Abstract
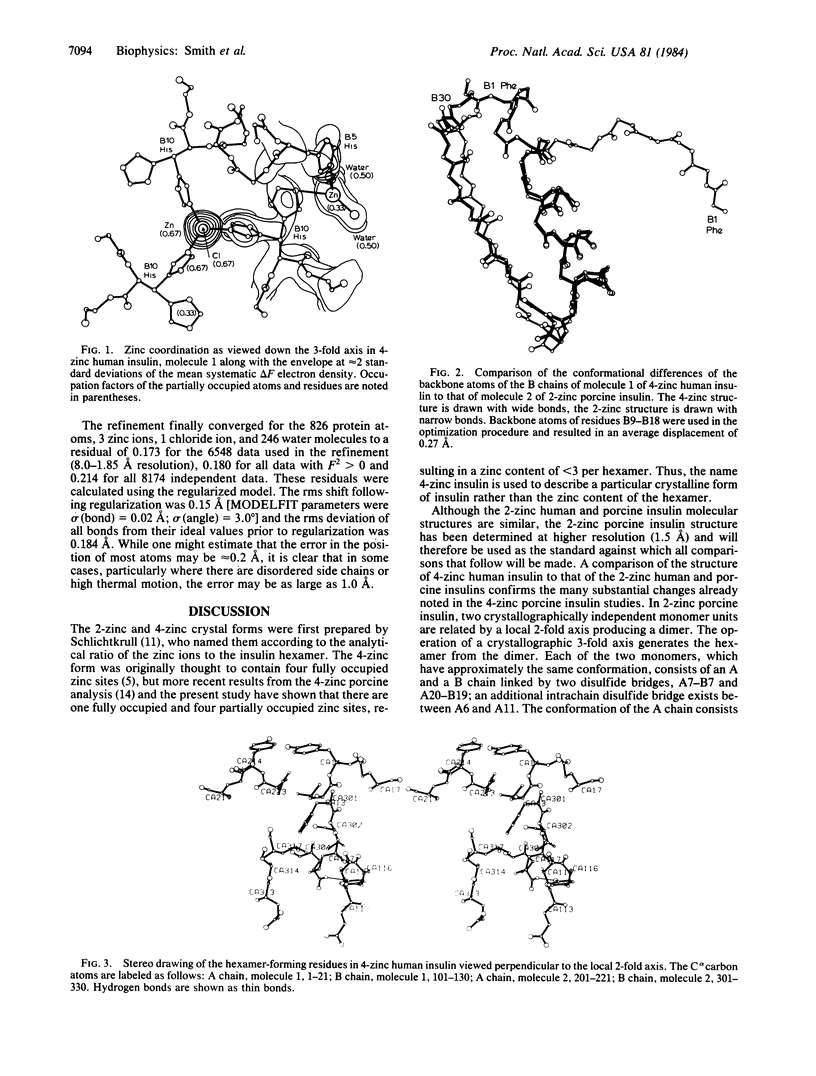
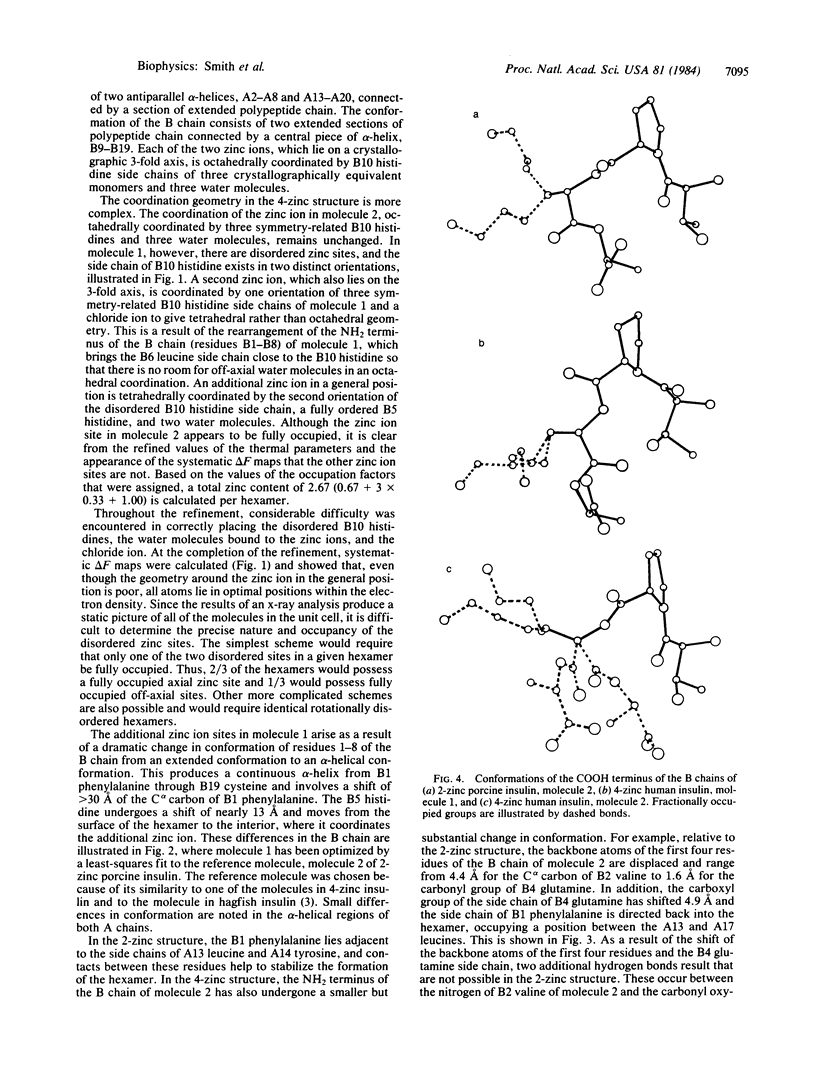
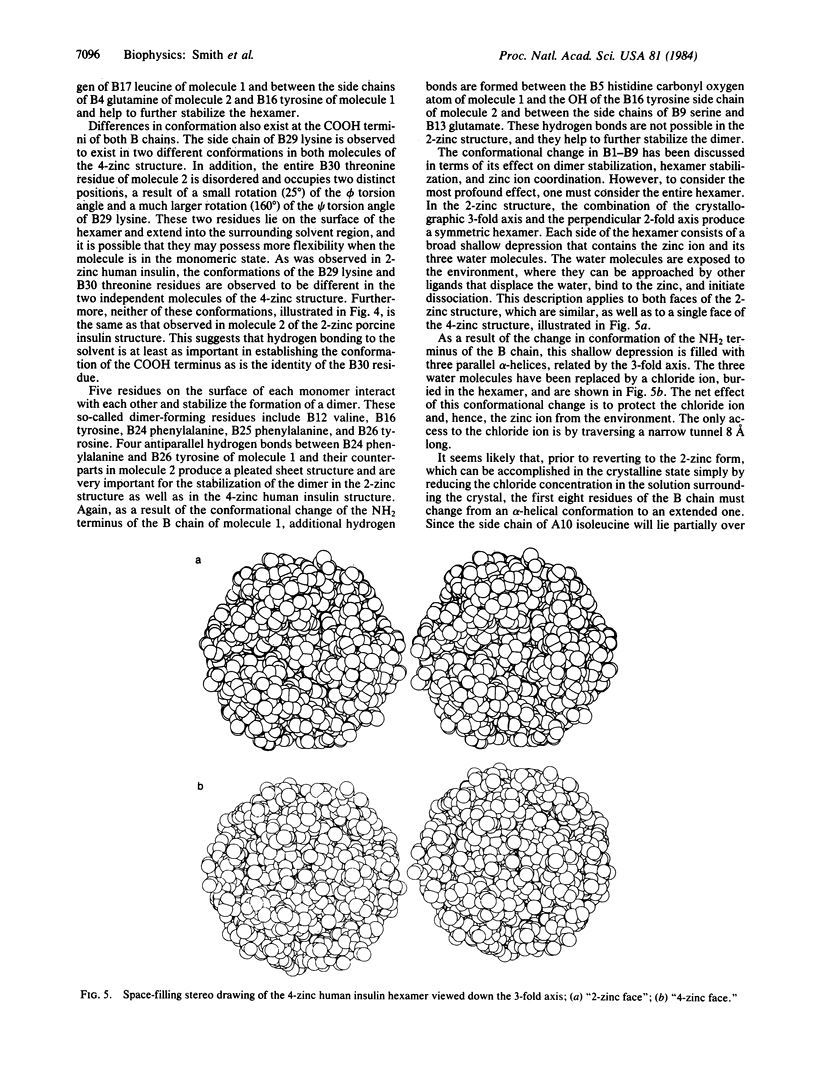
X-ray studies on human insulins prepared by semisynthetic and biosynthetic methods have recently been undertaken. Human insulin differs from porcine insulin only at the COOH terminus of the B-chain. The present study reports the crystal structure of 4-zinc human insulin, which is used clinically as a slow-acting preparation. The structure has been refined, using 1.85-A resolution data, to a residual of 0.173. The unit cell is rhombohedral, space group R3, with hexagonal cell constants a = 80.953 and c = 37.636 A, and it is nearly isomorphous with that of 4-zinc porcine insulin. As a result of a conformational change of the first eight residues of the B-chain of molecule 1 from an extended conformation observed in the 2-zinc structure to an alpha-helical one, the coordination around one of the zinc ions on the 3-fold axis has changed, an additional zinc ion in a general position is bound by the hexamer, and additional hydrogen-bonded interactions help stabilize dimer and hexamer formation. Unlike the surface of the 2-zinc insulin hexamer, which possesses a shallow depression containing a zinc ion and its coordinating water molecules, the 4-zinc human insulin hexamer contains a zinc and chloride ion at the bottom of an 8-A tunnel produced by three parallel alpha-helices. These alpha-helices shield the zinc ion from the environment, decreasing the rate of dissociation of the hexamer, and provide an explanation for the slow-acting aspect of the 4-zinc crystalline form.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley G., Dodson E., Dodson G., Hodgkin D., Mercola D. Structure of insulin in 4-zinc insulin. Nature. 1976 May 13;261(5556):166–168. doi: 10.1038/261166a0. [DOI] [PubMed] [Google Scholar]
- Chance R. E., Kroeff E. P., Hoffmann J. A., Frank B. H. Chemical, physical, and biologic properties of biosynthetic human insulin. Diabetes Care. 1981 Mar-Apr;4(2):147–154. doi: 10.2337/diacare.4.2.147. [DOI] [PubMed] [Google Scholar]
- Chawdhury S. A., Dodson E. J., Dodson G. G., Reynolds C. D., Tolley S. P., Blundell T. L., Cleasby A., Pitts J. E., Tickle I. J., Wood S. P. The crystal structures of three non-pancreatic human insulins. Diabetologia. 1983 Dec;25(6):460–464. doi: 10.1007/BF00284451. [DOI] [PubMed] [Google Scholar]
- Cutfield J. F., Cutfield S. M., Dodson E. J., Dodson G. G., Emdin S. F., Reynolds C. D. Structure and biological activity of hagfish insulin. J Mol Biol. 1979 Jul 25;132(1):85–100. doi: 10.1016/0022-2836(79)90497-2. [DOI] [PubMed] [Google Scholar]
- Cutfield J., Cutfield S., Dodson E., Dodson G., Hodgkin D., Reynolds C. Evidence concerning insulin activity from the structure of a cross-linked derivative. Hoppe Seylers Z Physiol Chem. 1981 Jun;362(6):755–761. doi: 10.1515/bchm2.1981.362.1.755. [DOI] [PubMed] [Google Scholar]
- Dodson E. J., Dodson G. G., Hodgkin D. C., Reynolds C. D. Structural relationships in the two-zinc insulin hexamer. Can J Biochem. 1979 Jun;57(6):469–479. doi: 10.1139/o79-060. [DOI] [PubMed] [Google Scholar]
- Dodson E. J., Dodson G. G., Hubbard R. E., Reynolds C. D. Insulin's structural behavior and its relation to activity. Biopolymers. 1983 Jan;22(1):281–291. doi: 10.1002/bip.360220137. [DOI] [PubMed] [Google Scholar]
- Goeddel D. V., Kleid D. G., Bolivar F., Heyneker H. L., Yansura D. G., Crea R., Hirose T., Kraszewski A., Itakura K., Riggs A. D. Expression in Escherichia coli of chemically synthesized genes for human insulin. Proc Natl Acad Sci U S A. 1979 Jan;76(1):106–110. doi: 10.1073/pnas.76.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]



