Abstract
Smooth pursuit of natural objects requires flexible allocation of attention to inspect features. However, it has been reported that attention is focused at the fovea during pursuit. We ask here if foveal attention is obligatory during pursuit, or if it can be disengaged. Observers tracked a stimulus composed of a central dot surrounded by four others, and identified one of the dots when it dimmed. Extinguishing the center dot before the dimming improved task performance, suggesting that attention was released from it. To determine if the center dot automatically usurped attention, we provided the pursuit system with an alternative sensory signal by adding peripheral motion that moved with the stimulus. This also improved identification performance, evidence that a central target does not necessarily require attention during pursuit. Identification performance at the central dot also improved, suggesting that the spatial extent of the background did not attract attention to the periphery; instead, peripheral motion freed pursuit attention from the central dot, affording better identification performance. The results show that attention can be flexibly allocated during pursuit, and imply that attention resources for pursuit of small and large objects come from different sources.
Keywords: motion, eye movements, peripheral retina, pursuit system, dual task, fovea
Introduction
Smooth pursuit is a type of eye movement used to follow moving objects and has been studied widely with a small, moving, spot stimulus. Despite being modeled as exclusively driven by visual motion (Robinson, Gordon & Gordon, 1986; Krauzlis & Lisberger, 1989), smooth pursuit is also under cognitive control (Kowler, 1990). Attention is an important cognitive factor at work during pursuit since diverting attention during it impairs pursuit performance (e.g., Acker & Toone, 1978; Brezinova & Kendell, 1977; Souto & Kerzel, 2008). Furthermore, more attention is allocated to pursuit stimuli than background stimuli (Khurana & Kowler, 1987) and attention during maintained pursuit is focused within a small 2º region centered on the fovea and decreases sharply at more eccentric locations (Lovejoy et al., 2009). However, most objects we pursue in the natural environment are different from the spot in that they extend beyond the fovea into the periphery. Moreover, it would be beneficial to be able to attend to eccentric features that require inspection, which could not occur if attention remained focused at the fovea. In the current study, we ask whether attention at a foveal target is obligatory or not.
It has been shown that extinguishing a fixation point before saccade target onset reduces saccade latency, a finding referred to as the gap effect (Saslow, 1967). There is evidence that the gap effect on saccades to static targets occurs because attention is released from the fixation point (Fischer & Weber, 1993; Mackeben & Nakayama, 1993; Pratt et al., 2006; Jin & Reeves, 2009). The gap effect has also been observed with the pursuit system. It has been shown that during pursuit, saccade latency to a moving or static target is reduced after a gap is imposed (Boman et al., 1996; Knox, 1996; Krauzlis & Miles, 1996; Tanaka et al., 1998). The occurrence of the gap effect during pursuit suggests that the gap might also release attention from the pursuit target, since attention is allocated to the fovea while pursuit is in progress (Lovejoy et al., 2009).
Motivated by the previous work, we used the gap paradigm to test whether attention at the fovea is obligatory or not. In the experiments, we employed a secondary, attention-demanding identification task on peripheral targets to allow direct assessment of attentional resource allocation. When a temporal gap was introduced before the target was specified, performance on the identification task during pursuit improved. In a second manipulation, we added peripheral motion consistent with the target motion to provide an alternative driving signal for pursuit. This manipulation also produced better performance on the identification task, suggesting that the mere presence of the central target does not necessarily capture attention. Taken together, the results suggest that attention at the fovea during pursuit can be released for allocation to other locations, and its engagement at the fovea is not obligatory even when a foveal stimulus is present.
Methods
Observers
Altogether, nine observers participated in the current study. Each experiment had four observers and some observers participated in more than one experiment. Among the nine observers were two of authors; each participated in one experiment (SW in the main experiment, and ZL in the supplementary experiment). The remaining observers were naïve to the purposes of the experiments. All observers had normal or corrected to normal visual acuity. All experiments were approved by the Smith-Kettlewell Institutional Review Board and all observers gave informed consent before participating.
Stimuli
All stimuli were generated on a Macintosh computer using Matlab (The MathWorks Inc., Natick MA) and functions from the PsychToolbox (Pelli, 1997) and were displayed at 60 Hz on a 17-inch high-resolution Nanao color monitor (1.76 min arc/pixel). Viewing distance was 48 cm. An identification task was performed on stimuli that were constructed of 5 small bright dots (0.2 deg diameter, 40.0 cd/m2) arranged in the shape of a plus sign (+) that spanned 6 deg vertically and horizontally. The stimulus was either presented alone on a dark screen (0.07 cd/m2, 99% Michelson contrast), or was superimposed on a large field of background dots (22.6 × 37.7 deg). The background dots were the same size as the target dots (0.2 deg diameter), but of slightly lower luminance (2.63 cd/m2, 94% Michelson contrast), and were displayed at a density of 1 dot/deg2. When present, the background dots appeared and disappeared with the task stimuli and when background dots moved off screen, new random dots were generated to maintain a constant dot density over the entire background region. Background dots were restricted from a square region surrounding the target whose borders extended one dot width beyond the target dots (see Figure 1B). Observers were initially tested on the identification task during pursuit with no background to individually set the dim level (range: 13.2 cd/m2 to 24.4 cd/m2) so that their performance was 65–75% correct. This ensured that identification task performance for every observer had room to increase or decrease with experimental manipulations.
Figure 1.
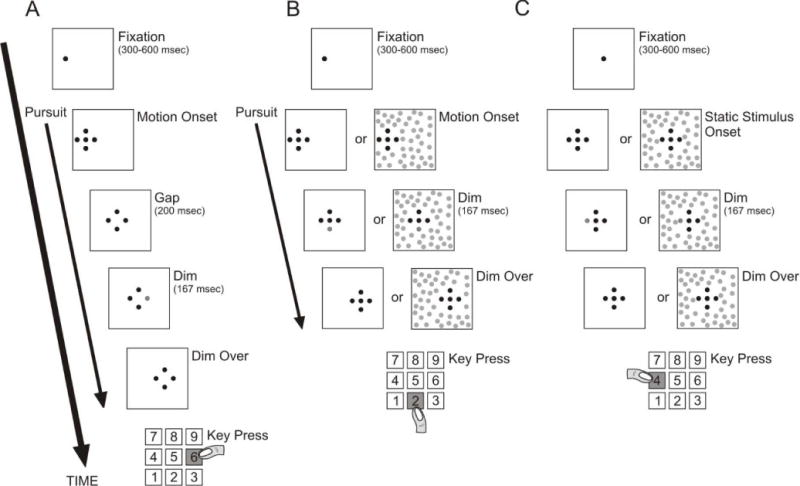
Experimental paradigms. In all cases, observers initially fixated for 300–600 msec. They next pursued the stimuli (except for in the static experiment) that moved from left to right at 10, 20 or 30 deg/sec, for a total duration of 1.0 sec. A keypress at the end of the trial indicated which dot dimmed. A) Gap paradigm. The center spot was turned off 0, 100 or 200 msec before the dimming, which occurred either 300 or 500 msec after target motion onset. Only the four peripheral dots were dim candidates. B) Pursuit task without a gap, with and without a textured background. Any one of the five dots could dim, either 100, 300 or 500 msec after target motion onset. When the background texture was present it moved at the same velocity as the task stimulus. Note that the background texture was restricted from the region occupied by the 5-dot target. C) Static task. The task was identical to that depicted in (B) but the stimulus and background were stationary.
Procedure
Basic task
The general procedure was the same for all experiments (Figure 1). Each trial began with only the center dot of the 5-dot target illuminated, which served as a fixation point. After a pseudo-random fixation interval (300–600 msec), the task phase of the trial began, which lasted 1000 msec. At the start of the task phase, the remaining four spots appeared, as well as the background dots if present, and after a random time, one of the dots became the identification target and was dimmed (i.e., decreased in luminance) for 167 msec. The target location and dim onset time were selected randomly on each trial. At the end of the trial, the observer was required to identify which dot dimmed by pressing a button on the computer’s numeric keypad. The spatial arrangement of the response buttons corresponded to that of the 5-dot target (i.e., 2=bottom, 4=left, 5=center, 6=right, 8=top).
For pursuit trials, the stimulus moved from left to right along the horizontal meridian. The fixation point appeared 3.5 deg away from the left edge of the display, and the stimulus began moving at a constant velocity when the other dots appeared. The speed of the stimulus was selected randomly on each trial to be 10, 20, or 30 deg/sec. In the gap experiment, the target dimmed 300 or 500 msec after the stimulus began to move and the central spot either remained on or was extinguished 0, 100 or 200 msec before the target dimmed and remained off. The center dot did not dim in this experiment, and therefore, only the surrounding four dots were potential targets. No background on-condition was used in the gap experiment. In the background on/off pursuit experiment, the target dimmed 100, 300 or 500 msec after the task stimulus began to move and the background moved at the same speed and in the same direction when present. Instead of only the four surrounding dots, all five dots had an equal probability of being a target. All conditions were randomized, except for the background-on and –off conditions which were constant within a block.
We also had observers perform the identification task when the stimuli were static as a control condition both with and without the background. In static trials, the stimuli were displayed at the center of the monitor. However, when using the same luminance decrement values that were used during pursuit, the performance level of all observers was 100%. We therefore reduced the magnitude of the decrement in the static trials so that observer’s performance was again 65–75% correct, allowing improvements or decrements caused by the independent variables to be detected. As in the background experiment, all five dots had an equal probability of being a target.
Each observer completed a minimum of 5 blocks of the gap experiment, with 216 trials per block. For the background on/off pursuit experiment, 15 blocks of 90 trials were run with the background on, and the same number with the background off. For the static control, 4 blocks of 90 trials were collected with the background on, and the same number with it off.
Eye Movement Measurement & Analysis
Eye position was sampled at 1000 Hz using a video-based Eyelink 1000 eye tracker. Prior to each block of trials, the eye tracker was calibrated by having observers fixate at a series of 9 positions on the display (the center and 8 surrounding peripheral positions). Forehead- and chin-rests maintained a constant viewing distance and stabilized the head for accurate eye tracking. Eye velocity was obtained by digital differentiation of eye position signals and filtered to reduce 60 Hz noise (2 pole Butterworth filter, cutoff = 50 Hz). Saccades were detected with an eye acceleration thresholding algorithm used in previous work (e.g., Badler & Heinen, 2006). Saccades were excised from the velocity traces when pursuit speed was characterized.
Pursuit initiation was first detected using an automatic algorithm that determined when eye velocity first exceeded 5 deg/sec. All traces were visually inspected and the latency measure was manually adjusted when necessary. Pursuit initiation was analyzed in the interval 200–400ms after motion onset to overlap the 100 msec target dim duration. Steady-state pursuit was analyzed in the interval 500–700ms after task stimulus onset, an epoch consistent with that used in other work investigating pursuit with a background and with similar duration stimuli (Spering & Gegenfurtner, 2007). When comparing background-on and background-off pursuit conditions, trials in which a saccade potentially could interfere with target dimming detection because of saccadic suppression were rejected from the analysis. For a trial to be rejected, a saccade had to be initiated in a time window that extended from 30 msec prior to target dimming until dimming ended. The reason for this criterion is that 3 deg saccades have durations of roughly 27.6 msec (Robinson, 1964), therefore saccades that began within this temporal window would have overlapped the dimmed target.
Results
Gap Experiment
Four observers participated in the gap experiment in which the central spot was turned off 0, 100 or 200 msec (gap) before the dot dimming, and gap duration was randomized within the same block to minimize the chances of a general warning effect. After being turned off, the central spot remained off. Since 200 msec is the optimum gap duration (Pratt et al., 1999), we defined the gap effect by the difference between the 0 msec and 200 msec gap durations. The 200 msec gap trials produced better task performance than 0 msec gap trials overall (gap 200 msec, 72.0% and gap 0 msec, 65.9%; an overall 6.1% change, Figure 2). A paired t-test showed that this difference in performance was significant (t(3) = 4.708, p < .02). We also applied Fisher’s exact test to individual observer data with a hypothesis of higher performance in the gap condition, and found significance for three of the four observers (HH p < .05; JH p < .05; AK p < .03; KS p = .256). The results of the gap experiment suggest that attention is not necessarily locked to the fovea during pursuit, at odds with the findings of Lovejoy et al. (2009).
Figure 2.
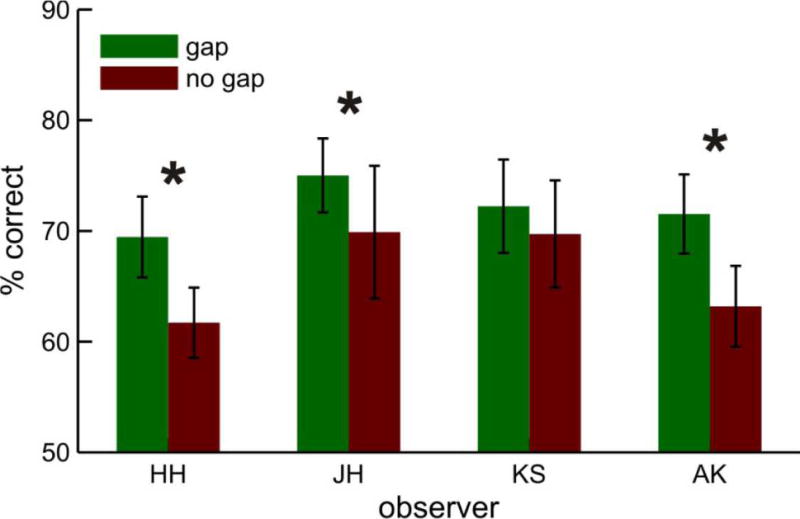
Identification performance with and without a temporal gap for four observers. The gap was 200 msec in duration, and immediately preceded the target dimming. Note better performance overall when the gap was present. Error bars represent ±1standard error of the mean computed from variations across blocks. Asterisks significantly better performance in the gap condition with p<.05.
It is known that the gap effect for saccades is partially due to the offset of the fixation point cuing observers as to when targets will appear (Kingstone & Klein, 1993; Pratt et al., 2000). To test whether the gap could act as a temporal cue to the target blink, we performed a control experiment and found significantly better identification on our task when a gap was used as opposed to when a color change in the central target occurred 200 msec before the dimming (Supplementary material, S1). Furthermore, if observers could merely be cued to direct their attention to the periphery, and the fixation point did not pull attention toward the fovea, the optimum default strategy would be to spread attention to the periphery, since observers knew the task was only performed on the four peripheral dots. Yet, observers did not do this. This observation further supports our contention that in the gap experiment, the presence of the foveal target attracted attention, but that removing the target released it to perform identification on the peripheral spots.
It is possible that the eyes moved more freely during the gap and that they were positioned better relative to the peripheral spots to perform the identification (see van Donkelaar, 1999). Therefore we compared horizontal eye position and velocity during the gap in 200 msec gap trials with those during the same period in no-gap trials using a repeated-measure ANOVA. Neither eye position (F(1, 3) = 0.243, p = .656) nor eye velocity (F(1, 3) = 2.427, p = .217) differed between the gap and no gap trials, evidence that better performance in the gap trials was not due to changes in the pattern of eye movements.
Eye movements during pursuit with an RDC background
This result suggests that attention can be released from the fovea during pursuit. We wondered if reducing reliance on the foveal target as a pursuit drive can also free up attention to perform the identification task even if the foveal target remains present. Since large, moving, random-dot cinematograms (RDCs) are sufficient to drive pursuit (Heinen & Watamaniuk, 1998), we added an RDC background to the 5-dot stimulus, and moved the two together as a coherent unit (see Methods). In the experiment, observers pursued the 5-dot stimulus with or without the background. Here, all five dots served as potential dimming targets with equal probability. Background-on and background-off trials were presented in separate blocks. Four observers participated in this experiment including two who had participated in the gap experiment.
First we present a comparison of the eye movement data in the background on and off conditions because differences that we found in the eye movements between these conditions are relevant to the hypothesis of the study, and also affected how we analyzed the task performance data. During pursuit of a single spot, saccades are often made to correct for position errors that develop between the retinal image of the spot and the fovea. When pursuing large RDCs without a prominent central spot, the frequency of catch-up saccades when the target begins to move is reduced (Heinen & Watamaniuk, 1998). If the background reduces the necessity to use the spot to pursue, it should also reduce catch-up saccades even when the spot is present.
To investigate this, we compared the eye movements obtained in the background-on and background-off conditions (Figure 3). In Figure 3A are typical eye velocity traces for each condition from one observer. Fewer catch-up saccades can be seen in the background-on condition (blue traces) than in the background-off condition (red traces). Note that in general saccades are restricted to the horizontal traces, evidence that the saccadic intrusions were standard “catch-up” saccades largely used to correct for position error during pursuit of a spot stimulus (de Brouwer et al., 2002). We analyzed the number of catch-up saccades during pursuit initiation (200–400 msec after target motion onset) and found fewer saccades in the background-on than the background-off condition at this time (Figure 3B) (background off M=0.384, background on M=0.256; paired t-test t(3) = −3.321, p < .05). Furthermore, although not statistically significant, eye acceleration during pursuit initiation tended to be greater when the background was present (background off M=60.50 deg/sec², background on M=70.57 deg/sec²; paired t-test t(3) = 2.529, p = .086) in agreement with previous work comparing pursuit of an RDC alone and pursuit of a spot (Heinen & Watamaniuk, 1998). These findings suggest that pursuit depends less on the foveal spot when there is a background moving with it.
Figure 3.
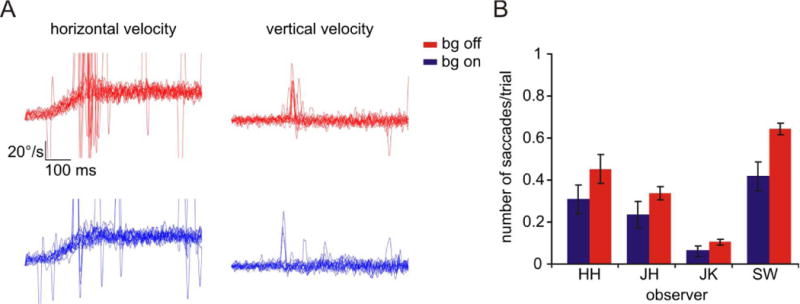
Eye movements while pursuing and performing the identification task with and without the RDC background. A) 15 representative horizontal (left) and vertical (right) eye velocity traces for one observer (HH) randomly sampled from two blocks of trials, one in which the RDC background was on and another in which it was off. Target speed was 30 deg/sec. Top traces (red) are from background-off trials; bottom traces (blue) are from background-on trials. Note fewer saccadic intrusions with the background on. Velocity traces were truncated at +/− 50 deg/sec for ease of viewing. B) Number of saccadic intrusions per trial for each observer averaged over all background-on/off trial blocks. Error bars represent ±1 standard error of the mean.
However, during steady-state pursuit (500–700 msec after target motion onset), the presence of the background did not affect pursuit. Saccade frequency during steady state was not different with the background (background off M=0.126 saccades/trial, background on M=0.130 saccades/trial; paired t-test t(3) = −0.455, p = .680) and paired t-tests for steady-state velocity gain and standard deviation also revealed no significant differences between the two background conditions (steady-state velocity gain, t(3) = −1.119, p = .3446; steady-state velocity standard deviation, t(3) = −.485, p = .661), consistent with previous results (Niemann & Hoffmann, 1997). In addition, the standard deviation of the position error between the eye and the central dot during steady-state pursuit did not differ in two conditions (background off M=0.162, background on 0 M=.178; paired t-test, t(3) = 1.040, p = .375), suggesting that the background did not better stabilize the eyes.
Task performance during pursuit with an RDC background
Next, we present the identification task performance results. The fact that more saccades occurred when the background was off could introduce a confound in assessing task performance; if a saccade occurred when the target dimmed, the dimming might not be perceived because of saccadic suppression, i.e. an elevated luminance threshold during a saccade (Dodge, 1900). Therefore, we characterized identification performance only in trials in which no saccades occurred around the time of target dimming (see Methods). We performed a 4-way repeated-measures ANOVA on the percent correct identification data with background (on, off), speed (10, 20, 30 deg/sec), dimming onset time (100, 300, 500 msec), and target position (center, up, down, left, right) as the independent variables (Table 1). For all observers, accuracy on the task was higher when the background was present than when it was not (Figure 4). As the speed of stimuli increased, task performance decreased which might be expected if more attention is required to pursue at higher speeds, but to our knowledge this has not been shown in the literature. Task performance was also better the later the dimming onset occurred (averaged across speed: 53.9% at 100 msec, 89.0% at 300 msec, 90.9% at 500 msec). Since the early dimming began 100 msec after motion onset and lasted until 270 msec, it roughly overlapped pursuit initiation. Poorer task performance at this time is consistent with previous literature showing that attention is needed for pursuit initiation, manifesting about 150 msec after pursuit onset (Recanzone & Wurtz, 2000), with maximal effects on pursuit velocity 180–300 msec after motion onset (Souto & Kerzel, 2008). There was also a significant interaction between the background and speed such that the difference in performance when the background was and was not present was greater at higher speeds.
Table 1.
Results of the 4-way repeated-measures ANOVA on the percent correct identification data.
| F value | df | p value | |
|---|---|---|---|
| background | 45.164 | 1, 3 | .0067 |
| speed | 9.349 | 2, 6 | .0143 |
| dim time | 42.532 | 2, 6 | .0003 |
| target position | 2.658 | 4, 12 | .0849 |
| background*speed | 6.385 | 2, 6 | .0327 |
| background*dim time | 2.809 | 2, 6 | .1377 |
| background*target position | 2.029 | 4, 12 | .1541 |
| speed*dim time | .927 | 4, 12 | .4805 |
| speed*target position | 1.130 | 8, 24 | .3796 |
| dim time*target position | 4.054 | 8, 24 | .0036 |
| background*speed*dim time | .302 | 4, 12 | .8713 |
| background*speed*target position | .489 | 8, 24 | .8658 |
| background*dim time*target position | .636 | 8, 24 | .7400 |
| speed*dim time*target position | 1.444 | 16, 48 | .1622 |
| background*speed*dim time*target position | 1.057 | 16, 48 | .4196 |
Figure 4.
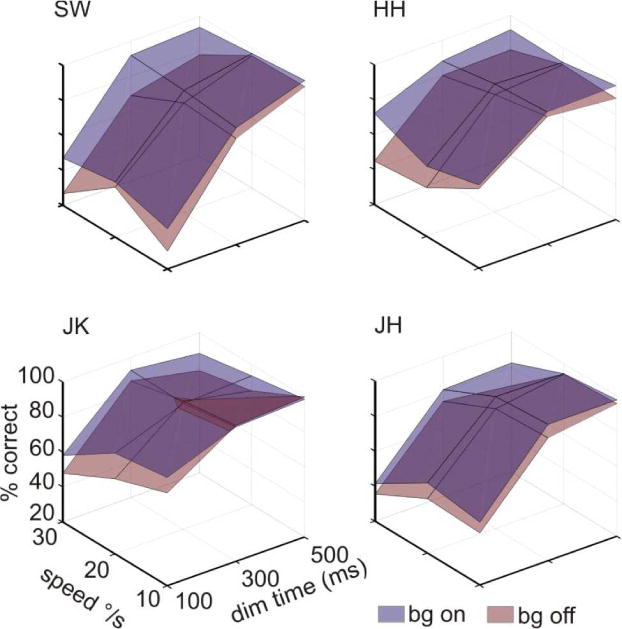
Task performance during pursuit with the RDC background on and off. Identification accuracy was better for all observers and at most target speeds and dimming times when the background was present.
Previous studies have demonstrated that attention is preferentially allocated ahead of the target during pursuit (van Donkelaar, 1999; van Donkelaar & Drew, 2002; Tanaka et al., 1998; Kanai, van der Geest, & Frens, 2003; Blohm, Missal & Lefèvre, 2005, Kahn, et al., 2010), although other work found it to be focused on the target (Khurana & Kowler, 1987; Kerzel, Souto, & Ziegler, 2008; Lovejoy et al., 2009). To test which of these alternatives our results support, we compared identification performance for targets that dimmed in front of or behind the central spot. There was a tendency for better performance when the dimmed dot was presented ahead of the spot, although this improvement was not significant (ahead 79.9%, behind 73.5%; paired t(3) = 1.924, p =.1501). We also tested for a difference in identification performance between the upper and lower dot-dimming positions and found none (upper 77.1%, lower 77.0%; paired t(3) = 0.086, p = .9396).
Does the RDC background release attention for the task?
The background appeared to release attention used to pursue the spot target by providing a sufficient signal to drive smooth pursuit, presumably because attention required to pursue peripheral motion is different from that required to pursue a spot. However, there are several alternative explanations. The first is that the background provided a better motion signal that led to better pursuit, and hence better image stabilization, which in turn enabled easier detection of the target dimming. In support of this we found fewer saccades and higher acceleration during pursuit initiation in background-on trials. However, we only analyzed identification performance in trials where no saccades intruded on the dim time, and when looking at the gain of pursuit initiation as a function of identification performance for the 100 msec dim, we found no gain difference between correct and incorrect trials (gain correct = 1.03, gain incorrect = 1.02; paired t(1) = 2.38, p = 0.25). During steady-state, we found no difference in saccade frequency or the quality of pursuit (see above). Therefore, better stabilization does not appear to account for our results.
Another possible explanation for the results is that the mere presence of the RDC provided a reference luminance that made it easier to detect the dimming of the target dot. If true, an RDC should also improve identification on a static target array as the reference luminance effect should not depend upon the presence or absence of motion. In our initial tests with a static array that had the same luminance decrement as did the one used in the pursuit trials, all observers performed the identification at 100% with the background either on or off, obscuring a potential benefit of the RDC by a ceiling effect. To eliminate this problem, we increased task difficulty by reducing the magnitude of the luminance decrement separately for each observer (see Methods). All other task parameters were the same as in the previous experiment. A paired t-test on the static stimuli data showed that accuracy of dimmed target identification was not improved by the background (t(3) = −.090, p = .437; Figure 6). Fisher’s exact test was done on individual observer’s data with a hypothesis of higher performance in the background on than off condition. This analysis yielded a significant result for only one subject (JH, p < .01) and insignificant results for the remaining three. Two of four subjects even showed significantly better performance on the identification task when no background was presented (SW, p < 0.04; JK, p < 0.001). These results suggest that the performance enhancement observed in the previous experiment in which the background moved with the task stimuli did not occur because the background provided a reference luminance that facilitated dimming detection.
Figure 6.
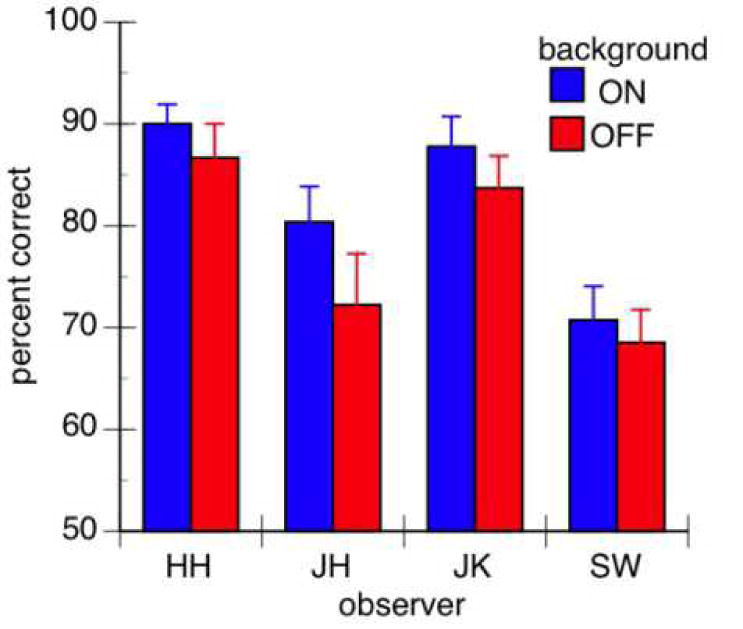
Identification performance at the central dot position. Note that performance was better in the background-on than background-off condition.
A potential explanation for better identification performance when the background is on is that the background attracts attention, and therefore draws attention to the periphery. This would lead to the prediction that performance at the center dot would be either the same or even worse when the background was present than when not since spreading the attention could make it more diffuse. To investigate this possibility, we compared performance at the center dot in the background-on and off conditions (Figure 6). As can be seen here, there is a tendency for performance to improve at the center dot with the background for all observers. This improvement was significant (background on: 84.51%, off: 80.05%. paired t(3)=4.305, p = .023). In fact, the lack of a significant interaction between background and position (see Table 1) suggests that identification performance improved uniformly at all dot positions when the background was moved with the 5-dot stimulus.
Discussion
In the present study we used a secondary identification task to manipulate attentional demands during smooth pursuit. We found that attention can be released from the fovea, as evidenced by an improvement in performance on the secondary task when the foveal stimulus was removed. Our results also demonstrate that during pursuit, the gap effect benefits a secondary perceptual task, and not just a saccadic one. In addition, we found that it is not obligatory for the foveal target to capture attention if an alternative driving signal for pursuit is provided. This was evidenced by our finding that adding peripheral motion also boosted performance on the secondary task. Since there was less interference between the identification task and pursuit with the peripheral motion than with just foveal motion, attention required to pursue in each condition was apparently allocated differentially to different components of the pursuit system.
Previous work found that attention was narrowly allocated within a 2 deg region centered on the pursuit target (Lovejoy et al., 2009). However, in our study we found that attention was displaced away from the central target, allowing successful performance on a task which employed a stimulus that spanned 6 deg. Other work has found that attention is allocated ahead of the pursuit target (van Donkelaar, 1999; van Donkelaar & Drew, 2002). A commonality between this experiment and ours is that the targets were specified by a transient luminance change. Lovejoy et al. (2009) argued that transient targets outside of the foveal window could be detected during pursuit because they invoked attentional capture. This could explain the discrepancy between our results and theirs. Alternatively, the scope of attention is flexible, and modulated by task demands. The pursuit target in the Lovejoy et al. (2009) experiment was a number, and the task required discrimination of a letter or number which could appear at the fovea and therefore may have required more focused foveal attention. Our gap and background manipulations apparently both decreased attentional demands at the fovea to enable better performance on the task, consistent with this view.
Several studies have demonstrated that during smooth pursuit, the latency of saccades from a moving foveal pursuit stimulus to peripheral targets decreases with the gap (Boman et al., 1996; Knox, 1996; Krauzlis & Miles, 1996; Tanaka et al., 1998) as it does with static stimuli (Saslow, 1967; Fischer & Boch, 1983; Fischer & Weber, 1993; Fischer & Rampsperger, 1984). It has been proposed that for static foveal stimuli, the gap reduces saccade latency because removing the fixation point allows for an earlier release of attention from the fovea (Fischer & Weber, 1993; Pratt et al., 2006; Jin & Reeves, 2009). This argument was also supported by improvement of performance on a peripheral vernier discrimination with the gap where no saccades where required (Mackeben & Nakayama, 1993). However, all studies showing a gap effect during pursuit used saccade tasks. Our results instead demonstrate a gap effect in a perceptual task during pursuit, and provide direct evidence that the gap releases attention from a moving foveal target.
The peripheral motion provided by the RDC also appeared to reduce the amount of attention allocated to the central spot. This implies that while the spot is attended when it is the sole pursuit stimulus, its mere presence during pursuit does not necessarily usurp attention, since adding the RCD motion appeared to release attention from it. A potential reason that attention is required to pursue a spot in isolation is that the pursuit system must correct position error in order to keep the eyes foveated on the spot (Pola & Wyatt, 1980; Blohm, et al., 2005). However, when the moving background is present, it may no longer be necessary to use position error as a drive for pursuit since motion provided by the background may be sufficient for this purpose. Therefore, although position error likely remains with the background present, it may be unnecessary to process it, thus reducing foveal attention and leaving the excess attention available for other tasks. Consistent with this idea, there were fewer catch-up saccades with the background (see Results), which are largely used to correct for position error (de Brouwer et al., 2002).
There a several possibilities as to how the background affects attention allocation to improve task performance. One is that it redistributes attention spatially, thereby improving performance at the peripheral dots. This might occur if the background attracted attention to the periphery merely by stimulating peripheral retina. However, this alternative seems unlikely given that the presence of the background improved performance at the center dot (see Figure 6). If the mere spatial extent of the background drew attention to the periphery, performance on our task should also improve when the background moved in a different direction from the 5-dot stimulus. Preliminary data from our laboratory where subjects performed the task with background motion orthogonal to that of the 5-dot stimulus show no benefit of the background, consistent with this idea (Supplementary data 2).
Instead, we think that attention used to pursue the background arises from a different pool than that used to pursue the central spot, and that the spot attention is shared with the identification task. Given, pursuit of the background would free up attention from the spot and lead to improved performance on the identification task. Different pools of attention may be exploited to pursue the spot and the background because different computations are performed for pursuit of these very different stimuli. When the spot is the goal of pursuit, it not only produces motion on the retina, but it also can introduce significant position error between it and the fovea, which is corrected by a position mechanism (Blohm, Missal & Lefèvre, 2005). However, the RDC background alone produces no consistent position error and therefore it is likely to activate more exclusively motion related mechanisms. Therefore, if it is used to drive the pursuit system, it might alleviate the need to correct position error that the spot might introduce. Our belief is that while the attention pool that is used to pursue the spot shares its resources with other cognitive functions such as the identification task in the current study, the attention pool used to pursue the background is outside the realm of attention used for such tasks.
Our results have implications for differential activation of structures in the pursuit system when large vs. small objects are pursued. When the main stimulus for pursuit is small and attention is directed towards it to drive pursuit, potential structures that are preferentially activated are the superior colliculus (SC), which processes position error (Krauzlis et al., 2000), and possibly pursuit areas in frontal cortex that are not part of the classic motion-processing pathway, such as the frontal eye fields (FEF) (MacAvoy et al., 1991; Gottlieb et al., 1993; Shi et al., 1998; Tanaka & Lisberger, 2001) and the supplementary eye fields (Schlag & Schlag-Rey, 1987; Schlag et al., 1992; Heinen, 1995; Lee & Tehovnik, 1995; Missal & Heinen, 2001). In the current experiments, we supplemented the small stimulus that is normally foveated during pursuit with a large RDC that also stimulated peripheral retina. Since our results suggest the RDC minimizes the necessity to attend to the spot in order to pursue, position error that develops between it and the fovea may be irrelevant for maintaining pursuit, and therefore pursuit regions that are more specifically involved in processing motion might be preferentially activated when the RDC is present. These would include the main cortical motion areas that have been implicated in pursuit, including the middle temporal area (MT) and the medial superior temporal area (MST) (Dürsteler & Wurtz, 1988, Newsome et al., 1988). Also activated might be subcortical structures in the accessory optic system, such as the nucleus of the optic tract (NOT), that have been implicated in pursuit, peripheral motion processing and the more reflexive optokinetic reflex (OKR) (Hoffmann & Distler, 1989; Mustari & Fuchs, 1990; Ilg et al. 1993; Inoue et al. 2000).
However, the colliculus might still be activated since it has been shown to be active in the absence of a small spot when explicit correction of position error is not required (Hafed & Krauzlis, 2008). In this study, observers pursued by maintaining gaze at the center of two peripheral spots that moved together as a unit. These authors interpreted the role of the colliculus as representing the goal of pursuit and not explicitly correcting for position error, since correcting position error would have placed the fovea on one of the peripheral dots. We speculate that the colliculus was active in this situation because it was correcting for “virtual” position error created by attempting to keep the fovea located between those spots, possibly because attention was directed here due to previous training. Given the hypothesis that the colliculus represents the goal of pursuit, we further speculate that it would not be active when a large RDC is used to drive pursuit, as was the case in our experiments, because an explicit goal is not inherent in the motion of the background.
How do our findings relate to ocular following, smooth eye movements that respond to full-field motion (Miles et al., 1986)? Natural pursuit objects, such as a colleague walking through the workplace, generally have a larger spatial extent than a small spot, as well as features that may require inspection. We believe that voluntary pursuit of the global motion of larger objects (simulated by our random-dot patterns) stimulates MT/MST, where pursuit neurons that respond to large texture motion are found (Komatsu & Wurtz, 1988), but also recruits circuitry in the system that generates the optokinetic reflex (OKR), a subsystem of ocular following that we think has been modified through evolution to follow an object selected for pursuit. The nucleus of the optic track (NOT), commonly thought to drive OKR (Hoffmann et al. 1988; Kato et al. 1988; Schiff et al. 1988), also has neurons that respond during pursuit (Mustari & Fuchs, 1990). We think that this modern OKR circuitry performs a function of a larger object to allow inspection of its features using an attentional, foveate system that utilizes fixation and saccades.
However, pursuit of natural objects is critically different from primitive OKR. Primitive OKR is driven by motion of the global visual scene on the retina and is used to supplement the vestibuloocular reflex (VOR) in stabilizing that scene during self-movement in afoveate animals such as fish (Cohen, 1974). OKR has been studied with textured stimuli, usually composed of gratings or random dot stimuli such as those which we used in our experiments. However, in foveate animals, this primitive circuitry can be more of a hindrance than a benefit, at least during pursuit of an object in the visual scene. When pursuing an object in the natural world, motion driving the pursuit system is almost always directed opposite to that which would drive primitive OKR, and therefore this reflex must either be inhibited or ignored.
Our results have implications for operation of the pursuit system outside of the laboratory. Specifically, they suggest that attention differentially activates different components of the pursuit system during pursuit of small, foveal objects such as birds, or airplanes at a distance, and pursuit of larger objects that stimulate peripheral retina such as people or other animals in our proximity.
Conclusions
Previous work has shown that attention during smooth pursuit is restricted to a narrow region centered on the fovea. In this study we perform two experiments which show that attention instead can be flexibly allocated during pursuit. Attention allocation was assessed by measuring identification performance on a dot-dimming task. Better performance on peripheral dot identification during pursuit was observed in a gap paradigm when the central dot was extinguished before the dot dimmed. Better performance was also found when consistent peripheral motion was provided as an alternative pursuit drive. Improved performance was not due to better image stabilization, or better visibility of the dimming in the presence of the background, rather the background appeared to free up attention from pursuit of the spot for the identification task. We conclude that attention can be flexibly allocated during pursuit, and that attention for pursuit of peripheral motion comes from a different source from that required to pursuit a small spot. Our results suggest that ocular pursuit in natural scenes may utilize neural mechanisms that require little conscious intervention, thereby allowing maximum allocation of resources to other tasks that require attention. Our work also has clinical implications for patients with age-related macular degeneration (AMD), as it suggests that oculomotor therapy for smooth pursuit should include peripheral motion.
Supplementary Material
Figure 5.
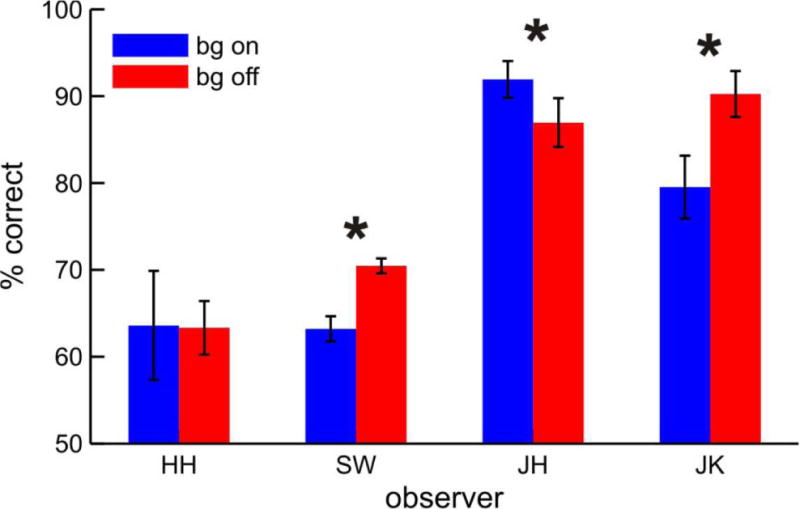
Identification performance in the static control condition. Note that there was no consistent difference in identification accuracy between the background-on and -off conditions. Error bars represent ±1 standard error of the mean computed from variations over blocks. Asterisks indicate significance p<.05.
Acknowledgments
Supported by NIH grant EY013886 and the Rachel C. Atkinson Fellowship award. We would like to thank Joel Ford for his valuable technical assistance on the project.
Contributor Information
Stephen J. Heinen, The Smith-Kettlewell Eye Research Institute, San Francisco, CA, 94115 USA
Zhenlan Jin, The Smith-Kettlewell Eye Research Institute, San Francisco, CA, 94115 USA.
Scott N.J. Watamaniuk, Department of Psychology, Wright State University, Dayton, OH, 45435 USA
References
- Badler JB, Heinen SJ. Anticipatory movement timing using prediction and external cues. J Neurosci. 2006;26:4519–4525. doi: 10.1523/JNEUROSCI.3739-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blohm G, Missal M, Lefèvre P. Direct evidence for a position input to the smooth pursuit system. J Neurophysiol. 2005;94:712–721. doi: 10.1152/jn.00093.2005. [DOI] [PubMed] [Google Scholar]
- Blohm G, Missal M, Lefèvre P. Processing of retinal and extraretinal signals for memory-guided saccades during smooth pursuit. J Neurophysiol. 2005;93:1510–1522. doi: 10.1152/jn.00543.2004. [DOI] [PubMed] [Google Scholar]
- Boman D, Braun D, Hotson J. Stationary and pursuit visual fixation share similar behavior. Vision Res. 1996;36:751–763. doi: 10.1016/0042-6989(95)00160-3. [DOI] [PubMed] [Google Scholar]
- Brown B. Dynamic visual acuity, eye movements and peripheral acuity for moving targets. Vision Res. 1972;12:305–321. doi: 10.1016/0042-6989(72)90120-4. [DOI] [PubMed] [Google Scholar]
- Cheng M, Outerbridge JS. Optokinetic nystagmus during selective retinal stimulation. Exp Brain Res. 1975;23:129–139. doi: 10.1007/BF00235455. [DOI] [PubMed] [Google Scholar]
- Cohen B. The vestibulo-ocular reflex arc. In: Kornhuber HH, editor. Handbook of Sensory Physiology, Vestibular System: Basic Mechanisms. Vol. 6. New York: Springer-Verlag; 1974. pp. 478–540. [Google Scholar]
- Collewijn H, Tamminga EP. Human smooth and saccadic eye movements during voluntary pursuit of different target motions on different backgrounds. J Physiol. 1984;351:217–250. doi: 10.1113/jphysiol.1984.sp015242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler GH, Ley AH. Kinetic visual acuity. British Journal of Physiological Optics. 1963;20:119–127. [PubMed] [Google Scholar]
- de Brouwer S, Missal M, Barnes G, Lefèvre P. Quantitative analysis of catch-up saccades during sustained pursuit. J Neurophysiol. 2002;87:1772–1780. doi: 10.1152/jn.00621.2001. [DOI] [PubMed] [Google Scholar]
- Dodge R. Visual perception during eye movement. Psych Rev. 1900;7:454–465. [Google Scholar]
- Downing CJ, Movshon JA. Spatial and temporal summation in the detection of motion in stochastic random dot displays. Investigative Ophthalmology and Visual Science. 1989;30(supplement):72. [Google Scholar]
- Dürsteler MR, Wurtz RH. Pursuit and optokinetic deficits following chemical lesions of cortical areas MT and MST. J Neurophysiol. 1988;60:940–965. doi: 10.1152/jn.1988.60.3.940. [DOI] [PubMed] [Google Scholar]
- Fischer B, Boch R. Saccadic eye movements after extremely short reactions in the monkey. Brain Res. 1983;260:21–26. doi: 10.1016/0006-8993(83)90760-6. [DOI] [PubMed] [Google Scholar]
- Fischer B, Ramsperger E. Human express saccades: extremely short reaction times of goal directed eye movements. Exp Brain Res. 1984;57:191–195. doi: 10.1007/BF00231145. [DOI] [PubMed] [Google Scholar]
- Fischer B, Weber H. Express saccades and visual attention. Behav & Brain Sci. 1993;16:553–567. [Google Scholar]
- Flechtner KM, Steinacher B, Sauer R, Mackert A. Smooth pursuit eye movements in schizophrenia and affective disorder. Psychol Med. 1997;27:1411–1419. doi: 10.1017/s0033291797005709. [DOI] [PubMed] [Google Scholar]
- Geer I, Robertson KM. Measurement of central and peripheral dynamic visual acuity thresholds during ocular pursuit of a moving target. Optom Vis Sci. 1993;70:552–560. doi: 10.1097/00006324-199307000-00006. [DOI] [PubMed] [Google Scholar]
- Gottlieb JP, Bruce CJ, MacAvoy MG. Smooth eye movements elicited by microstimulation in the primate frontal eye field. J Neurophysiol. 1993;69:786–799. doi: 10.1152/jn.1993.69.3.786. [DOI] [PubMed] [Google Scholar]
- Haarmeier T, Thier P. Impaired analysis of moving objects due to deficient smooth pursuit eye movements. Brain. 1999;122:1495–1505. doi: 10.1093/brain/122.8.1495. [DOI] [PubMed] [Google Scholar]
- Hafed ZM, Krauzlis RJ. Goal representations dominate superior colliculus activity during extrafoveal tracking. J Neurosci. 2008;28:9426–9439. doi: 10.1523/JNEUROSCI.1313-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen SJ. Single-neuron activity in dorsomedial frontal cortex during smooth pursuit eye movements. Exp Brain Res. 1995;104:357–361. doi: 10.1007/BF00242022. [DOI] [PubMed] [Google Scholar]
- Heinen SJ, Watamaniuk SNJ. Spatial integration in human smooth pursuit. Vision Res. 1998;38:3785–3794. doi: 10.1016/s0042-6989(97)00422-7. [DOI] [PubMed] [Google Scholar]
- Hoffmann KP, Distler C, Erickson RG, Mader W. Physiological and anatomical identification of the nucleus of the optic tract and dorsal terminal nucleus of the accessory optic tract in monkeys. Exp Brain Res. 1988;69:635–644. doi: 10.1007/BF00247315. [DOI] [PubMed] [Google Scholar]
- Hoffmann KP, Distler C. Quantitative analysis of visual receptive fields of neurons in nucleus of the optic tract and dorsal terminal nucleus of the accessory optic tract in macaque monkey. J Neurophysiol. 1989;62:416–428. doi: 10.1152/jn.1989.62.2.416. [DOI] [PubMed] [Google Scholar]
- Holzman PS, Kringlen E, Levy DL, Proctor LR, Haberman SJ, Yasillo NJ. Abnormal-pursuit eye movements in schizophrenia: Evidence for a genetic indicator. Arch Gen Psychiatry. 1977;34:802–805. doi: 10.1001/archpsyc.1977.01770190064005. [DOI] [PubMed] [Google Scholar]
- Ilg UJ, Bremmer F, Hoffmann KP. Optokinetic and pursuit system: a case report. Behav Brain Res. 1993;57:21–29. doi: 10.1016/0166-4328(93)90057-w. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Takemura A, Kawano K, Mustari MJ. Role of the pretectal nucleus of the optic tract in short-latency ocular following responses in monkeys. Exp Brain Res. 2000;131:269–281. doi: 10.1007/s002219900310. [DOI] [PubMed] [Google Scholar]
- Khan AZ, Lefèvre P, Heinen SJ, Blohm G. Default allocation of attention is broadly ahead of smooth pursuit. Journal of Vision. doi: 10.1167/10.13.7. In press. [DOI] [PubMed] [Google Scholar]
- Kanai R, van der Geest JN, Frens MA. Inhibition of saccade initiation by preceding smooth pursuit. Exp Brain Res. 2003;148(3):300–307. doi: 10.1007/s00221-002-1281-8. [DOI] [PubMed] [Google Scholar]
- Kathmann N, Hochrein A, Uwer R, Bondy B. Deficits in gain of smooth pursuit eye movements in schizophrenia and affective disorder patients and their unaffected relatives. Am J Psychiatry. 2003;160:696–702. doi: 10.1176/appi.ajp.160.4.696. [DOI] [PubMed] [Google Scholar]
- Kato I, Harada K, Hasegawa T, Ikarashi T. Role of the nucleus of the optic tract of monkeys in optokinetic nystagmus and optokinetic after-nystagmus. Brain Res. 1988;474:16–26. doi: 10.1016/0006-8993(88)90665-8. [DOI] [PubMed] [Google Scholar]
- Kerzel D, Souto D, Ziegler NE. Effects of attention shifts to stationary objects during steady-state smooth pursuit eye movements. Vision Res. 2008;48:958–969. doi: 10.1016/j.visres.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Khurana B, Kowler E. Shared attentional control of smooth eye movement and perception. Vision Res. 1987;27:1603–1618. doi: 10.1016/0042-6989(87)90168-4. [DOI] [PubMed] [Google Scholar]
- Kingstone A, Klein RM. Visual offsets facilitate saccadic latency – Does predisengagement of visuospatial attention mediate this gap effect? J Exp Psych Hum Percep Perf. 1993;19(6):1251–1265. doi: 10.1037//0096-1523.19.6.1251. [DOI] [PubMed] [Google Scholar]
- Knox PC. The effect of the gap paradigm on the latency of human smooth pursuit of eye movement. Neuroreport. 1996;7:3027–3030. doi: 10.1097/00001756-199611250-00046. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Wurtz RH. Relation of cortical areas MT and MST to pursuit eye movements. III. Interaction with full-field visual stimulation. J Neurophysiol. 1988;60:621–644. doi: 10.1152/jn.1988.60.2.621. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ. Neuronal activity in the rostral superior colliculus related to the initiation of pursuit and saccadic eye movements. J Neurosci. 2003;23:4333–4344. doi: 10.1523/JNEUROSCI.23-10-04333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzlis RJ, Basso MA, Wurtz RH. Discharge properties of neurons in the rostral superior colliculus of the monkey during smooth-pursuit eye movements. J Neurophysiol. 2000;84:876–891. doi: 10.1152/jn.2000.84.2.876. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Lisberger SG. A control system model of smooth pursuit eye movements with realistic emergent properties. Neural Comp. 1989;1:116–122. [Google Scholar]
- Krauzlis RJ, Miles FA. Decreases in the latency of smooth pursuit and saccadic eye movements produced by the “gap paradigm” in the monkey. Vision Res. 1996;36:1973–1985. doi: 10.1016/0042-6989(95)00307-x. [DOI] [PubMed] [Google Scholar]
- Koenderink JJ, van Doorn AJ, van de Grind WA. Spatial and temporal parameters of motion detection in the peripheral visual field. J Opt Soc Am A. 1985;2:252–259. doi: 10.1364/josaa.2.000252. [DOI] [PubMed] [Google Scholar]
- Kowler E. Eye movements and their role in visual and cognitive processes. Elsevier; Amsterdam: 1990. The role of visual and cognitive processes in the control of eye movement. [PubMed] [Google Scholar]
- Kowler E, Murphy BJ, Steinman RM. Velocity matching during smooth pursuit of different targets on different backgrounds. Vision Res. 1978;18:603–605. doi: 10.1016/0042-6989(78)90211-0. [DOI] [PubMed] [Google Scholar]
- Kowler E, van der Steen J, Tamminga EP, Collewijn H. Voluntary selection of the target for smooth eye movement in the presence of superimposed, full-field stationary and moving stimuli. Vision Res. 1984;24:1789–1798. doi: 10.1016/0042-6989(84)90010-5. [DOI] [PubMed] [Google Scholar]
- Lawden MC, Bagelmann H, Crawford TJ, Matthews TD, Kennard C. An effect of structured backgrounds on smooth pursuit eye movements in patients with cerebral lesions. Brain. 1995;118:37–48. doi: 10.1093/brain/118.1.37. [DOI] [PubMed] [Google Scholar]
- Lee K, Tehovnik EJ. Topographic distribution of fixation-related units in the dorsomedial frontal cortex of the rhesus monkey. Eur J Neurosci. 1995;7:1005–1011. doi: 10.1111/j.1460-9568.1995.tb01088.x. [DOI] [PubMed] [Google Scholar]
- Lovejoy LP, Fowler GA, Krauzlis RJ. Spatial allocation of attention during smooth pursuit eye movements. Vision Res. 2009;49:1275–1285. doi: 10.1016/j.visres.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvigh E, Miller JW. Study of visual acuity during the ocular pursuit of moving test objects. J Opt Soc Am. 1958;48:799–802. doi: 10.1364/josa.48.000799. [DOI] [PubMed] [Google Scholar]
- Luebke AE, Robinson DA. Transition dynamics between pursuit and fixation suggest different systems. Vision Res. 1988;28:941–946. doi: 10.1016/0042-6989(88)90103-4. [DOI] [PubMed] [Google Scholar]
- MacAvoy MG, Gottlieb JP, Bruce CJ. Smooth-pursuit eye movement representation in the primate frontal eye field. Cereb Cortex. 1991;1:95–102. doi: 10.1093/cercor/1.1.95. [DOI] [PubMed] [Google Scholar]
- Mackeben M, Nakayama K. Express attentional shifts. Vision Res. 1993;33:85–90. doi: 10.1016/0042-6989(93)90061-z. [DOI] [PubMed] [Google Scholar]
- Miles FA, Kawano K, Optican LM. Short-latency ocular following responses of monkey. I. Dependence on temporospatial properties of visual inputs. J Neurophysiol. 1986;56:1321–1353. doi: 10.1152/jn.1986.56.5.1321. [DOI] [PubMed] [Google Scholar]
- Missal M, Heinen SJ. Facilitation of smooth pursuit initiation by electrical stimulation in the region of the supplementary eye fields. J Neurophysiol. 2001;86:2413–2425. doi: 10.1152/jn.2001.86.5.2413. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Fixation cells in monkey superior colliculus. I. Characteristics of cell discharge. J Neurophysiol. 1993;70:559–575. doi: 10.1152/jn.1993.70.2.559. [DOI] [PubMed] [Google Scholar]
- Mustari MJ, Fuchs AF. Discharge patterns of neurons in the pretectal nucleus of the optic tract (NOT) in the behaving primate. J Neurophysiol. 1990;64:77–90. doi: 10.1152/jn.1990.64.1.77. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Wurtz RH, Komatsu H. Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. J Neurophysiol. 1988;60:604–620. doi: 10.1152/jn.1988.60.2.604. [DOI] [PubMed] [Google Scholar]
- Niemann T, Hoffmann KP. The influence of stationary and moving textured backgrounds on smooth-pursuit initiation and steady state pursuit in humans. Exp Brain Res. 1997;115:531–540. doi: 10.1007/pl00005723. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The video toolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- Pivik RT. Smooth pursuit eye tracking dysfunction in schizophrenia: Subcortical implications. J Psychiatry Neurosci. 1991;16:123–130. [PMC free article] [PubMed] [Google Scholar]
- Pola J, Wyatt HJ. Target position and velocity: The stimuli for smooth pursuit eye movements. Vision Res. 1980;20:523–534. doi: 10.1016/0042-6989(80)90127-3. [DOI] [PubMed] [Google Scholar]
- Pratt J, Bekkering H, Abrams RA, Adam J. The gap effect for spatially oriented responses. Acta Psychol (Amst) 1999;102:1–12. doi: 10.1016/s0001-6918(99)00014-1. [DOI] [PubMed] [Google Scholar]
- Pratt J, Bekkering H, Leung M. Estimating the components of the gap effect. Exp Brain Res. 2000;130:258–263. doi: 10.1007/s002219900243. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Wurtz RH. Effects of attention on MT and MST neuronal activity during pursuit initiation. J Neurophysiol. 2000;83:777–790. doi: 10.1152/jn.2000.83.2.777. [DOI] [PubMed] [Google Scholar]
- Robinson DA. The mechanics of human saccadic eye movement. J Physiol. 1964;174:245–264. doi: 10.1113/jphysiol.1964.sp007485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DA, Gordon JL, Gordon SE. A model of the smooth pursuit eye movement system. Biol Cybern. 1986;55:43–57. doi: 10.1007/BF00363977. [DOI] [PubMed] [Google Scholar]
- Saslow MG. Effects of components of displacement-step stimuli upon latency for saccadic eye movement. J Opt Soc Am. 1967;57:1024–1029. doi: 10.1364/josa.57.001024. [DOI] [PubMed] [Google Scholar]
- Schiff D, Cohen B, Raphan T. Nystagmus induced by stimulation of the nucleus of the optic tract in monkey. Exp Brain Res. 1988;70:1–14. doi: 10.1007/BF00271841. [DOI] [PubMed] [Google Scholar]
- Schlag J, Schlag-Rey M. Evidence for a supplementary eye field. J Neurophysiol. 1987;57:179–200. doi: 10.1152/jn.1987.57.1.179. [DOI] [PubMed] [Google Scholar]
- Schlag J, Schlag-Rey M, Pigarev I. Supplementary eye field: influence of eye position on neural signals of fixation. Exp Brain Res. 1992;90:302–306. doi: 10.1007/BF00227242. [DOI] [PubMed] [Google Scholar]
- Shi D, Friedman HR, Bruce CJ. Deficits in smooth-pursuit eye movements after muscimol inactivation within the primate’s frontal eye field. J Neurophysiol. 1998;80:458–464. doi: 10.1152/jn.1998.80.1.458. [DOI] [PubMed] [Google Scholar]
- Souto D, Kerzel D. Dynamics of attention during the initiation of smooth pursuit eye movements. J Vis. 2008;8(14):3. doi: 10.1167/8.14.3. [DOI] [PubMed] [Google Scholar]
- Spering M, Gegenfurtner KR. Contextual effects on smooth-pursuit eye movements. J Neurophysiol. 2007;97:1353–1367. doi: 10.1152/jn.01087.2006. [DOI] [PubMed] [Google Scholar]
- Spering M, Gegenfurtner KR, Kerzel D. Distractor interference during smooth pursuit eye movements. J Exp Psychol Hum Percept Perform. 2006;32:1136–1154. doi: 10.1037/0096-1523.32.5.1136. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Clementz BA, Haas GL, Escobar MD, Drake K, Frances AJ. Eye tracking dysfunction in schizophrenia: characterization of component eye movement abnormalities, diagnostic specificity, and the role of attention. J Abnorm Psychol. 1994;103:222–230. doi: 10.1037//0021-843x.103.2.222. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Lisberger SG. Regulation of the gain of visually-guided smooth pursuit eye movements by frontal cortex. Nature. 2001;409:191–194. doi: 10.1038/35051582. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Yoshida T, Fukushima K. Latency of saccades during smooth-pursuit eye movement in man. Exp Brain Res. 1998;121:92–98. doi: 10.1007/s002210050440. [DOI] [PubMed] [Google Scholar]
- Van den Berg AV, Collewijn H. Human smooth pursuit effects of stimulus extent and of spatial and temporal constraints of the pursuit trajectory. Vision Res. 1986;26:1209–1222. doi: 10.1016/0042-6989(86)90102-1. [DOI] [PubMed] [Google Scholar]
- van Donkelaar P. Spatiotemporal modulation of attention during smooth pursuit eye movements. Neuroreport. 1999;10:2523–2526. doi: 10.1097/00001756-199908200-00016. [DOI] [PubMed] [Google Scholar]
- van Donkelaar P, Drew AS. The allocation of attention during smooth pursuit eye movements. Prog Brain Res. 2002;140:267–277. doi: 10.1016/S0079-6123(02)40056-8. [DOI] [PubMed] [Google Scholar]
- Van Doorn AJ, Koenderink JJ. Spatiotemporal integration in the detection of coherent motion. Vision Res. 1984;24:47–53. doi: 10.1016/0042-6989(84)90143-3. [DOI] [PubMed] [Google Scholar]
- Van Doorn AJ, Koenderink JJ. Spatial properties of the visual detectability of moving spatial white noise. Exp Brain Res. 1982;45:189–195. doi: 10.1007/BF00235778. [DOI] [PubMed] [Google Scholar]
- Verghese P. Visual search and attention: a signal detection theory approach. Neuron. 2001;31:523–535. doi: 10.1016/s0896-6273(01)00392-0. [DOI] [PubMed] [Google Scholar]
- Watamaniuk SNJ, Heinen SJ. Human smooth pursuit direction discrimination. Vision Res. 1999;39:59–70. doi: 10.1016/s0042-6989(98)00128-x. [DOI] [PubMed] [Google Scholar]
- Watamaniuk SNJ, Sekuler R. Temporal and spatial integration in dynamic random-dot stimuli. Vision Res. 1992;32:2341–2347. doi: 10.1016/0042-6989(92)90097-3. [DOI] [PubMed] [Google Scholar]
- Watamaniuk SNJ, Sekuler R, Williams DW. Direction perception in complex dynamic displays: The integration of direction information. Vision Res. 1989;29:47–59. doi: 10.1016/0042-6989(89)90173-9. [DOI] [PubMed] [Google Scholar]
- Westheimer G, McKee SP. Visual acuity in the presence of retinal-image motion. Journal of the Optical Society of America. 1975;65:847–850. doi: 10.1364/josa.65.000847. [DOI] [PubMed] [Google Scholar]
- Yee RD, Daniels SA, Jones OW, Baloh RW, Honrubia V. Effects of an optokinetic background on pursuit eye movements. Invest Ophthalmol Vis Sci. 1983;24:1115–1122. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


