. CD4+CD45RbhiT cells were transferred to Rag−/− mice. Following the onset of enterocolitis at 7.5 weeks, mice were gavaged with LL-IL-27 or were intraperitoneally injected with rmIL-27 for 5 consecutive days. Tissues were harvested three days following the last treatment. (A) Clinical disease activity index (DAI) was determined at day of harvest. (B) Histopathological scores were determined for the distal colon. (C) Plasma was analyzed for IL-27 and IL-10. (D) Tissue homogenates were analyzed for IL-10. Duo: duodenum, Jej: jejunum, Ile: Ileum, PC: proximal colon, DC: distal colon. (n=3–5, data representative of two independent experiments) Data represents mean ± s.e.m. *P ≤ .05, *** P ≤ .005, determined by two -tailed Student’s t test.
LL-IL-27 reduces inflammatory cytokines and increases IL-10 in vivo
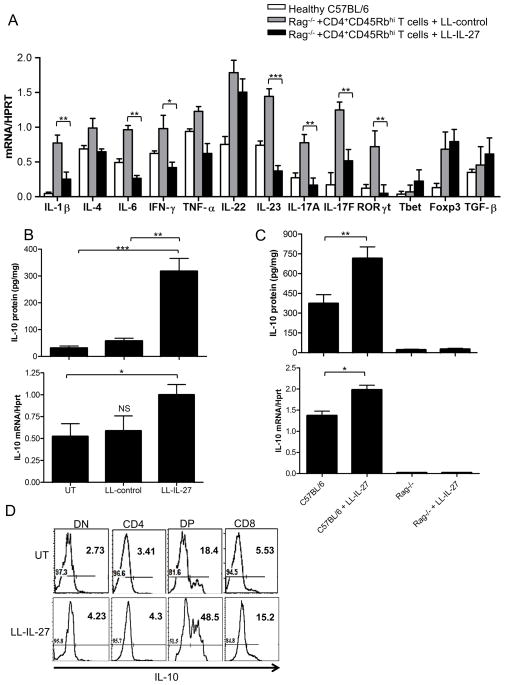
To address the protective mechanism of LL-IL-27, gene expression for inflammatory cytokines and transcription factors was quantified in distal colons (Fig. 4A). Reductions in gene expression for IL-1α, IL-6, IFN-γ, and IL-23 were seen in the LL-IL-27-treated group relative to the LL-control-treated group. IL-17A, IL-17F, and RORγt, all of which are markers of TH17 cells, were also reduced. Tbet, Foxp3, and TGF-β gene expression was not affected.
Figure 4.
LL -IL-27 decreases inflammatory cytokines, increases IL-10 in vivo. (A) Gene expression in distal colons of healthy C57BL/6 mice and mice with enterocolitis treated with LL-control or LL-IL-27 was determined by RT-PCR. (B) Rag−/− mice with enterocolitis were untreated (UT) or treated with LL-control or LL-IL-27 for 14 days. Colons were harvested 7 days following the last gavage or at death. (C) Healthy C57BL/6 and Rag−/− mice were serially gavaged with LL-IL-27 every half hour for 5 hours on Day 1 and one gavage on Day 2. Colons were harvested 1 hour following the last gavage. Untreated C57BL/6 and Rag−/− mice were used as controls. Top, IL-10 protein levels in distal colon homogenate were determined by ELISA. Bottom, IL-10 gene expression in whole distal colons was semi-quantitated by RT-PCR. Levels were normalized to HPRT. (D) LL-IL-27 increases IL-10 expression in DP and CD8 cells. Healthy Vert-X IL-10/eGFP mice were treated with 3 serial gavages on Day 1 and one gavage on Day 2 of BM9 media (UT) or LL-IL-27. Mice were euthanized and small intestines were harvested an hour following the last gavage. IL-10 expression was analyzed on CD3+ lymphocytes from Peyer’s Patches. DN: CD4−CD8− cells, DP: CD4+CD8α+ cells. (n=4, data representative of three independent experiments). Data represents mean ± s.e.m. *P ≤ .05 **P ≤ .01, ***P ≤ .005, determined by two -tailed Student’s t test. NS=no significance.
IL-10 is required to establish and maintain immune tolerance towards enteric bacteria as shown by studies in which mice with a targeted disruption of the IL-10 gene develop spontaneous enterocolitis5, 28. The IL-10 pathway is also implicated in IBD based on GWAS studies29, 30. Because some effects of IL-27 act via IL-1012, 17, 18, we investigated the role of LL-IL-27-induced IL-10 in T cell transfer enterocolitis. LL-IL-27 induced higher IL-10 protein (Fig. 4B, top) and transcript (Fig. 4B, bottom) levels than untreated or LL-control-treated mice.
IL-10 can be produced by a variety of immune cells including lymphocytes and macrophages; therefore, we investigated which cell type produced IL-10. C57BL/6 mice and Rag−/− mice were given serial gavages of LL-IL-27 for 2 days. There was an increase in IL-10 protein levels (Fig. 4C, top) and gene expression (Fig. 4C, bottom) in distal colons of LL-IL-27-treated C57BL/6 mice compared to the untreated C57BL/6 control. However, there were no detectable levels of IL-10 in the LL-IL-27-treated Rag−/− mice, thus, LL-IL-27-induced IL-10 in the T cell transfer model was dependent on T cells and presumably derives from T cells themselves. We also treated macrophages with LPS and varying concentrations of rmIL-27 to determine if macrophages were a source of IL-10. We did not observe a difference in IL-10 induction between LPS-only and LPS with rmIL-27 (Supplementary Fig. 8); therefore it is unlikely that macrophages were the source of LL-IL-27 induced IL-10 in vivo. We next sought to identify the IL-10-producing T cell population. Healthy IL-10 reporter mice were treated with serial inoculations of LL-IL-27 for 2 days. Increased reporter expression was observed in CD8+ and CD4+CD8α+(double positive, DP) from Peyer’s patches of LL -IL-27-treated mice compared to untreated mice (Fig. 4D).
IL-10 is required for LL-IL-27’s therapeutic effect, but LL-IL-10 is ineffective
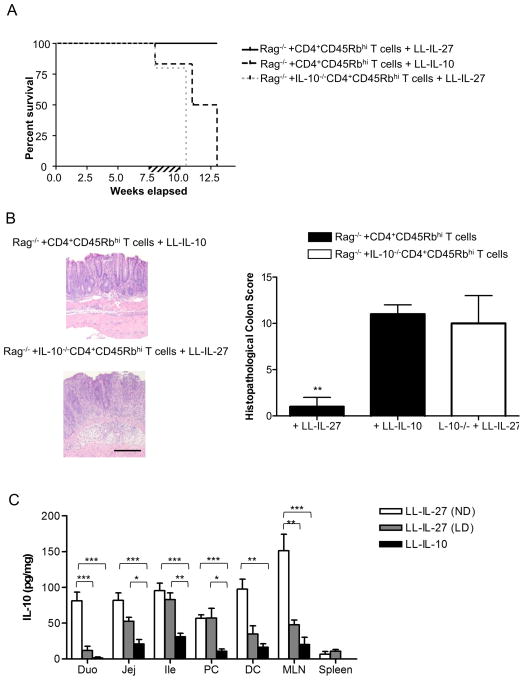
To assess whether IL-10 induction was required for LL-IL-27’s therapeutic effect, we transferred CD4+CD45Rbhi T cells from IL-10−/− mice to Rag−/− mice, and treated them with LL-IL-27 once enterocolitis was established. All mice had succumbed to disease by 10.5 weeks following transfer; therefore IL-10 is required for LL-IL-IL-27’s therapeutic effect (Fig. 5A). Steidler et al. demonstrated that LL-IL-10 alleviates DSS colitis and the onset of colitis in IL-10−/− mice23. Since LL-IL-27’s therapeutic efficacy depended on IL-10, we investigated whether LL-IL-10 was as effective as LL-IL-27 in treating T cell transfer enterocolitis. LL-IL-10-treated mice began to die or had to be euthanized by 8 weeks and by week 13, all had succumbed (Fig. 5A). LL-IL-10 also had a higher DAI than LL-IL-27 (Supplementary Fig. 9). Microscopically, the gut had extensive pathology in both the LL-IL-27-treated IL-10−/−CD4+CD45Rbhi T cell transferred mice and the LL-IL-10-treated mice (Fig. 5b, left), whereas LL-IL-27-treatment lowered the histopathological score (Fig. 5b, right). IL-10 levels in GI tissues and MLN were lower in LL-IL-10-treated mice compared to LL-IL-27-treated mice (Fig. 5c). We also assessed IL-10 induction by a 10-fold lower dose of LL-IL-27 (LD) and found that it was still able to induce higher levels of IL-10 compared to LL-IL-10 (Fig. 5c), although it did not lower the DAI as the normal dose of LL-IL-27 (ND) did (Supplementary Fig. 9). Therefore, although IL-10 is required for LL-IL-27’s therapeutic effect, LL-IL-27 is much more effective than LL-IL-10, at least in part due to LL-IL-27’s ability to induce higher levels of IL-10.
Figure 5.
IL-10 is necessary for LL-IL-27’s therapeutic effect. CD4+CD45Rbhi T cells from C57BL/6 mice or IL-10−/− mice were transferred to Rag−/− mice. Following the onset of enterocolitis at 7.5 weeks, mice were gavaged with LL-IL-27 or LL-IL-10 for 14 days. Colons were harvested upon death (UT and LL-control) or the day after the last gavage (LL-IL-27). (A) Percent survival following T cell transfer (B) Representative H&E-stained sections of distal colons (left). Scale bar = 100 μm. Histopathological scores were determined for the distal colon (right) (C) Tissue homogenates were analyzed for IL-10 in mice gavaged with LL-IL-27 at the normal dose (ND) or at a 10-fold lower dose (LD), or LL-IL-10. Duo: duodenum, Jej: jejunum, Ile: Ileum, PC: proximal colon, DC: distal colon. (n=3–5, data representative of three independent experiments). Data represents mean ± s.e.m. *P ≤ .05, **P ≤ .01, determined by two-tailed Student’s t test.
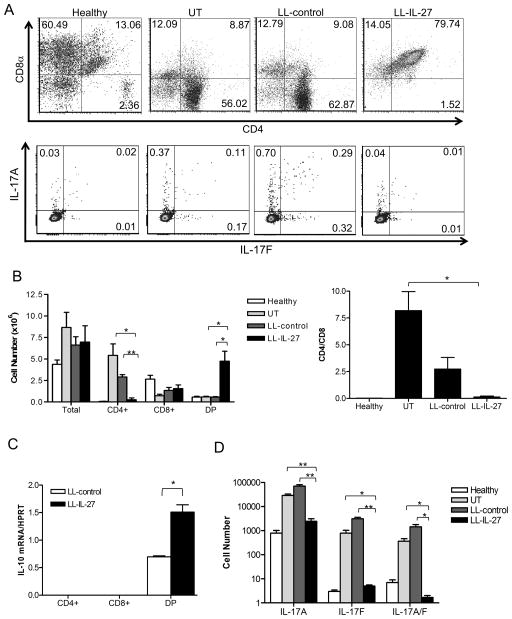
LL-IL-27 decreases CD4+ and IL-17+ small intestinal IELs
IELs play an important role in suppressing enterocolitis in the T cell transfer model, potentially by polarizing CD4+ cells toward a regulatory phenotype31, thus we investigated the effect of LL-IL-27 treatment of mice with enterocolitis on T cell subsets in the intraepithelium. Decreased percentages (Fig. 6A, top) and total cell number (Fig. 6B, left) of CD4+ T cells and increased CD4+CD8α+ T cells (DP) in LL-IL-27-treated mice were observed compared to untreated and LL-control-treated mice (Fig. 6A). Additionally, LL-IL-27-treated mice had a lower CD4/CD8 ratio than untreated mice (Fig. 6B, right). In contrast to colitic mice, this effect on T cell subsets was not observed in healthy mice that received serial gavages of LL-IL-27 (Supplementary Fig. 10). Healthy mice showed no effect of LL-IL-27 on Foxp3, the regulatory T cell CXCR3/Tbet32, CD25, CD44, CD62L, or CD69 expression. In colitic mice, IL-10 mRNA was analyzed in each T cell subset and we found that LL-IL-27 increased levels in the DP subset compared to LL-control (Fig. 6C). No effects of LL-IL-27 were found on IFN-γ, Tbet, GATA-3, Foxp3, or PD-L1 mRNA in any T cell subset (data not shown). To compare the effects of LL-IL-10 and rmIL-27 treatment with LL-IL-27 on T cell phenotype, mice were treated for 7 days with LL-IL-27, LL-IL-10, or rmIL-27. LL-IL-27 treatment increased CD8+ and DP frequency (Supplementary Fig. 11A) and total cell number (Supplementary Fig. 11B) and decreased CD4+ frequency in SI IEL, MLN, and the spleen compared to LL-IL-10 and rmIL-27; however, the number of CD4+ cells was not decreased by LL-IL-27 as seen after 14 days of treatment (Fig. 6A, top). Foxp3 and Tbet/CXCR3 was not affected by 7 days of treatment (data not shown).
Figure 6.
LL -IL-27 treatment decreases CD4+ and IL-17+ small intestinal intraepithelial lymphocytes. CD45Rbhi T cells from C57BL/6 mice or IL-17A/F dual-color reporter mice were transferred to Rag−/− mice to induce enterocolitis. Rag−/− mice with enterocolitis were treated with LL-control or LL-IL-27 for 14 days. Organs were harvested upon death (UT and LL-control) or the day after the last gavage (LL-IL-27). C57BL/6 mice were used as healthy controls. Lymphocytes were isolated from the small intestine intraepithelium and were pooled from 2 mice. (A) Representative dot plot analysis of CD4+ and CD8α+ cells (top) and IL-17A and IL-17F cells (bottom). Numbers in dot plot quadrants indicate percent of CD3+ cells. Average total cells and CD4 single positive (CD4+), CD8α single positive (CD8 +), and CD4+CD8α+ (double-positive, DP) cells (B, left), CD4/CD8α ratio (B, right), and IL-17A, IL-17F, and IL-17A/F double-expressor cells (C). T cell subset numbers were calculated from the total cell count multiplied by the percentage of T lymphocytes obtained from flow cytometric analyses. (D) IL-10 gene expression was semi-quantitated for CD4+, CD8α+ and DP purified populations. DP: CD4+CD8α+ cells. (n=3, data representative of two independent experiments). Data represents mean ± s.e.m. *P ≤ .05 ** P ≤ .01 determined by two -tailed Student’s t test.
TH17 cells are involved in driving the onset and the development of IBD in mouse models33 and in patients34. Recently, IL-27 treatment was shown to decrease IL-17A-expressing cells in a mouse model of colitis21, thus we examined the effect of LL-IL-27 treatment of mice with colitis on TH17 cells using IL-17A/F dual-color reporter mice. LL-IL-27-treated mice had decreased percentages (Fig. 6A, bottom) and total number (Fig. 6D) of IL-17A, IL-17F, and IL-17A/F expressing cells compared to untreated and LL-control-treated mice.
Following LL-IL-27 treatment, decreased percentages of phagocytic cells were observed (Supplementary Fig. 12). LL-IL-27 treatment decreased Gr1+CD11b+CD11c− cell (predominately granulocytes) frequency in MLNs and colon lamina propria (LP) (Supplementary Fig. 12A) and Gr1−CD11b+CD11c− cell (predominately monocytes) frequency decreased in the spleen, MLNs, and cLP (Supplementary Fig. 12B).
In addition to inhibiting TH17 cells, IL-27 can control inflammation by promoting development of IL-10-producing Tr1 regulatory cells17. We investigated the expression of Tr1-associated genes in intestinal lymphocytes of LL-IL-27-treated mice. We did not find any differences in ICOS, IL-21, or IL-21R between LL-control and LL-IL-27-treated mice (Supplementary Fig. 13). We did observe an increase in IL-27R gene expression in LL-IL-27-treated mice.
Discussion
A localized delivery of the immunosuppressive cytokine, IL-27, was developed using L. lactis to treat T cell-dependent chronic enterocolitis and T cell-independent acute colitis. In the T cell transfer model of enterocolitis, LL-IL-27 improved survival, lessened colon and small intestine pathology, and decreased inflammatory cytokine gene expression in the colon. The therapeutic effect of LL-IL-27 was found to be dependent on T cell-derived IL-10 production. LL-IL-27 decreased CD4+ and IL-17+ colitogenic T cells in the intestinal intraepithelium. LL-IL-27 treatment improved DAI in the T cell-independent acute model of colitis induced by DSS. By comparison to mucosal delivery, systemic rmIL-27 treatment increased IL-10 levels in the circulation but not in the distal colon, which may contribute to its failure to decrease disease activity and colon pathology. LL-IL-27 treatment was not associated with any pathology, it did not affect intestinal barrier function, nor did it exacerbate an intestinal infection caused by C. rodentium. Genetically modified L. lactis have been shown to be safe in clinical trials (ClinicalTrials.gov identifiers NCT00729872 and NCT00938080). Therefore, LL-IL-27 is potentially a more effective and safer treatment of IBD than current treatment options.
Standard therapy for IBD involves lifelong treatment of immunosuppressive agents administered systemically, often with surgical resection of sections of bowel. Inefficient drug delivery and intolerable side effects, particularly from manipulating cytokines, such as TNF-α35 has contributed to limited treatment options for IBD patients. The indispensable role of the anti-inflammatory cytokine, IL-10, in the regulation of mucosal immunity is most aptly demonstrated by the development of spontaneous enterocolitis in IL-10−/− mice5 and the occurrence of genetic variants of IL-10 in IBD patients29, 36. Clinical trials in which IBD patients were given systemic recombinant IL-10, however, did not display clinical benefit, possibly due to the low intestinal bioavailability and dose-limiting side effects8, 37. Delivery of IL-10 locally by LL-IL-10 had shown promise by alleviating colitis in IL-10−/− mice and mice exposed to DSS23, however it was shown to be much less effective than LL-IL-27 in the T cell-induced colitis described in the present study. In our study, following LL-IL-27 treatment, IL-10 levels were elevated locally throughout the intestinal tract. In healthy mice, serial gavages of LL-IL-27 induced IL-10 levels in the GI tract nearly 20 times greater than the level delivered by LL-IL-1023 and further, LL-IL-27-treated mice had enhanced survival, decreased disease activity, and improved mucosal healing of the colon to a greater degree than LL-IL-10. Even at a 10-fold lower dose, LL-IL-27 induced higher levels of IL-10 than LL-IL-10 in the areas of the GI tract. This may explain why LL-IL-27, despite acting via IL-10, was a better therapeutic than LL-IL-10.
LL-IL-27 reduced the percentage of CD4+ T cells in the intraepithelium of the small intestine and increased the percentage of DP cells. IL-10 mRNA was increased in the DP subset of LL-IL-27-treated mice, and following serial gavages of healthy IL-10 reporter mice, the DP subset of T cells was the highest IL-10 producer. Extrathymic DP cells, specifically CD4+CD8αα+CD8β−TCRαβ+ cells, have been described as a unique cell type localizing in the intestinal intraepithelial layer. These DP have been attributed a regulatory function in inhibiting Th1-induced intestinal inflammation, primarily through the production of IL-1038. They were also reported to express TGF-β, IFN-γ, and no IL-2, IL-4, or TNF-α. We found that CD4+CD8αα+CD8β−TCRαβ+ cells make up the majority of the DP population in healthy and colitic mice as previously reported38; however we did not observe an LL-IL-27 effect on any of the cytokines that contribute to this cell population’s regulatory function other than increased IL-10. Whether this DP population is able to regulate expansion of colitogenic CD4+ will require further investigation. Our characterization of the DP cell type is similar to the findings of Kamanaka et al., in which anti-CD3 treatment induced T regulatory cell 1 (Tr1)-like cells in SI intraepithelium39. Briefly, transferred CD4+ cells into immunodeficient mice gained CD8+ expression in the SI IEL compartment, and these cells expressed IL-10, but not Foxp3, IL-2, IL-4, and IFN-γ. Our data suggest that the transferred naïve CD4+ T cells travel to the SI intraepithelium, and following a 14-day dosing regimen of LL-IL-27, the CD4+ T cells gain CD8 expression, either directly via IL-27 or secondary to IL-10 induction, then produce high levels of IL-10 that contribute to the efficacy of LL-IL-27 treatment for enterocolitis. While IL-10 is not required for the CD4+CD8αα+CD8β−TCRαβ+ phenotype, it is critical for their function38. Interestingly, T cell phenotype differed greatly between mice treated with LL-IL-27 for 7 days (Supplementary Fig. 11A) and 14 days (Fig. 6A, top). At some point after 7 days of treatment, the number of CD4+ cells decreased markedly.
Currently, the role of IL-27 and its receptors in IBD has been interpreted differently based on different models. Several studies have shown a pro-inflammatory role for IL-27 in experimental colitis40–43, while others have shown anti-inflammatory effects44, 45. Two studies have reported that IL-27Rα−/− CD4+CD45Rbhi T cells are unable to induce colitis40, 46. Cox et al. concluded that the inability to induce colitis in Balb/c mice was due to the increase of Foxp3+ cells converted from the naïve donor cells and low expansion of IL-27Rα−/− donor cells in the large intestine40, while Kim et al. found that the inability to induce colitis in C57Bl/6 mice was due to activated IL-27Rα−/− donor cells being unable to survive, particularly in the large intestine, despite normal Foxp3 expression46. In our model, mucosal delivery of IL-27 has an anti-inflammatory effect once enterocolitis is established, possibly through the conversion of CD4+ effector cells to IL-10 producing-DP cells, and without increasing Foxp3 expression. We did not observe an increase in CD4+ cells when healthy mice were treated with LL-IL-27 (Supplementary Figure 10), nor did any signs of colitis develop following a 30-day treatment of LL-IL-27 to healthy mice (data not shown); therefore, our findings suggest that mucosal delivery of IL-27 has an anti-inflammatory effect in T cell-dependent colitis. Consistent with our findings that IL-27 has therapeutic efficacy, a GWAS study implicated a single nucleotide polymorphisms in the IL-27 regulatory region that reduces expression and increases susceptibility to IBD22.
In designing therapeutics for IBD patients, a balance is sought to inhibit enough immunity to reduce IBD symptoms without rendering the patient systemically immunocompromised. These results suggest that mucosal delivery of LL-IL-27 is potentially a more effective and safer treatment of IBD in humans.
Methods
Induction of enterocolitis by T cell transfer, LL administration
The T cell transfer model was used to induce enterocolitis as reported in Ostanin et al.47. Male Rag−/− were used for recipients, while female C57BL/6, IL-10−/−, or IL-17A/F dual reporter mice were used for donors (see Supplementary Methods for details). Enterocolitis was induced 7–7.5 weeks following cell transfer. We determined that the onset of enterocolitis occurred when mice lost ≥5% body weight and had pasty, semi formed stools. For experiments where C57BL/6 or IL-10−/− mice were cell donors, L. lactis administration began following enterocolitis induction and continued with 14 daily gavages (5 days/week). Tissues were either harvested immediately following death (Untreated, LL-control) or at 1 or 7 days post-gavage (LL-IL-27). For experiments where IL-17A/F dual-reporter mice were cell donors, L. lactis administration began at 4 weeks and continued with 14 daily gavages. Tissues were harvested 8 weeks following cell transfer. C57BL/6 and Rag−/− mice not receiving a T cell transfer were serially gavaged every half hour for five hours on day 1 and one gavage on day 2. Tissues were harvested an hour after gavage.
Systemic treatment with rmIL-27
Seven weeks following T cell transfer, Rag−/− mice were injected intraperitoneally daily for 5 days with PBS, 500 ng or 1 μg murine rmIL-27 (R & D Systems). Mice were euthanized three days after the final injection and their colons were processed for histopathology analysis.
Histological analysis
Tissues (small and large intestine) from mice were fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. H&E tissue sections were evaluated and graded in coded fashion by a veterinary pathologist (M.R.A.). See Supplementary Methods for scoring criteria.
Statistics
Statistical analysis was performed using the GraphPad Prism software (version 5.00; GraphPad, San Diego, CA). Data are expressed as ± s.e.m. The Student two-tailed unpaired, parametric t test was used to assess statistical differences between two experimental groups. Asterisks indicate statistical differences, * P ≤ .05, ** P ≤ .01, *** P ≤ .005.
Supplementary Material
Acknowledgments
We thank Kelli Czarra and Megan Karwan for animal technical assistance, Kathleen Noer Roberta Matthai, and Guity Mohammadi, for flow cytometry assistance, Christopher Karp for use of Vert-X mice, and Giorgio Trinchieri for use of IL-10−/− mice. We are also grateful to Joost J. Oppenheim for critical review of the manuscript. This research was supported in part by grants from the Crohn’s and Colitis Foundation of America and the Eli and Edythe Broad Foundation, the Intramural Research Program of the NIH, NCI, and with federal funds from the NCI, NIH, under Contract No. HHSN261200800001E.
Footnotes
Conflict of Interest: None to declare.
Author contributions: M.L.H. designed and performed the experiments, analyzed and interpreted data, and wrote the manuscript; J.A.H. provided technical expertise and assisted with experiments; W.L. and B.K.F. designed and generated mouse IL27 hyperkine; C.A.S. and W.S. performed reporter mouse experiments and analyzed data; M.R.A provided pathology support and performed H&E staining and scoring; C.A.S., provided scientific advice; B.M.J and S.K. Datta performed the Citrobacter rodentium experiment; M.H.M assisted with experiments and provided scientific advice; and S.K. Durum directed the entire study. All authors reviewed the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–35. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Packey CD, Sartor RB. Interplay of commensal and pathogenic bacteria, genetic mutations, and immunoregulatory defects in the pathogenesis of inflammatory bowel diseases. Journal of Internal Medicine. 2008;263:597–606. doi: 10.1111/j.1365-2796.2008.01962.x. [DOI] [PubMed] [Google Scholar]
- 4.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 5.Kuhn R, Lohler J, Rennick D, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 6.Tagore A, Gonsalkorale WM, Pravica V, et al. Interleukin-10 (IL-10) genotypes in inflammatory bowel disease. Tissue antigens. 1999;54:386–90. doi: 10.1034/j.1399-0039.1999.540408.x. [DOI] [PubMed] [Google Scholar]
- 7.Fedorak RN, Gangl A, Elson CO, et al. Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn’s disease. Gastroenterology. 2000;119:1473–1482. doi: 10.1053/gast.2000.20229. [DOI] [PubMed] [Google Scholar]
- 8.Schreiber S, Fedorak RN, Nielsen OH, et al. Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn’s disease. Gastroenterology. 2000;119:1461–1472. doi: 10.1053/gast.2000.20196. [DOI] [PubMed] [Google Scholar]
- 9.Pflanz S, Timans JC, Cheung J, et al. IL-27, a heterodimeric cytokine composed of EB13 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 10.Pflanz S, Hibbert L, Mattson J, et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. Journal of Immunology. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 11.Villarino AV, Larkin J, Saris CJM, et al. Positive and negative regulation of the IL-27 receptor during lymphoid cell activation. Journal of Immunology. 2005;174:7684–7691. doi: 10.4049/jimmunol.174.12.7684. [DOI] [PubMed] [Google Scholar]
- 12.Batten M, Kljavin NM, Li J, et al. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. Journal of immunology. 2008;180:2752–6. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- 13.Batten M, Li J, Yi S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nature Immunology. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 14.Stumhofer JS, Laurence A, Wilson EH, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nature Immunology. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald DC, Ciric B, Touil T, et al. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. Journal of immunology. 2007;179:3268–75. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 16.Diveu C, McGeachy MJ, Boniface K, et al. IL-27 Blocks RORc Expression to Inhibit Lineage Commitment of Th17 Cells. Journal of Immunology. 2009;182:5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 17.Awasthi A, Carrier Y, Peron JPS, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nature Immunology. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 18.Stumhofer JS, Silver JS, Laurence A, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nature Immunology. 2007;8:1363–U5. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 19.Hall AO, Beiting DP, Tato C, et al. The Cytokines Interleukin 27 and Interferon-gamma Promote Distinct Treg Cell Populations Required to Limit Infection-Induced Pathology. Immunity. 2012;37:511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niedbala W, Cai B, Wei X, et al. Interleukin 27 attenuates collagen-induced arthritis. Annals of the rheumatic diseases. 2008;67:1474–9. doi: 10.1136/ard.2007.083360. [DOI] [PubMed] [Google Scholar]
- 21.Sasaoka T, Ito M, Yamashita J, et al. Treatment with IL-27 attenuates experimental colitis through the suppression of the development of IL-17-producing T helper cells. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2011;300:G568–G576. doi: 10.1152/ajpgi.00329.2010. [DOI] [PubMed] [Google Scholar]
- 22.Imielinski M, Baldassano RN, Griffiths A, et al. Common variants at five new loci associated with early-onset inflammatory bowel disease. Nature Genetics. 2009;41:1335–U107. doi: 10.1038/ng.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steidler L, Hans W, Schotte L, et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352–5. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- 24.Caluwaerts S, Vandenbroucke K, Steidler L, et al. AG013, a mouth rinse formulation of Lactococcus lactis secreting human Trefoil Factor 1, provides a safe and efficacious therapeutic tool for treating oral mucositis. Oral Oncology. 2010;46:564–570. doi: 10.1016/j.oraloncology.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Vandenbroucke K, de Haard H, Beirnaert E, et al. Orally administered L. lactis secreting an anti-TNF Nanobody demonstrate efficacy in chronic colitis. Mucosal Immunology. 2010;3:49–56. doi: 10.1038/mi.2009.116. [DOI] [PubMed] [Google Scholar]
- 26.Powrie F, Correa-Oliveira R, Mauze S, et al. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. The Journal of Experimental Medicine. 1994;179:589–600. doi: 10.1084/jem.179.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naito Y, Takagi T, Kuroda M, et al. An orally active matrix metalloproteinase inhibitor, ONO-4817, reduces dextran sulfate sodium-induced colitis in mice. Inflammation Research. 2004;53:462–468. doi: 10.1007/s00011-004-1281-1. [DOI] [PubMed] [Google Scholar]
- 28.Rennick D, Davidson N, Berg D. Interleukin-10 gene knock-out mice: a model of chronic inflammation. Clinical immunology and immunopathology. 1995;76:S174–8. doi: 10.1016/s0090-1229(95)90144-2. [DOI] [PubMed] [Google Scholar]
- 29.Franke A, Balschun T, Karlsen TH, et al. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nature Genetics. 2008;40:1319–1323. doi: 10.1038/ng.221. [DOI] [PubMed] [Google Scholar]
- 30.Moran CJ, Kugathasan S, Klein C, et al. Interleukin-10 Receptor Gene Polymorphisms Associate with Early-Onset Inflammatory Bowel Disease. Gastroenterology. 2011;140:S486–S486. [Google Scholar]
- 31.Laroux FS, Norris HH, Houghton J, et al. Regulation of chronic colitis in athymic nu/nu (nude) mice. International Immunology. 2004;16:77–89. doi: 10.1093/intimm/dxh006. [DOI] [PubMed] [Google Scholar]
- 32.Hall AO, Beiting DP, Tato C, et al. The cytokines interleukin 27 and interferon-gamma promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. 2012;37:511–23. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hue S, Ahern P, Buonocore S, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. Journal of Experimental Medicine. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duerr RH, Taylor KD, Brant SR, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Targan SR, Hanauer SB, vanDeventer SJH, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. New England Journal of Medicine. 1997;337:1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 36.van der Linde K, Boor PPC, Sandkuijl LA, et al. A Gly15Arg mutation in the interleukin-10 gene reduces secretion of interleukin-10 in Crohn disease. Scandinavian Journal of Gastroenterology. 2003;38:611–617. [PubMed] [Google Scholar]
- 37.vanDeventer SJH, Elson CO, Fedorak RN. Multiple doses of intravenous interleukin 10 in steroid-refractory Crohn’s disease. Gastroenterology. 1997;113:383–389. doi: 10.1053/gast.1997.v113.pm9247454. [DOI] [PubMed] [Google Scholar]
- 38.Das G, Augustine MM, Das J, et al. An important regulatory role for CD4+CD8 alpha alpha T cells in the intestinal epithelial layer in the prevention of inflammatory bowel disease. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:5324–9. doi: 10.1073/pnas.0831037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamanaka M, Kim ST, Wan YY, et al. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941–52. doi: 10.1016/j.immuni.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 40.Cox JH, Kljavin NM, Ramamoorthi N, et al. IL-27 promotes T cell-dependent colitis through multiple mechanisms. The Journal of Experimental Medicine. 2011;208:115–23. doi: 10.1084/jem.20100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nieuwenhuis EES, Neurath MF, Corazza N, et al. Disruption of T helper 2-immune responses in Epstein-Barr virus-induced gene 3-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:16951–16956. doi: 10.1073/pnas.252648899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Honda K, Nakamura K, Matsui N, et al. T helper 1-inducing property of IL-27/WSX-1 signaling is required for the induction of experimental colitis. Inflammatory bowel diseases. 2005;11:1044–52. doi: 10.1097/01.mib.0000191611.05466.1f. [DOI] [PubMed] [Google Scholar]
- 43.Villarino AV, Artis D, Bezbradica JS, et al. IL-27R deficiency delays the onset of colitis and protects from helminth-induced pathology in a model of chronic IBD. International Immunology. 2008;20:739–752. doi: 10.1093/intimm/dxn032. [DOI] [PubMed] [Google Scholar]
- 44.Troy AE, Zaph C, Du YR, et al. IL-27 Regulates Homeostasis of the Intestinal CD4(+) Effector T Cell Pool and Limits Intestinal Inflammation in a Murine Model of Colitis. Journal of Immunology. 2009;183:2037–2044. doi: 10.4049/jimmunol.0802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diegelmann J, Olszak T, Goke B, et al. A Novel Role for Interleukin-27 (IL-27) as Mediator of Intestinal Epithelial Barrier Protection Mediated via Differential Signal Transducer and Activator of Transcription (STAT) Protein Signaling and Induction of Antibacterial and Anti-inflammatory Proteins. Journal of Biological Chemistry. 2012;287:286–298. doi: 10.1074/jbc.M111.294355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim G, Shinnakasu R, Saris CJ, et al. A novel role for IL-27 in mediating the survival of activated mouse CD4 T lymphocytes. Journal of immunology. 2013;190:1510–8. doi: 10.4049/jimmunol.1201017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ostanin DV, Bao JX, Koboziev I, et al. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2009;296:G135–G146. doi: 10.1152/ajpgi.90462.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.