Abstract
Background
Pulmonary arterial hypertension (PAH) is a rare but fatal lung disease of diverse etiologies. PAH is now further subclassified as idiopathic (IPAH), familial (FPAH) and associated (APAH) varieties. Heterozygous mutations in BMPR2 can be detected in 50-70% of patients with FPAH and 10-40% of patients with IPAH. Although endothelial cells have been suspected as the cellular origin of PAH pathogenesis, no direct in vivo evidence has been clearly presented. The present study was designed to investigate whether endothelial Bmpr2 deletion can predispose to PAH.
Methods and Results
The Bmpr2 gene was deleted in pulmonary endothelial cells (pECs) using Bmpr2 conditional knockout mice and a novel endothelial Cre transgenic mouse line. Wide ranges of right ventricular systolic pressure (RVSP) were observed in mice with heterozygous (21.7 - 44.1 mmHg, median: 23.7 mmHg) and homozygous (20.7- 56.3 mmHg, median: 27 mmHg) conditional deletion of Bmpr2 in pECs in comparison with control mice (19.9 - 26.7 mmHg, median: 23 mmHg) at two to seven months of age. A subset of mice with RVSP greater than 30 mmHg exhibited right ventricular hypertrophy and an increase in the number and wall thickness of muscularized distal pulmonary arteries. In the lungs of these high RVSP mice, expression of proteins involved in the pathogenesis of PAH, such as serotonin transporter and tenacin-C, were elevated in distal arteries, and had a high incidence of perivascular leukocyte infiltration and in situ thrombosis.
Conclusions
Conditional hetero or homozygous Bmpr2 deletion in pECs predisposes mice to develop PAH.
Keywords: endothelium, genetics, hypertension, pulmonary, BMP receptor, animal model
Introduction
Pulmonary hypertension (PH) is a lung disorder, in which mean pulmonary arterial pressure rises above normal levels (25 mmHg at rest and 30 mmHg during exercise). PH is classified into arterial, venous, hypoxic, thromboembolic, and miscellaneous varieties. Of these varieties, pulmonary arterial hypertension (PAH) typically carries the worst prognosis. PAH is further subclassified as idiopathic (IPAH), familial (FPAH), and associated (APAH) subtypes 1. The previous term primary pulmonary hypertension (PPH) comprises IPAH, FPAH, and the anorexigen-induced subset of APAH 1. Pathological features of PAH appear in small pulmonary arteries, and include intimal fibrosis, distal localization and proliferation of vascular smooth muscle, and pulmonary arterial occlusion 2. Appearing in severe end-stage of PAH are plexiform lesions that consist of multiple, irregular vascular lumens with highly proliferative endothelial cells. Increased pulmonary vascular resistance and tone associated with thickening of medial layer and occlusions of small arteries lead to right ventricular hypertrophy and eventually to right heart failure. The prognosis of PAH still remains poor, despite recent advances in therapeutic approaches that appear to prolong survival in some PAH patients 3.
The pathogenesis of PAH is largely unknown, but there is ample evidence implicating the involvement of diverse vascular effectors. In general, PAH can be promoted by hormones, growth factors, neurotransmitters and environmental stresses that induce pulmonary vascular constriction, cell proliferation, or remodeling 4. More specifically, pathways involved in a decrease of vasodilatory and/or antiproliferative effects such as prostacyclin, nitric oxide and voltage-gated potassium channels, or in an increase of vasoconstrictory and/or mitogenic effects such as Eodothelin-1 and Serotonin (5-HT) have been implicated in PAH 4.
Genetic studies have shown that bone morphogenetic protein receptor type II (BMPR2) signaling plays a critical role in the pathogenesis of IPAH and FPAH. FPAH accounts for at least 6% of all cases of PAH, and shows an autosomal-dominant manner of inheritance 5. Heterozygous mutations of the BMPR2 gene were found in about 50-70% of FPAH cases 5. Furthermore, 11-40% of apparently sporadic IPAH cases also carry germline BMPR2 mutations 5. In addition to the BMPR2 gene, PAH also develops in a subset of hereditary hemorrhagic telangiectasia (HHT) patients harboring heterozygous mutations in ALK1 (Activin receptor-like kinase 1) or ENG (Endoglin) gene 6;7. Since ALK1 and ENG are components of BMP signaling pathway, these genetic data suggest that the deficiency of BMP signaling is a primary factor predisposing to FPAH.
Pedigree studies of FPAH families have shown that only about 20% of people who are expected to harbor a heterozygous BMPR2 mutation exhibit pulmonary hypertension 5. Genetic anticipation (more severe forms and earlier onset of clinical manifestations in successive generations) was also observed in FPAH families 5. The molecular mechanisms governing the low penetrance and genetic anticipations in FPAH cases are unknown. However, these genetic data suggest that the heterozygous BMPR2 mutations are by themselves insufficient to account for the clinical manifestation of IPAH, and that environmental or genetic multiple `hits' may play a pivotal role in triggering the disease.
Several attempts have been made to investigate the impact of BMPR2 deficiency in the development of PAH in mice. Since the characteristics of BMPR2 mutations in human PAH patients indicate haploinsufficiency (reduced functional protein) as the molecular mechanism of disease 8, mice heterozygous for a Bmpr2-null allele (Bmpr2+/-) received much attention. Bmpr2+/- mice showed moderately elevated mean pulmonary artery pressure and pulmonary vascular resistance 9. More recent studies using the same mouse strain, however, showed no significant difference of RVSP between Bmpr2+/- and control mice 10;11. Mice having smooth muscle-specific down regulation of BMPR2 signaling using a dominant negative form of BMPR2, Tg(SM22α-dnBmpr2), showed elevated RVSP 12. Both Bmpr2+/- and Tg(SM22α-dnBMPR2) mice exhibited moderate increases in muscularization of small arteries, but these mice did not fully recapitulate the pathological features of severe PAH in patients, such as intimal fibrosis, occlusion of arteries or plexiform lesions 9;12. It is unknown why the lung histology of Bmpr2+/- mice containing a Bmpr2 mutation essentially the same as humans does not recapitulate the histological characteristics seen in human PAH patients. Perhaps, mice, in comparison with humans, may have a lower threshold level of BMPR2 signaling required for the maintenance of pulmonary vascular homeostasis. Bmpr2-knockdown mice with a shRNA technology which showed up to 90% reduction of Bmpr2 expression compared to normal had no significant elevation in pulmonary pressure 13. Unfortunately, no additional information regarding the role of BMPR2 deficiency in PAH could be obtained from mice having further reduction of BMPR2 level, such as Bmpr2 hypomorphs and Bmpr2-/- mice, due to their embryonic lethal phenotypes 14;15. A report showed that BMPR2 expression was almost completely absent in endothelial cells of plexiform or concentric vascular lesions of FPAH patients harboring heterozygous BMPR2 mutations, suggesting that further reduction of BMPR2 expression or function may play a role in PAH pathogenesis16;17.
Here we show using the Cre/loxP system that deficiency of BMPR2 signaling in pulmonary endothelial cells (pECs) can elicit PAH. On the other hand, even homozygous Bmpr2 deletion in pECs is not sufficient to cause PAH in all mice by 7 months. Our data suggest that genetic and environmental factors beyond those that modulate expression or function of BMPR2 in pECs play an important role in development of PAH. This novel genetic model represents a valuable resource with which to further our understanding of the etiology and pathogenesis of PAH.
Methods
All animal procedures performed were reviewed and approved by the University of Florida Institutional Animal Care and Use Committee.
Homozygous deletion of Bmpr2 gene in pECs
Generation of a conditional Bmpr2 allele (Bmpr2f) was previously described 18. Generation of Tg(Alk1-cre)-L1 (L1cre) and Tg(Alk1-cre)-B1 (B1cre) lines was also recently described 19. R26R mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Bmpr2f/f mice were intercrossed with L1cre and R26R mice. The L1cre(+);Bmpr2+/f or L1cre(+);Bmpr2+/f; R26R males were further intercrossed with Bmpr2f/f;R26R females to produce L1cre(-);Bmpr2f/f, L1cre(+);Bmpr2+/f, and L1cre(+);Bmpr2f/f. More than a half of the experimental and control mice contained the R26R allele which served to monitor the Cre activities. PCR primer sets detecting the conditional as well as null alleles of Bmpr2 were as previously described 18. Primer sets either detecting the Cre or lacZ gene were used to genotype L1cre or R26R, respectively.
Hemodynamic analysis
Systemic blood pressure was recorded non-invasively using the tail-cuff method. A pneumatic pulse sensor was placed on the tail distal to an occlusion cuff controlled by a Programmed Electro-Sphygmomanometer (PE-300, Narco Bio-Systems, TX), which was connected to the Powerlab system (ADinstrument, CO). To evaluate pulmonary artery pressure, right ventricular systolic pressure (RVSP) was measured by right heart catheterization through the right jugular vein. Briefly, each mouse was anesthetized by ketamine (100 mg/kg) and xylazine (15 mg/kg) and placed with supine position. A Mikro-Tip pressure transducer (SPR-835, Millar Instrument, TX) was inserted into right external jugular vein and advanced into the right ventricle. All electrical outputs from the tail cuff, the pulse sensor and transducer were recorded and analyzed by Powerlab 8/30 data acquisition system and associated Chart software (ADinstrument, CO).
Pulmonary vessel morphometry
After hemodynamic analysis, mice were euthanized, and the heart and lungs were isolated for measuring heart weight and for lung morphometry studies as described in Supplementary methods.
Western blotting and immunohistochemistry
Detailed reagents and procedures used for immunoblotting and immunohistochemistry are described in Supplementary methods.
Statistical analysis
Data are presented as mean±SEM, except for the RVSP data. One-way ANOVA was used to determine a statistical significance among groups, and multiple pairwise comparisons were made by post-hoc tests (Tukey and Tukey-Kramer) using SigmaStat and Minitab. For RVSP data (Fig. 2a), nonparametric one-way ANOVA (Dunn's Method) was used followed by Kruskal-Wallis test for pairwase comparisons. The Z-test was used to evaluate the differences of proportion of PH mice. For comparison of PCNA-positive vascular cells between two genotype groups, the t-test was used.
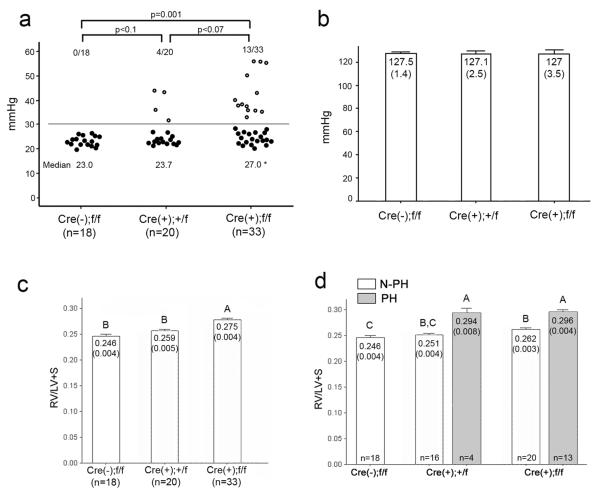
Figure 2.
Endothelial-specific Bmpr2 deletion resulted in elevation of RVSP and RV hypertrophy. a, RV systolic pressure of control, L1cre(+);Bmpr2+/f and L1cre(+);Bmpr2f/f mice was measured at 2-6 months of age. Each circle represents the RVSP of an individual mouse. RVSPs greater than 30 mmHg (the PH group) were shown as open circles. Proportions of PH mice were indicated above the circles. RVSP of L1cre(+);Bmpr2f/f was significantly higher than that of controls (p = 0.001). Median value of RVSPs was used for statistical evaluation due to divergent distribution in L1cre(+);Bmpr2+/f and L1cre(+);Bmpr2f/f groups. Mean values of RVSPs were 23.3±0.5, 26.7±1.5, and 31.7±1.9 for control, +/f and f/f groups, respectively. b, Systemic systolic arterial pressure was not different by genotypes of mice. Mean and SEM (in parenthesis) was shown in each bar. c, The RV/(LV+S) ratio was greater in homozygous group than in control or heterozygous group (p<0.05). d, Non-PH (N-PH; open bars) and PH (solid bars) groups were evaluated separately. Statistical differences (p<0.05) were indicated by letters above each bar (e.g. `A' is different from `B' or `C'; `B,C' is different from `A', but not from `B' nor `C').
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Bmpr2 deletion in pulmonary ECs by a novel Cre transgenic mouse line
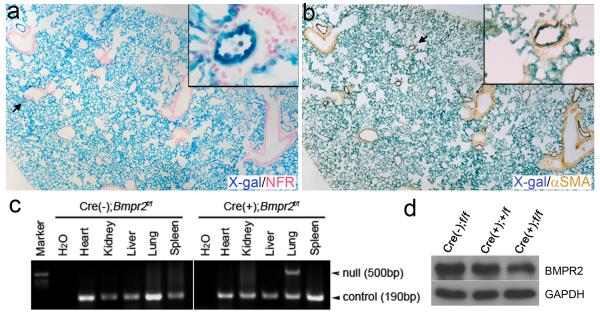
To investigate the role of BMPR2 signaling in pulmonary vascular endothelium, we utilized a Bmpr2 conditional knockout mouse strain18 and a novel Cre transgenic mouse line, designated as Tg(Alk1-cre)-L1. The Tg(Alk1-cre)-L1 line was established during the screening of multiple transgenic founder lines in which the Cre recombinase is driven by a 9.2kb Alk1 genomic fragment 20. Unlike other independent Tg(Alk1-cre) founder lines and previously reported endothelium-specific Cre lines, Cre-mediated gene excision (Cre activity, hereafter) was not detected in the endocardial cells and their progeny in Tg(Alk1-cre)-L1 mice 19. On the other hand, the Cre activity was observed in pECs from the emergence of patent pulmonary vessel formation during embryogenesis till adulthood 19. We have monitored the Cre activity of neonatal and adult Tg(Alk1-cre)-L1 mice (designated as L1cre hereafter) by crossing them with ROSA26-lacZ (R26R) mice in which constitutive and permanent lacZ expression is designed to be activated by Cre. As shown in Supplementary Figure 1, strong and homogeneous lacZ activity was detected in the lungs of L1cre(+);R26R in a comparable level to an endothelial Cre line B1cre(+);R26R newborn pups (Sup. Fig. 1b, and c). Unlike B1cre mice, however, the lacZ activity of L1cre in systemic vessels, except for brain (Sup. Fig. 1d, e), appeared to be mosaic or absent (Sup. Fig. 1f-r). The Cre activity is persistent in adult lungs, as it was widely detected in the lung of 5 month-old L1cre;R26R mice. Histological sections showed that pECs were positive for the Cre activity, whereas the bronchial epithelia and smooth muscle layers were negative (Figure 1a, b). We crossed L1cre mice with Bmpr2f/f females to generate L1cre(+);Bmpr2+/f male mice, that were subsequently crossed with Bmpr2f/f;R26R females.
Figure 1.
Tg(Alk1-cre)-L1 (L1cre) mice express the Cre recombinase in the pulmonary vascular endothelial cells. a and b, Lungs of 5-month-old L1cre;R26R bigenic mice were stained with X-gal for the β-galactosidase expression to visualize the cells in which Cre-mediated recombination has occurred. Sections of whole mount X-gal stained lungs were counter stained with nuclear fast red (NFR; a), or with anti-αSMA antibodies (b). Insets are magnified views of the area indicated by arrows. Note that X-gal positive cells reside in vascular ECs, but not in airway epithelial cells or in smooth muscle cells. c, PCR detection of the Bmpr2 null allele (i.e., Bmpr21loxP: deletion of exons 4 and 5 of Bmpr2 by Cre)18 from genomic DNA isolated from multiple organs/tissues of 2-month-old L1cre(-);Bmpr2f/f (left) and L1cre(+);Bmpr2f/f (right), demonstrating a lung-specific Cre activity. A primer set amplifying an Alk1 locus (~190 bps) was used as a control for PCR reaction. d, Representative Western blotting analysis with anti-BMPR2 antibody on whole lung lysate shows reduced levels of BMPR2 protein (~120 kd) in L1cre(+);Bmpr2f/f lungs compared to L1cre(-);Bmpr2f/f lungs: about 30% reduction (P<0.09, n=3). GAPDH (~36 kd) was used as a loading control.
The L1cre(+);Bmpr2f/f mice were viable over 8 months. Genomic PCR analysis on multiple organs/tissues of two month-old L1cre(+);Bmpr2f/f mice revealed that the Cre-mediated Bmpr2 gene excision was detected only in the lung, indicating that Cre expression was lung-specific in the L1cre line (Figure 1c). Western blot analysis on whole lung extracts with anti-BMPR2 antibodies revealed about 30% reduction of BMPR2 protein in L1cre(+);Bmpr2f/f lungs compared to L1cre(-);Bmpr2f/f controls (Figure 1d).
Bmpr2 deletion in pulmonary ECs can induce elevation of RVSP and RV hypertrophy
To assess the pulmonary artery pressure, right ventricular pressure was measured by right heart catheterization through the right jugular vein in 2-7 month old mice. Median right ventricular systolic pressure (RVSP) of control [L1cre(-);Bmpr2f/f] mice was 23 mmHg (n=18; Figure 2a). The median RVSP of L1cre(+);Bmpr2+/f mice (23.7 mmHg; n=20) was similar to that of control mice, but RVSP in L1cre(+);Bmpr2f/f mice (27 mmHg, n=33) was significantly higher than that in control mice (Figure 2a). There was no difference in the systemic pressure among the different genotype groups (Figure 2b).
In a subset of L1cre(+);Bmpr2+/f (4/20) and L1cre(+);Bmpr2f/f (13/33) mice, RVSPs were greater than 30 mmHg (Supplementary Table 1, 2). These high RVSP mice were designated as pulmonary hypertensive (PH) group, while those mice with RVSP below 30 mmHg were designated as Non(N)-PH group. The RV/(LV+septum) ratio, an indicator of right ventricular (RV) hypertrophy, was higher in L1cre(+);Bmpr2f/f mice compared to the controls or L1cre(+);Bmpr2+/f mice (Figure 2c). When the data were analyzed by the PH and N-PH groups, the PH mice had greater RV/(LV+septum) ratios than did N-PH mice, indicating the presence of sustained elevation of RV pressure in the PH mice (Figure 2d).
Increased number and medial wall thickness of αSMA-positive distal arteries in the mutant mice with elevated RVSP
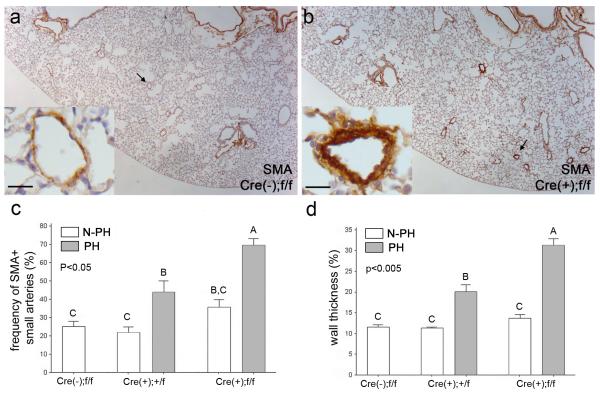
To determine whether the elevation of RVSP and right ventricular hypertrophy were associated with pulmonary vascular remodeling, lung tissue sections were examined by H&E staining and immunohistochemistry. General aspects of lung development and morphology were indistinguishable among genotypes and between the PH and N-PH groups. Immunostaining of the lung sections with anti-α-smooth muscle actin (αSMA) antibodies revealed increased number and wall thickness of αSMA-positive small arteries in the PH group compared to the N-PH group (Figure 3a, b). Quantitative morphometric analysis demonstrated that the percentages of αSMA-positive small arteries (30-70 μm outer diameter) in the PH lungs were greater than those in the N-PH lungs within each genotype group (Figure 3c). Likewise, the wall thickness of αSMA-positive small arteries in the PH group was also greater than that in the N-PH group (Figure 3d). Both the percentage of αSMA-positive small arteries and wall thickness were greater in lungs of L1cre(+);Bmpr2f/f PH mice compared to L1cre(+);Bmpr2+/f PH mice, suggesting that loss of both alleles induced more marked pulmonary vascular remodeling than did loss of one allele.
Figure 3.
Mice in the PH group exhibited increased number of muscularized distal arteries and thickening of medial layers. a and b, Representative histological sections of control (a) and PH (b) lungs stained with anti-αSMA antibody. Insets in (a) and (b) are magnified views of the areas indicated by arrows. Note, readily identifiable strong αSMA-positive small arteries in the PH group lungs (b) and thickening of arterial walls (inset in b). Scale bars in insets represent 25 μm. c and d, Percentages of small arteries positive for αSMA staining (c) and medial thickness of distal arteries (d) were compared among five groups, i.e. control, PH and N-PH groups in each mutant genotype. Statistical differences were indicated by letters above each bar.
Histopathological features in the mutant mice with elevated RVSP
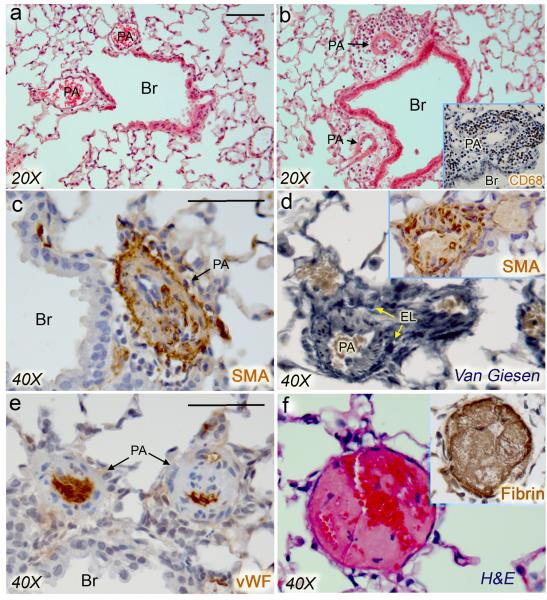
A high incidence of focal leukocyte infiltrations at the surrounding of pulmonary vessels was found in L1cre(+);Bmpr2+/f (46%; 6/13) and L1cre(+);Bmpr2f/f (50%; 12/24) mice, but not in the control mice (0/11) (Figure 4a,b; Supplementary Table 3). Among L1cre(+);Bmpr2f/f mice, frequency of the infiltration tended to be greater in the PH group (8/13) than that in the N-PH (4/11) group. Most of the infiltrated cells were CD68-positive monocyte/macrophages (Figure 4b inset). Conspicuous thickening of αSMA-positive cell layers was observed in small arteries of the PH lungs (Figure 4c,d), and some arteries appeared to be occluded (Figure 4e), resembling the concentric vascular lesion in human PAH lung samples 16. In situ thrombosis was also observed in the PH lungs. As shown in Figure 4f, lumens of some affected vessels were partially or completely occluded by fibrin(ogen)-positive thrombi. The frequency of one or more thrombotic lesions was higher in PH lungs (53%; 9/17) than in N-PH lungs (14%; 3/22) of L1cre(+);Bmpr2f/f and L1cre(+);Bmpr2+/f mice (P<0.05) (Supplementary Table 3). In addition we have examined expression of 5-hydroxytryptamine transporter (5HTT) and tenascin-C (Tn-C) in PH lungs, because elevated serotonin signaling and Tn-C expression have been implicated in the pathogenesis of PAH 21;22. As shown in Supplementary Fig. 2, elevated 5HTT and Tn-C expression was detected in the vascular SMC (vSMC) layer of PH lungs but not in N-PH or control lungs.
Figure 4.
Vascular lesions of the PH lungs mimic some pathological features of human PAH. a and b, H&E staining of control and PH lungs, showing focal leukocyte infiltration around pulmonary arteries of PH lungs of L1cre(+);Bmpr2f/+ or L1cre(+);Bmpr2f/f mice. Immunohistochemical studies revealed that the infiltrated cells were mostly CD68-positive mononuclear cells (inset in b). Br, Bronchus; PA, pulmonary artery. c and d, Immunostaining with anti-αSMA (c, d) and elastic van Giesen staining (d) showed excessive thickening of aSMA-positive layers of small arteries. EL, external elastic lamina. e, Anti-von Willebrand factor (vWF; d) antibodies showed occluded arteries resembling concentric vascular lesions. f, H&E staining showing a representative in situ thrombotic lesion in a PH lung. Lumen of the affected vessel was occluded by formation of fibrin(ogen)-positive thrombus (inset). Scale bars represent 50 μm.
Increased proliferation index in ECs and SMCs of L1cre(+);Bmpr2f/f mice
In order to investigate whether the histological alteration of pulmonary vessels were associated with an increase in proliferation and/or a decrease in (or resistance to) apoptosis of vascular cells, proliferation and apoptosis indexes were examined on 2-7 month old mice. We found no significant differences between PH and N-PH lungs in the percentage of cells positive for a proliferation or apoptosis marker in distal arteries. Since muscularizations of distal arteries in PH lungs were readily detectable in 2-7 month old mice, the processes of proliferation or apoptosis might have already occurred in this age group. We then examined a younger set of mice at about 4 weeks of age. Since we could not perform cardiac catheterization in this young age group, we randomly chose 7 mice each from L1cre(+);Bmpr2f/f and L1cre(-);Bmpr2f/f groups, and performed PCNA or TUNEL assay on their lung sections. We found only a very small number of TUNEL positive vascular cells in the lung sections regardless of their genotypes (data not shown), and no significant difference of TUNEL staining between two genotype groups. On the other hand, both PCNA-positive EC and SMC populations in distal arteries of the L1cre(+);Bmpr2f/f group appeared to be greater than those of controls (p < 0.001) (Fig. 5).
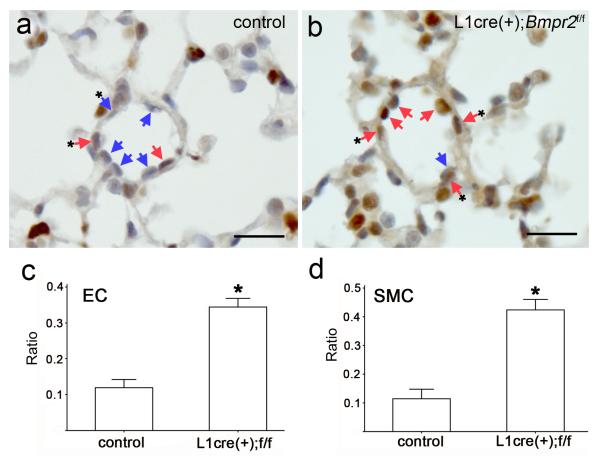
Figure 5.
L1cre(+);Bmpr2f/f mice showed a higher proliferation index in ECs and SMCs of 4week-old lungs. a and b, Lung samples from four week old L1cre(-);Bmpr2f/f (a) and L1cre(+);Bmpr2f/f (b) mice were immunostained with PCNA antibodies followed by light hemotoxylin counter staining. Distal small arteries of about 50 μm diameter are shown as representative images. ECs and SMCs are marked with arrows and arrows with asterisks, respectively. The purplish staining represents PCNA-negative nuclei (indicated by blue arrows), while brown staining represents PCNA-positive nuclei (indicated by red arrows). Scale bars represent 30 μm. c and d, The ratios of PCNA-positive nuclei to total nuclei of ECs (c) and of SMCs (d) at distal arteries were compared between two groups. Significantly higher ratios of PCNA-positive ECs and SMCs were detected (p<0.001, n=7).
Pulmonary hypertension (PH) is a lung disease of diverse etiologies. PH is classified into arterial, venous, hypoxic, thromboembolic, and miscellaneous varieties. Of these varieties, pulmonary arterial hypertension (PAH) typically carries the worst prognosis. PAH is promoted by the imbalance of hormones, growth factors, neurotransmitters, or environmental stresses that leads to pulmonary vascular constriction, cell proliferation, or remodeling. Bone morphogenetic protein receptor type II (BMPR2) signaling plays a critical role in PAH pathogenesis, as germline mutations of BMPR2 are associated with about 25-30% of all PAH cases. PAH pathogenesis involves multiple vascular and non-vascular cell types. We show that mice with the genetic ablation of Bmpr2 in pulmonary endothelial cells (pECs) exhibited an elevation of RVSP, RV hypertrophy, and histopathological features reminiscent of human PAH lungs, demonstrating for the first time in vivo that Bmpr2 mutation in endothelium is sufficient to predispose to PAH. Our data suggest that impaired BMPR2 signaling in pECs may increase the risk of pECs to damages that render the pulmonary vessels more susceptible to dysregulated remodeling. One of the major impediments for PAH studies is limited access to biological samples because pathological samples are available only from lung explants and autopsy specimens, at the very late stage of the disease. Animal models that reproduce key features of PAH provide relevant pathological samples from early to late stages of PAH. Development of non-invasive monitoring systems to detect PH from these mutant mice at early phase will facilitate the usefulness of this animal model for various PAH studies.
Discussion
We show here that some, but not all, mice with the genetic ablation of Bmpr2 in pECs exhibited an elevation of RVSP, RV hypertrophy, and histopathological features reminiscent of human PAH lungs, demonstrating for the first time in vivo that Bmpr2 mutation in endothelium is sufficient to predispose to PAH. We also show that homozygous Bmpr2 deletion is in itself not sufficient to cause PAH, but can increase the susceptibility to PAH to a greater extent than that observed with heterozygous deletion.
PAH pathogenesis is associated with dysregulation of pulmonary arterial remodeling, which involves multiple vascular (endothelial, smooth muscle, and adventitial fibroblasts) and non-vascular cell types (leukocytes, platelets, and circulating endothelial progenitor cells)2. BMPR2 is expressed in most of these cell types 16;23;24. Predisposition to PAH is associated with mutations in ALK1 or ENG genes6;7, which are expressed primarily in endothelial cells, suggesting that pECs may be a primary cell type in which BMPR2 mutations elicit PAH.
In order to examine whether Bmpr2 deletion in pEC is sufficient to predispose mice to PAH, we exploited the Cre/loxP system. For the endothelial-specific Cre driver, we used the Tg(Alk1-cre)-L1 line (L1cre) for following three reasons. First, the Alk1 promoter could be the most relevant promoter to drive Cre expression because Alk1 is persistently expressed in pECs from embryonic to adult stages and ALK1 mutations are linked to IPAH. Second, unlike currently available transgenic mouse lines expressing Cre in endothelium by Tie1, Tie2, Flk, or Vecad regulatory sequences, L1cre mice do not express the Cre recombinase in the endocardial cells, progeny of which constitute atrioventricular cushions, during cardiogenesis stages 19. Conditional deletion of several TGFβ/BMP receptors using these known EC-specific Cre lines cause cardiac malformations, such as cardiac valve or ventricular septal defects 25;26. When we deleted the Bmpr2 gene using Tg(Tie2-cre) mice, most Tg(Tie2-cre);Bmpr2f/- mice died before the weaning age, because of cardiac malformations (unpublished; Beppu et al), which makes this model unsuitable for studying the pathogenesis of PAH. Third, the L1cre mice show strong Cre activities in pulmonary ECs but mosaic or absent Cre activities in most systemic ECs (Supplementary Fig. 1). The L1cre line is superior to the known endothelial Cre lines for evaluating the impact of a gene deletion preferentially in pulmonary ECs without affecting cardiogenesis. The L1cre line will also be useful to assess the role of other BMPR2 signaling partners (e.g BMPR1A and SMAD1) and of potential downstream mediators of BMPR2 in the pathogenesis of PAH.
L1cre(+);Bmpr2f/f mice at 2-7 months of age showed a wide range of RVSP (20.7- 56.3 mmHg; n=33), compared to control mice (19.9 - 26.7 mmHg; n=18) (Supplementary Table1, 2). Although there is no conventional definition of PH in mice, we designated the mice having RVSP greater than 30 mmHg, beyond the range observed in the control mice, as `PH' group (33.2 - 56.3 mmHg; 41%, 13/33). The PH mice displayed higher right ventricular weight, increased number of muscularized distal arteries, and thicker smooth muscle layers, compared to those of N-PH mice or control mice (Fig. 2d; Fig. 3). Similar, but in a less extent, to the L1cre(+);Bmpr2f/f group, 20% (4/20) of L1cre(+);Bmpr2+/f mice also exhibited the PH phenotype. Interestingly, this frequency is similar to human studies showing that about 20% of people harboring heterozygous BMPR2 mutations develop PAH 5. While there are technical limitations in mice to fully satisfy all clinical criteria for PAH, the strong correlations between RVSP and morphometric data infer that these PH mice may represent PAH.
It is unclear why some, but not all, mice with endothelial BMPR2 deficiency develop PAH. The frequency of PAH phenotypes in this study did not differ by the gender of mice. To examine the possibility that the phenotypic variation resulted from inconsistency of Bmpr2 deletion in pECs, we monitored the Cre activity by introducing the R26R allele. No differences in the intensity and pattern of X-gal staining were detected between PH and N-PH groups regardless of their Bmpr2 genotype, indicating that the phenotypic variation should not be attributed to incomplete excision of Bmpr2 gene in pECs. Moreover, the PAH phenotype was not more frequent in mice carrying the R26R allele than those without the allele. When the data is analyzed by two age groups, 2-4 months and 5-7 months of age, the older age group tends to have a higher frequency of the PH mice than younger age group (67% (6/9) vs. 30% (7/24)), indicating that the N-PH mice at 2-7 months of age may eventually develop PAH when they get older. Studies on 12 month- and 18 month-old mice may address whether incomplete penetrance is the onset issue. Since the mice used in this present study were on 129Sv, C57BL/6, and FVB mixed background, strain-specific genetic modifiers may have modulated the propensity to developing PAH in mice carrying mutant Bmpr2 alleles. Candidate modifiers may include genes involved in modulating the balance between TGF-β and BMP signal transduction pathways 27.
We have shown that both pECs and vSMCs in distal arteries of 4 week-old L1cre(+);Bmpr2f/f lungs had a higher number of proliferating cells compared to controls (Fig. 5). Since the BMPR2 signaling is intact in vSMCs of L1cre(+);Bmpr2f/f mice, the higher proliferation index of vSMCs in the mutants is likely due to indirect effect of defects in endothelial cells. It is conceivable that impaired BMPR2 signaling in pECs may result in dysregulated production of cytokines that in turn promote vSMC growth. We observed a higher incidence of infiltration of CD68-positive mononuclear cells in the perivascular regions of L1cre(+);Bmpr2f/f (12/24) and L1cre(+);Bmpr2+/f mice (6/13) compared to L1cre(-);Bmpr2f/f controls (0/11) (Supplementary Table 3). Moreover, in situ thrombosis was observed more frequently in the PH group than the N-PH group or control group. These observations are consistent with pathological findings that human PAH lungs often contain inflammatory and/or thrombotic vascular lesions. Taken together, our data suggest that Bmpr2 deletion in pECs may increase the risk of pECs to damages that render the pulmonary vessels more susceptible to inflammation or thrombosis. A recent report suggested that BMP signaling in EC may protect them from apoptosis, and thus BMPR2 deficiency may predispose the ECs to apoptosis 28. Endothelial cell death can be a triggering point of endothelial dysfunction and PAH pathogenesis. In our system, however, no significant difference in the number of TUNEL-positive cells between normal and mutant lungs at presymptomatic and symptomatic phases.
One of the major impediments for understanding the underlying pathogenetic mechanisms for PAH is limited access to biological samples. IPAH is a rare disease (2-3 per million per year), and pathological samples are available only from lung explants and autopsy specimens, at the very late stage of the disease. Animal models that reproduce key features of PAH provide relevant pathological samples from early to late stages of PAH. L1cre(+);Bmpr2f/f mouse lines presented here may serve as a useful genetic resources to further our knowledge about PAH. Incomplete penetrance of this genetic model is a limitation for studying PAH pathogenesis but it can be a great opportunity to identify environmental or genetic factors that influence PAH pathogenesis in terms of frequency, onset, or severity. It was shown that Bmpr2+/- mice are susceptible to an inflammatory mediator or serotonin in development of PH 10;11. Genetic crosses or pharmacological treatments that modulate inflammation, cell growth, or endothelial functions on L1cre(+);Bmpr2f/f mice may provide valuable insights regarding the source of `hits' for PAH pathogenesis. Because the readouts used in the current study, i.e. right heart catheterization for RVSP, Fulton index, and histopathology, are terminal procedures, it is impossible to monitor the progression of disease, which is a major limitation of this model for preclinical translational studies. Future work should include development of non-invasive monitoring systems such as echocardiographic techniques for monitoring RV wall thickness and/or hemodynamics, and identification of serum biomarkers for the PH group mice.
Supplementary Material
Acknowledgement
We thank Rebecca Howard for editorial assistance, Dr. Kamal K. Mubarak for critical reading of the manuscript, and Dr. Tsugio Seki, Daesong Jang and Naime Fliess for technical assistance.
Sources of Funding This work was supported by the NIH HL64024 and American Heart Association (AHA) grant-in-aid (0455336B) to Dr. Oh, AHA predoctoral fellowship to Dr. Hong, AHA postdoctoral fellowship to Dr. Park, HL74352 to Dr. Bloch, and NIH HL56921 to Dr. Raizada.
Footnotes
Conflict of Interest Disclosures None.
Reference List
- 1.Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M, Rich S, Fishman A. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 2.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin VV, Presberg KW, Doyle RL, Abman SH, McCrory DC, Fortin T, Ahearn G. Prognosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:78S–92S. doi: 10.1378/chest.126.1_suppl.78S. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006;114:1417–1431. doi: 10.1161/CIRCULATIONAHA.104.503540. [DOI] [PubMed] [Google Scholar]
- 5.Austin ED, Loyd JE. Genetics and mediators in pulmonary arterial hypertension. Clin Chest Med. 2007;28:43–viii. doi: 10.1016/j.ccm.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison RE, Flanagan JA, Sankelo M, Abdalla SA, Rowell J, Machado RD, Elliott CG, Robbins IM, Olschewski H, McLaughlin V, Gruenig E, Kermeen F, Laitinen T, Morrell NW, Trembath RC, Halme M, Raisanen-Sokolowski A. Molecular and functional analysis identifies ALK-1 as the predominant cause of pulmonary hypertension related to hereditary haemorrhagic telangiectasia. J Med Genet. 2003;40:865–871. doi: 10.1136/jmg.40.12.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison RE, Berger R, Haworth SG, Tulloh R, Mache CJ, Morrell NW, Aldred MA, Trembath RC. Transforming growth factor-beta receptor mutations and pulmonary arterial hypertension in childhood. Circulation. 2005;111:435–441. doi: 10.1161/01.CIR.0000153798.78540.87. [DOI] [PubMed] [Google Scholar]
- 8.Thomson J, Machado R, Pauciulo M, Morgan N, Yacoub M, Corris P, McNeil K, Loyd J, Nichols W, Trembath R. Familial and sporadic primary pulmonary hypertension is caused by BMPR2 gene mutations resulting in haploinsufficiency of the bone morphogenetic protein tuype II receptor. J Heart Lung Transplant. 2001;20:149. doi: 10.1016/s1053-2498(01)00259-5. [DOI] [PubMed] [Google Scholar]
- 9.Beppu H, Ichinose F, Kawai N, Jones RC, Yu PB, Zapol WM, Miyazono K, Li E, Bloch KD. BMPR-II heterozygous mice have mild pulmonary hypertension and an impaired pulmonary vascular remodeling response to prolonged hypoxia. Am J Physiol Lung Cell Mol Physiol. 2004 doi: 10.1152/ajplung.00239.2004. [DOI] [PubMed] [Google Scholar]
- 10.Song Y, Jones JE, Beppu H, Keaney JF, Jr., Loscalzo J, Zhang YY. Increased susceptibility to pulmonary hypertension in heterozygous BMPR2-mutant mice. Circulation. 2005;112:553–562. doi: 10.1161/CIRCULATIONAHA.104.492488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long L, MacLean MR, Jeffery TK, Morecroft I, Yang X, Rudarakanchana N, Southwood M, James V, Trembath RC, Morrell NW. Serotonin increases susceptibility to pulmonary hypertension in BMPR2-deficient mice. Circ Res. 2006;98:818–827. doi: 10.1161/01.RES.0000215809.47923.fd. [DOI] [PubMed] [Google Scholar]
- 12.West J, Fagan K, Steudel W, Fouty B, Lane K, Harral J, Hoedt-Miller M, Tada Y, Ozimek J, Tuder R, Rodman DM. Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ Res. 2004;94:1109–1114. doi: 10.1161/01.RES.0000126047.82846.20. [DOI] [PubMed] [Google Scholar]
- 13.Liu D, Wang J, Kinzel B, Mueller M, Mao X, Valdez R, Liu Y, Li E. Dosage-dependent requirement of BMP type II receptor for maintenance of vascular integrity. Blood. 2007;110:1502–1510. doi: 10.1182/blood-2006-11-058594. [DOI] [PubMed] [Google Scholar]
- 14.Delot EC, Bahamonde ME, Zhao M, Lyons KM. BMP signaling is required for septation of the outflow tract of the mammalian heart. Development. 2003;130:209–220. doi: 10.1242/dev.00181. [DOI] [PubMed] [Google Scholar]
- 15.Beppu H, Kawabata M, Hamamoto T, Chytil A, Minowa O, Noda T, Miyazono K. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol. 2000;221:249–258. doi: 10.1006/dbio.2000.9670. [DOI] [PubMed] [Google Scholar]
- 16.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, Morrell NW. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105:1672–1678. doi: 10.1161/01.cir.0000012754.72951.3d. [DOI] [PubMed] [Google Scholar]
- 17.Machado RD, James V, Southwood M, Harrison RE, Atkinson C, Stewart S, Morrell NW, Trembath RC, Aldred MA. Investigation of second genetic hits at the BMPR2 locus as a modulator of disease progression in familial pulmonary arterial hypertension. Circulation. 2005;111:607–613. doi: 10.1161/01.CIR.0000154543.07679.08. [DOI] [PubMed] [Google Scholar]
- 18.Beppu H, Lei H, Bloch KD, Li E. Generation of a floxed allele of the mouse BMP type II receptor gene. Genesis. 2005;41:133–137. doi: 10.1002/gene.20099. [DOI] [PubMed] [Google Scholar]
- 19.Park SO, Lee YJ, Seki T, Hong KH, Fliess N, Jiang Z, Park A, Wu X, Kaartinen V, Roman BL, Oh SP. ALK5- and TGFBR2-independent role of ALK1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2 (HHT2) Blood. 2008;111:633–642. doi: 10.1182/blood-2007-08-107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seki T, Hong KH, Yun J, Kim SJ, Oh SP. Isolation of a regulatory region of activin receptor-like kinase 1 gene sufficient for arterial endothelium-specific expression. Circ Res. 2004;94:e72–e77. doi: 10.1161/01.RES.0000127048.81744.31. [DOI] [PubMed] [Google Scholar]
- 21.Guignabert C, Izikki M, Tu LI, Li Z, Zadigue P, Barlier-Mur AM, Hanoun N, Rodman D, Hamon M, Adnot S, Eddahibi S. Transgenic mice overexpressing the 5-hydroxytryptamine transporter gene in smooth muscle develop pulmonary hypertension. Circ Res. 2006;98:1323–1330. doi: 10.1161/01.RES.0000222546.45372.a0. [DOI] [PubMed] [Google Scholar]
- 22.Ihida-Stansbury K, McKean DM, Lane KB, Loyd JE, Wheeler LA, Morrell NW, Jones PL. Tenascin-C is induced by mutated BMP type II receptors in familial forms of pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;291:L694–L702. doi: 10.1152/ajplung.00119.2006. [DOI] [PubMed] [Google Scholar]
- 23.Zakrzewicz A, Hecker M, Marsh LM, Kwapiszewska G, Nejman B, Long L, Seeger W, Schermuly RT, Morrell NW, Morty RE, Eickelberg O. Receptor for activated C-kinase 1, a novel interaction partner of type II bone morphogenetic protein receptor, regulates smooth muscle cell proliferation in pulmonary arterial hypertension. Circulation. 2007;115:2957–2968. doi: 10.1161/CIRCULATIONAHA.106.670026. [DOI] [PubMed] [Google Scholar]
- 24.Jeffery TK, Upton PD, Trembath RC, Morrell NW. BMP4 inhibits proliferation and promotes myocyte differentiation of lung fibroblasts via Smad1 and JNK pathways. Am J Physiol Lung Cell Mol Physiol. 2005;288:L370–L378. doi: 10.1152/ajplung.00242.2004. [DOI] [PubMed] [Google Scholar]
- 25.Park C, Lavine K, Mishina Y, Deng CX, Ornitz DM, Choi K. Bone morphogenetic protein receptor 1A signaling is dispensable for hematopoietic development but essential for vessel and atrioventricular endocardial cushion formation. Development. 2006;133:3473–3484. doi: 10.1242/dev.02499. [DOI] [PubMed] [Google Scholar]
- 26.Jiao K, Langworthy M, Batts L, Brown CB, Moses HL, Baldwin HS. Tgfbeta signaling is required for atrioventricular cushion mesenchyme remodeling during in vivo cardiac development. Development. 2006;133:4585–4593. doi: 10.1242/dev.02597. [DOI] [PubMed] [Google Scholar]
- 27.Newman JH, Phillips JA, III, Loyd JE. Narrative review: the enigma of pulmonary arterial hypertension: new insights from genetic studies. Ann Intern Med. 2008;148:278–283. doi: 10.7326/0003-4819-148-4-200802190-00006. [DOI] [PubMed] [Google Scholar]
- 28.Teichert-Kuliszewska K, Kutryk MJ, Kuliszewski MA, Karoubi G, Courtman DW, Zucco L, Granton J, Stewart DJ. Bone morphogenetic protein receptor-2 signaling promotes pulmonary arterial endothelial cell survival: implications for loss-of-function mutations in the pathogenesis of pulmonary hypertension. Circ Res. 2006;98:209–217. doi: 10.1161/01.RES.0000200180.01710.e6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.