Abstract
Human embryonic stem cells (hESCs), due to their self-renewal capacity and pluripotency, have become a potential source of transplantable β-cells for the treatment of diabetes. However, it is imperative that the derived cells fulfill the criteria for clinical treatment. In this study, we replaced common Matrigel with a synthetic peptide-acrylate surface (Synthemax) to expand undifferentiated hESCs and direct their differentiation in a defined and serum-free medium. We confirmed that the cells still expressed pluripotent markers, had the ability to differentiate into three germ layers, and maintained a normal karyotype after 10 passages of subculture. Next, we reported an efficient protocol for deriving nearly 86% definitive endoderm cells from hESCs under serum-free conditions. Moreover, we were able to obtain insulin-producing cells within 21 days following a simple three-step protocol. The results of immunocytochemical and quantitative gene expression analysis showed that the efficiency of induction was not significantly different between the Synthemax surface and the Matrigel-coated surface. Thus, we provided a totally defined condition from hESC culture to insulin-producing cell differentiation, and the derived cells could be a therapeutic resource for diabetic patients in the future.
Introduction
Type I diabetes is an autoimmune disease that results from the destruction of insulin-producing β-cells in the pancreas. Islet transplantation is an effective therapy for diabetic patients and frees them from dependency on insulin. However, due to the shortage of donor pancreatic tissue for transplantation, the demand for an alternative source of β-cells is urgent [1,2].
Human embryonic stem cells (hESCs), derived from the inner cell mass of the blastocyst, offer extraordinary potential for regenerative medicine, drug screening, and clinical applications due to their ability to proliferate indefinitely and differentiate into all three germ layers [3–5]. However, the clinical application of these cells is limited because they are exposed to animal-derived components during derivation, cultivation, cryopreservation, and differentiation. Thus, these cells cannot be used to meet the requirements for clinical treatment, and this problem must be addressed [6–8].
Until now, most hESC lines were commonly cultured on a feeder layer of mouse embryonic fibroblasts (MEFs) to maintain their characteristics. With the aim of eliminating the xeno-contamination derived from MEFs, numerous studies have demonstrated the maintenance of hESCs on primary feeder cells from humans, such as adult skin fibroblasts [9] and placental fibroblasts [10]. Regardless of where the feeder cells are derived from, they are only viable for a few days; thus, the hESCs must be routinely transferred onto fresh feeder cells for continued culture. This routine work is a time- and labor-intensive procedure. Therefore, Matrigel has become a popular substitute as an extracellular matrix (ECM) for a feeder-free culture system. Matrigel is derived from Engelbreth-Holm-Swarm mouse tumors and is much easier and more convenient to use in practice. However, the concern regarding xeno-derived components persists. Recent studies have shown that long-term culture of hESCs was possible on a synthetic surface, and the cells had a normal karyotype [11,12]. These newly developed synthetic surfaces have become a potential substrate that may help meet the demand for clinical-grade cells by eliminating both xeno-contamination and lot-to-lot variation. After achieving the goal of long-term culture under defined conditions, the means by which cells can be differentiated should be considered in the next step.
To date, although many studies have reported that hESCs can be induced to differentiate into ectodermal and mesodermal lineages in vitro, such as neuronal cells and cardiomyocytes [13–16], reports on endodermal lineage differentiation were relatively less frequent and more inconsistent, especially with regard to pancreatic lineage induction. D'Amour et al. reported the induction of hESCs into endocrine cells by mimicking pancreatic organogenesis in vivo following a five-step differentiation process involving definitive endoderm (DE), gut-tube endoderm, pancreatic endoderm, endocrine precursor, and hormone-expressing endocrine cells [17]. Subsequently, many scientists attempted to develop much faster or more efficient processes in their cell systems [18–22]. For example, Zhang et al. developed a four-step induction protocol and obtained nearly 25% insulin-producing cells from hESCs [19]. Kunisada et al. also developed a four-step protocol utilizing small molecules to achieve a similar level of efficiency from different hESC lines [20]. However, the hESCs or human induced pluripotent stem cells (hiPSCs) used in these studies were always differentiated on MEFs or Matrigel.
In this study, we replaced common Matrigel with a synthetic peptide-acrylate surface (Synthemax) to maintain undifferentiated hESCs in a defined and serum-free medium for long-term culture. Furthermore, we developed a three-step protocol to induce hESCs into insulin-producing cells. The efficiency of induction on the Synthemax surface is equivalent to that on a Matrigel-coated surface. These findings could provide a stable means for long-term culture of hESCs, and the differentiated insulin-producing cells will be a therapeutic resource for diabetic patients in the future.
Materials and Methods
Maintenance and long-term culture of hESCs
The hESC TW line [23] was obtained from the Bioresource Collection and Research Center (BCRC; www.bcrc.firdi.org.tw), Taiwan, and it was utilized following the guidelines for the conduct of human embryos and embryonic stem cells (Department of Health, Taiwan). The cells were maintained on culture dishes coated with Matrigel (BD Biosciences, San Jose, CA) in the serum-free medium mTeSR (StemCell Technologies, Vancouver, Cananda) at 37°C in a humidified atmosphere containing 5% CO2. Similarly, hESCs were also subcultured on a synthetic peptide-acrylate surface (Synthemax®; Corning, Lowell, MA) in the mTeSR medium. The culture medium was changed daily. The hESCs were subcultured every 5–7 days after treatment with 1 U/mL dispase (Invitrogen, Carlsbad, CA). The cell morphology was observed every day to ensure that the cells were maintained in an undifferentiated state. The karyotype of the hESC TW cells was checked after every five passages.
Differentiation of hESCs into insulin-producing cells
First, hESCs were seeded on a Matrigel-coated culture plate or the Synthemax plate at a density of 2.5×104 cells/cm2 in the mTeSR medium with 2 μM thiazovivin (Tzv; BioVision, Milpitas, CA) for 48 h. Subsequently, the cells were cultured in the mTeSR medium without Tzv for an additional 24 h. Next, we followed the protocol published by D'Amour for DE differentiation [24]. In brief, the cells were cultured in the RPMI 1640 medium (Sigma, St. Louis, MO) with 2% bovine serum albumin (BSA; Sigma), 0.2% fetal bovine serum (FBS; Invitrogen), or 1% B27 (Invitrogen) to improve the low attachment of TW hESCs under serum-free conditions containing 100 ng/mL activin A (R&D Systems, Minneapolis, MN) and 25 ng/mL Wnt3a (R&D Systems) for 24 h and subsequently transferred to the RPMI 1640 medium containing 100 ng/mL activin A only for an additional 48 h. (Fig. 1A). Then, we modified the Zhang's protocol to establish a three-step induction process for insulin-producing cell differentiation (Fig. 2A) [19]. Briefly, following the DE induction method mentioned above, the differentiated cells were cultured in the Dulbecco's modified Eagle's medium/nutrient mixture F-12 (DMEM/F12, 1:1; Invitrogen) supplemented with 1% B27, 1% nonessential amino acids (Invitrogen), 1 mM GlutaMax (Invitrogen), 0.1 mM β-mercaptoethanol (Invitrogen), 20 ng/mL keratinocyte growth factor (KGF; Peprotech, London, United Kingdom), 100 ng/mL noggin (R&D Systems), and 2 μM retinoic acid (RA; Sigma) for 7 days. Finally, to enhance insulin-producing cell differentiation, the cells were cultured in the DMEM/F12 medium containing 1% insulin–transferrin–selenium (ITS; Invitrogen), 10 ng/mL basic fibroblast growth factor (bFGF; Peprotech), 10 ng/mL bone morphogenetic protein4 (BMP4; Peprotech), 50 ng/mL exendin-4 (Gibco, Grand Island, NY), and 10 mM nicotinamide (Sigma) for an additional 11 days. The medium was changed every 2 days.
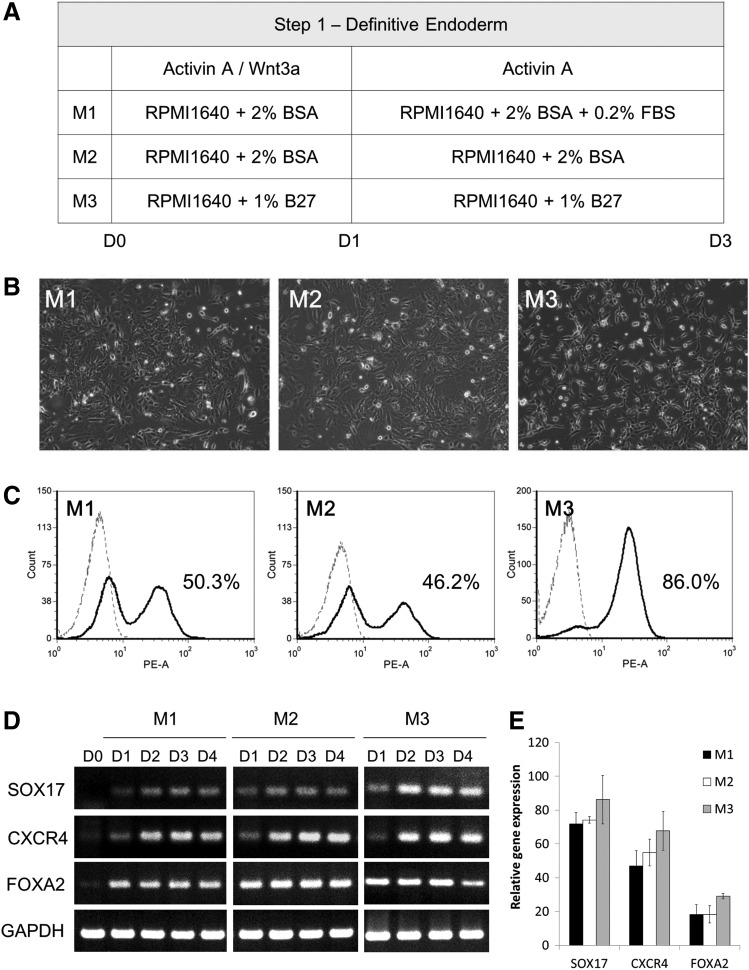
FIG. 1.
Comparison of three differentiation protocols from human embryonic stem cells (hESCs) into definitive endoderm (DE) cells on a Matrigel-coated surface. (A) Schematic representation of the three differentiation media for hESCs into DE cells. (B) Cell morphology of DE cells on day 3. Scale bar: 100 μm. (C) Immunophenotyping profile by flow cytometry of FOXA2 of DE cells on day 3. (D) RT-PCR analysis showing that the differentiated cells expressed DE cell markers such as SOX17, FOXA2, and CXCR4 following the 3-day induction. (E) Quantitative RT-PCR analysis of SOX17, FOXA2, and CXCR4 on day 0 and 4 populations. Data presented as mean±SD (n=3).
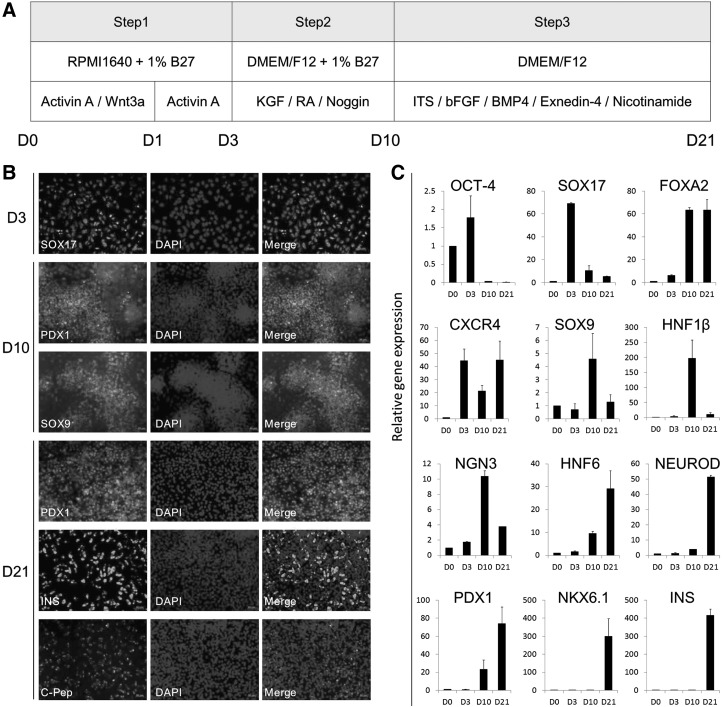
FIG. 2.
Illustration of differentiation of hESCs into insulin-producing cells on a synthetic surface. (A) Schematic representation of the three-step differentiation protocol from hESCs (D0), definitive endoderm (D3), pancreatic specification (D10), and finally, endocrine progenitor and maturation (D21), with applied media and factors. (B) Immunocytochemistry staining showing that cells expressed SOX17 on day 3, PDX1 and SOX9 on day 10, and PDX1, insulin (INS), and c-peptide (C-Pep) on day 21. Scale bar: 50 μm. (C) Quantitative RT-PCR analysis of OCT-4, SOX17, FOXA2, CXCR4, SOX9, HNF1β, NGN3, HNF6, NEUROD, PDX1, NKX6.1, and insulin (INS) on day 0, 3, 10, and 21 populations. Data presented as mean±SD (n=3).
Immunocytochemistry staining
The cells were fixed with 4% paraformaldehyde for 15 min and subsequently permeabilized with 0.5% Triton X-100 (Sigma) for 10 min at room temperature (RT). After incubating with a block buffer (5% serum corresponding to the secondary antibody species) for 1 h at room temperature, the cells were incubated with primary antibodies (listed in Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/scd) at 4°C overnight. The corresponding secondary antibodies were then applied to the cells for 1 h at room temperature in the dark. The cell nuclei were counterstained with 300 nM 4′,6-diamidino-2-phenylindole (DAPI) for 5 min. The stained cells were observed by fluorescence microscopy using Axio Observer (Zeiss, Göttingen, Germany), and the images were acquired using specific software (AxioCam ICm 1; Zeiss).
Flow cytometry
The cells were harvested using TrypLE (Invitrogen) and washed once in Dulbecco's phosphate-buffered saline (D-PBS; Invitrogen). A total of 1×106 cells were incubated with phycoerythrin (PE)-conjugated anti-human FOXA2 (BD Biosciences) for 30 min at 4°C. After washing twice in D-PBS, the cells were analyzed by flow cytometry using a FACSCalibur analyzer (BD FACSCanto™ II; BD Biosciences) with CellQuest software (BD Biosciences). A replicate sample was stained with PE-mouse IgG1 (BD Biosciences) as an isotype control to ensure specificity.
RT-PCR and quantitative RT-PCR
Total RNA was isolated from undifferentiated or differentiated hESCs using Tri-Reagent (Molecular Research Center, Cincinnati, OH) following the manufacturer's instructions. Total RNA (1 μg) was reverse transcribed into cDNA using the Maxima® First Strand cDNA Synthesis Kit (Fermentas, Glen Burnie, MD). Specific cDNA was amplified by PCR using DreamTaq™ PCR Master Mix (Fermentas) and subsequently analyzed by gel electrophoresis. Quantitative RT-PCR was performed using Maxima SYBR Green/ROX qPCR Master Mix (Thermo Scientific, Lafayette, CO) on the ABI Prism™ 7700 Sequence Detection System. The primer sequences are listed in Supplementary Table S2, and the expression of each target gene was normalized to the level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression.
In vitro differentiation (embryoid body formation)
The hESCs detached in clumps upon treatment with collagenase IV (Gibco) and were resuspended in the DMEM/F12 containing 10% FBS in an ultralow attachment plate (Corning). The medium was changed every 2 days. After 14 days, the embryoid bodies (EBs) were transferred to a 0.1% gelatin-coated plate and cultured in the same medium for an additional 7 days. The samples were then fixed for immunocytochemistry staining.
In vivo differentiation (teratoma formation)
The animal study conformed to the Animal Protection Law (amended on October 1, 2010) published by the Council of Agriculture, Taiwan and was approved by the Animal Care and Use Committee of the Food Industry Research and Development Institute, Hsinchu, Taiwan. The hESCs were harvested using TrypLE, and approximately 1×106 cells were injected intramuscularly into severe combined immunodeficient (SCID) mice (Biolasco, Taipei, Taiwan). The mice were euthanized 8–12 weeks later, and the tumors were dissected and fixed in 10% formaldehyde (Sigma). For histological examination, the tumors were embedded in paraffin and sectioned with hematoxylin and eosin staining (H&E staining) by the Taipei Institute of Pathology (Taipei, Taiwan).
Karyotype analysis
The hESCs were treated with 0.02 μg/mL colcemid (Gibco) for up to 2 h at 37°C. After dissociation using 0.25% trypsin/EDTA (Invitrogen), the cells were resuspended in 0.075 M KCl and incubated for 30 min at 37°C. The cells were then fixed by means of three consecutive immersions in ice-cold fixative (methanol:acetic acid, 3:1) and subsequently dropped onto precleaned chilled glass slides. The samples were stored at 4°C, and the chromosomes were visualized using standard G-band staining and analyzed by the Prenatal Diagnostic Laboratory, Cathay General Hospital (Taipei, Taiwan). The karyotype description followed the International System for Human Cytogenetic Nomenclature (ISCN:2009; Karger Ag, Basel, Switzerland).
Statistical analysis
At least three separate experiments were performed for each test condition. The results are presented as the mean±SD. Statistical analysis was performed using the Student's t-test, and differences between the groups were considered statistically significant when the P value was less than 0.05.
Results
Long-term culture of hESCs on a synthetic peptide-acrylate surface
First, we determined that the hESCs could be maintained on a synthetic peptide-acrylate surface in a defined medium for long-term culture (over 10 passages). As shown in Supplementary Fig. S1A, the cells formed a compact colony and showed a high nuclear to cytoplasmic ratio. Alkaline phosphatase staining of the cells produced a positive result (Supplementary Fig. S1B). The cells also displayed a normal karyotype after 10 passages of subculture (Supplementary Fig. S1C). According to the immunocytochemical analysis, the colonies expressed the surface markers SSEA-4, TRA-1-60, and TRA-1-81 and the nuclear marker OCT-4 (Supplementary Fig. S1D). In addition, the gene expression of the hESC markers and the early differentiation markers was examined by RT-PCR, and the results showed that the cells grown on the Synthemax surface or the Matrigel-coated surface expressed OCT-4 and SOX-2, while without expressing Brachyury (T), SOX17, and Goosecoid (GSC) (Supplementary Fig. S1E). Additionally, we examined the pluripotency of the cells in vitro and in vivo after long-term culture. Based on immunocytochemical analysis, the EBs formed, and three germ layers were generated: nestin (ectoderm), smooth muscle actin (mesoderm), and albumin (endoderm) (Supplementary Fig. S1F). Furthermore, as observed by H&E staining, the teratomas formed in SCID mice comprised three germ layers, including endodermal epithelium (endoderm), muscle tissue (mesoderm), and neuronal tissue (ectoderm) (Supplementary Fig. S1G).
Differentiation of hESCs into DE cells on a Matrigel-coated surface
Before allowing differentiation into insulin-producing cells, we need to improve the induction efficiency at the first step: the DE. It was previously reported that hESCs could be induced to differentiate into DE using activin A and Wnt3a under low serum conditions [24]; therefore, we adapted this protocol with slight modifications. We utilized different combinations of supplements in an attempt to solve the problem of the low attachment of TW hESCs on a Matrigel-coated surface under serum-free conditions (Fig. 1A). The hESCs were induced to differentiate into DE by activin A and Wnt3a for 24 h and subsequently by activin A only for an additional 48 h. After 3 days of induction, the cells grew and spread out from the colonies. Based on microscopic observations, the cells induced by method 3 had a smaller and more homogeneous morphology than those induced by methods 1 and 2 (Fig. 1B). Using flow cytometry analysis, the proportion of the positive FOXA2 population was found to be 50.3%, 46.2%, and 86.0% from methods 1, 2, and 3, respectively (Fig. 1C). The gene expression levels of DE markers were assayed by RT-PCR and quantitative RT-PCR, and the results showed that FOXA2 expression was also highest in method 3, in which the supplement is 1% B27 without BSA or FBS (Fig. 1D, E). These findings suggest that method 3 effectively induces hESC differentiation into DE cells.
Directed differentiation of hESCs into insulin-producing cells
We next combined Zhang's protocol [19] with the efficient protocol we established above to a three-step procedure carried out within 21 days to direct the differentiation of hESCs into insulin-producing cells on the Synthemax surface or the Matrigel-coated surface (Fig. 2A). At the end of each step, key markers were examined by immunocytochemical analysis. As shown in Fig. 2B, the derived cells on the Synthemax surface positively stained for SOX17 on day 3, PDX1 and SOX9 on day 10, and PDX1, insulin, and c-peptide on day 21. Moreover, we also examined gene expression by quantitative RT-PCR as shown in Fig. 2C. We found that the expression levels of SOX17 and CXCR4 were the highest on day 3, and FOXA2 expression was induced on day 3 and continued to increase in the following days. The expression levels of HNF1β, SOX9, and NGN3 were the highest on day 10, and the expression levels of HNF6, NEUROD, PDX1, and insulin were the highest on day 21. These findings show that the cells differentiated following the step-by-step procedure, and insulin-producing cells could be derived from hESCs after 21 days of induction.
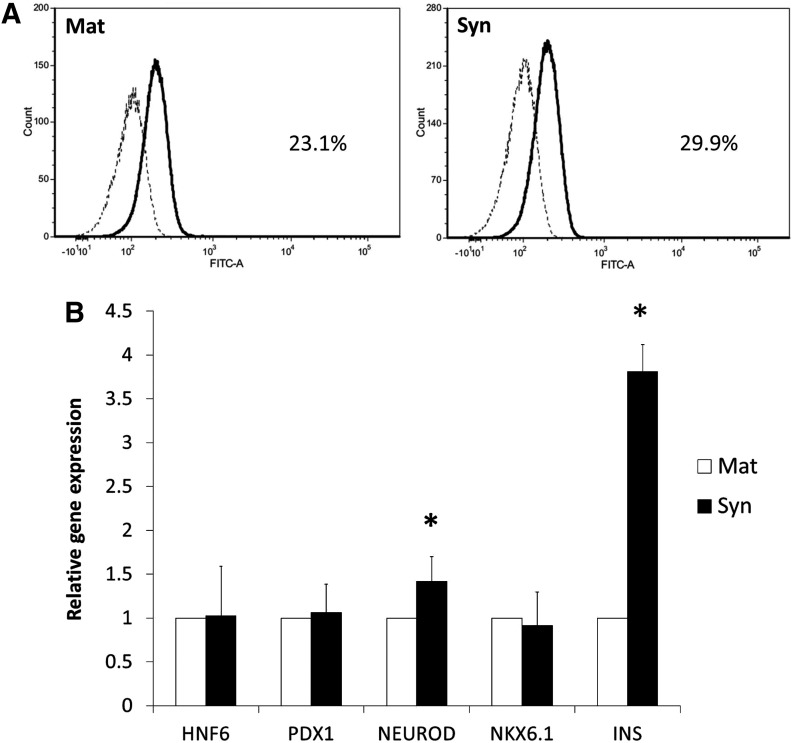
We also estimated the population of insulin-producing cells after 21 days of induction by flow cytometry, and the results were found to be 23.1% (Matrigel) and 29.9% (Synthemax) (Fig. 3A) Additionally, we compared the gene expression of insulin-producing cell markers between cells on the Synthemax surface and those on the Matrigel-coated surface after 21 days. Quantitative RT-PCR analysis revealed that the expression levels of NKX6.1, HNF6, and PDX1 genes were not significantly different between the two surfaces after 21 days of induction. Interestingly, in differentiated cells on the Synthemax surface, the NEUROD and insulin gene showed a 1.4- and 3.8-fold change in expression compared with its level in cells on the Matrigel-coated surface (Fig. 3B).
FIG. 3.
Gene expression of derived insulin-producing cells on Matrigel and Synthemax. (A) Immunophenotyping profile by flow cytometry of insulin (INS) of insulin-producing cells after 21 days of induction. (B) Comparison of gene expression of NKX6.1, PDX1, and insulin (INS) on day 21 between the Matrigel-coated surface (Mat) and the Synthemax surface (Syn) by quantitative RT-PCR analysis. Data presented as mean±SD (n=3). *P<0.05.
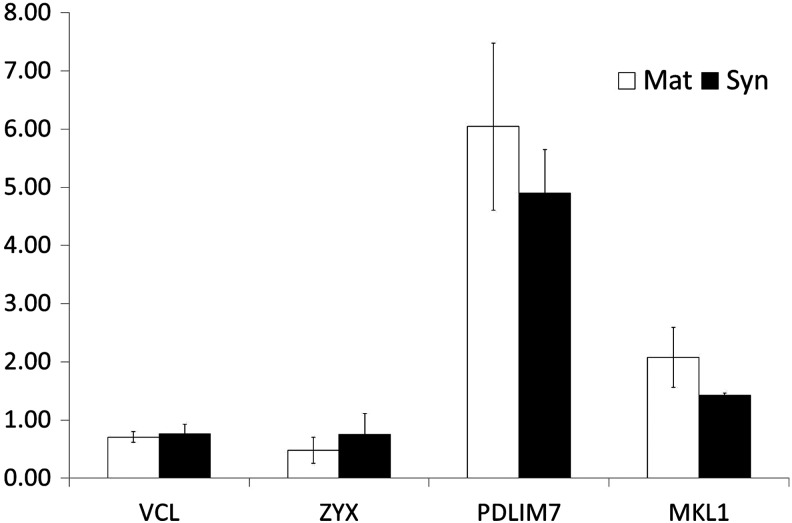
In addition, we compared the gene expression of the cytoskeleton structures of the hESCs differentiated on the Synthemax surface and on the Matrigel-coated surface after 21 days of induction. In our results, the expression levels of vinculin (VCL), zyxin (ZYX), PDZ and LIM domain 7 (PDLIM7), and megakaryoblastic leukemia (MKL1) were not significantly different on these two surfaces (Fig. 4).
FIG. 4.
Gene expression of cytoskeleton of differentiated cells on Matrigel and Synthemax. Comparison of gene expression of VCL, ZYX, PDLIM7, and MKL1 on day 21 between the Matrigel-coated surface (Mat) and the Synthemax surface (Syn) by quantitative RT-PCR analysis. Data presented as mean±SD (n=3).
Discussion
In this study, we confirmed that hESCs could be maintained on a synthetic surface in the defined medium mTeSR for long-term culture. We also developed a simple protocol for the differentiation of hESCs into insulin-producing cells within 21 days of induction. In each of the steps, we induced the hESCs using only small molecules and growth factors. Most importantly, we carried out the differentiation procedure on the Synthemax surface without serum to achieve a much more stable environment throughout the entire process. The results showed that the efficiency of induction was not significantly different between the Synthemax surface and the Matrigel-coated surface. Therefore, we provided a totally defined condition from hESC culture to insuling-producing cell differentiation.
To overcome the obstacles facing the clinical application of hESCs, many researchers have focused on improving various technical aspects, including derivation, cultivation, differentiation, and cryopreservation. Regardless of hESC cultivation and differentiation methods, the choice of an optimal ECM upon which to maintain the cells is important. Although hESCs and hiPSCs could be maintained on MEFs or Matrigel, xenogeneic contamination was still a problem. Thus, many scientists attempted to find synthetic substrates that could eliminate the concern [25]. Villa-Diaz et al. reported a fully defined synthetic polymer coating, PMEDSAH, on which hESCs could be maintained long term [26]. Additionally, Klim et al. developed a substrate that displayed heparin-binding peptides, which can interact with cell surface glycosaminoglycans to effectively maintain hESCs for more than 3 months [11]. In this study, we selected Synthemax, which is a synthetic vitronectin-mimicking surface with RGD-containing peptides (Ac-KGGNGEPRGDTYRAY and Ac-KGGPQVTRGDVFTMP). It has been reported that H1 and H7 hESCs were successfully maintained on Synthemax for over ten passages, and the cells were able to differentiate into functional cardiomyocytes [12]. Subsequently, Li et al. showed that hESCs could differentiate into oligodendrocyte progenitor cells on a Synthemax surface [27]. Jin et al. also confirmed that not only hESCs, but hiPSCs could be successfully maintained in an undifferentiated state for more than ten passages [28]. Until now, we were unable to find any reports describing the pancreatic lineage differentiation procedure on a Synthemax surface or any other synthetic surface. In this study, we not only repeatedly confirmed that the hESCs could be maintained on a Synthemax surface using different hESC lines derived from Taiwanese species, but we also showed that the cells could be effectively directed to differentiate into insulin-producing cells.
We know that there are many steps involved in the derivation of insulin-producing cells; however, the first step, which involves the generation of DE, is a crucial step for pancreas genesis in vivo [18]. D'Amour et al. first reported that they could obtain up 80% DE cells in the presence of activin A under low serum conditions. Subsequently, other groups have explored compounds such as activin B, small molecules, and others that may be used to replace activin A and achieve a higher induction efficiency [29,30]. We first followed the protocol published by D'Amour [17]; however, the hESC line that we used in this study showed poor cell attachment under low serum conditions. Surprisingly, we improved the cell attachment and concurrently obtained nearly 90% FOXA2-positive cells by substituting 1% B27 for serum or BSA throughout the entire process. Therefore, we were able to proceed to differentiate insulin-producing cells from the derived DE cells.
Current mainstream methods for the differentiation of insulin-producing cells that have been reported in the literature can be classified into two major types: suspension and adhesion. In the former, the cells formed aggregates (EBs) in suspension, and this method more closely mimicked the 3D environment in vivo [31–33]. However, the transport of nutrients and oxygen in aggregates and the control of aggregate size remain unsolved problems. Thus, we used the adhesion method to carry out our differentiation. Recently, several groups have shown that a large number of factors are involved in pancreatic lineage differentiation, including noggin, RA, KAAD-cyclopamine, KGF, FGF10, dorsomorphin, and SB431542 for pancreatic specialization and nicotinamide, bFGF, exedine-4 distortion less enhanced by polarization transfer, forskolin, hepatocyte growth factor, ITS, and BMP4 for the maturation of hormone-expressing cells [17,19,20]. We chose to simplify Zhang's protocol to a three-step induction procedure that is carried out within 21 days [19]. In brief, we executed only two steps—pancreatic specialization and maturation—to obtain insulin-producing cells from DE cells. Based on our results, we obtained approximately 29.9% insulin-producing cells after 21 days of induction. Although our efficiency of induction was similar to that reported in the literature, our procedure was simplified and did not require serum. Most importantly, we induced the hESCs on a Synthemax surface instead of a Matrigel-coated surface, and the quantity and quality of the derived insulin-producing cells on the Synthemax surface were similar to that obtained with the Matrigel-coated surface. These findings show that we can decrease the batch-to-batch variation and alleviate safety concerns related to Matrigel and serum in this environment, which is closer to a clinical-grade environment.
In conclusion, hESCs were maintained in an undifferentiated, pluripotent state on the Synthemax surface for long-term culture and were also successfully directed to differentiate into insulin-producing cells after 21 days of induction. These findings will be useful for the clinical application of hESCs in the future.
Supplementary Material
Acknowledgments
We thank Mr. Ming-Yuan Hung and Miss Yi-Ting Chen of the Food Industry Research and Development Institute (Hsinchu, Taiwan) for assistance in SCID mouse experiment and the Prenatal Diagnostic Laboratory, Cathay General Hospital, Taiwan for chromosome karyotype analysis. This work was supported by grants from the National Research Project for Biopharmaceuticals, National Science Council, Taiwan (NSC 102-2325-B-080-001), and the Ministry of Economic Affairs, Taiwan (102-EC-17-A-01-04-0525).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Weir GC, Cavelti-Weder C. and Bonner-Weir S. (2011). Stem cell approaches for diabetes: towards beta cell replacement. Genome Med 3:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner RT, Lewis J, Cooney A. and Chan L. (2010). Stem cell approaches for the treatment of type 1 diabetes mellitus. Transl Res 156:169–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomson JA. (1998). Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147 [DOI] [PubMed] [Google Scholar]
- 4.Reubinoff BE, Pera MF, Fong CY, Trounson A. and Bongso A. (2000). Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol 18:399–404 [DOI] [PubMed] [Google Scholar]
- 5.Vazin T. and Freed WJ. (2010). Human embryonic stem cells: derivation, culture, and differentiation: a review. Restor Neurol Neurosci 28:589–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murry CE. and Keller G. (2008). Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132:661–680 [DOI] [PubMed] [Google Scholar]
- 7.Pera M, Tannenbaum SE, Tako Turetsky T, Singer O, Aizenman E, Kirshberg S, Ilouz N, Gil Y, Berman-Zaken Y, et al. (2012). Derivation of xeno-free and GMP-grade human embryonic stem cells–platforms for future clinical applications. PLoS One 7:e35325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunt CJ. (2011). Cryopreservation of human stem cells for clinical application: a review. Transfus Med Hemother 38:107–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tecirlioglu RT, Nguyen L, Koh K, Trounson AO. and Michalska AE. (2010). Derivation and maintenance of human embryonic stem cell line on human adult skin fibroblast feeder cells in serum replacement medium. In Vitro Cell Dev Biol Anim 46:231–235 [DOI] [PubMed] [Google Scholar]
- 10.Ilic D, Kapidzic M. and Genbacev O. (2008). Isolation of human placental fibroblasts. Curr Protoc Stem Cell Biol Chapter 1:Unit 1C6. [DOI] [PubMed] [Google Scholar]
- 11.Klim JR, Li L, Wrighton PJ, Piekarczyk MS. and Kiessling LL. (2010). A defined glycosaminoglycan-binding substratum for human pluripotent stem cells. Nat Methods 7:989–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melkoumian Z, Weber JL, Weber DM, Fadeev AG, Zhou Y, Dolley-Sonneville P, Yang J, Qiu L, Priest CA, et al. (2010). Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat Biotechnol 28:606–610 [DOI] [PubMed] [Google Scholar]
- 13.Van Orman JR, Si-Tayeb K, Duncan SA. and Lough J. (2012). Induction of cardiomyogenesis in human embryonic stem cells by human embryonic stem cell-derived definitive endoderm. Stem Cells Dev 21:987–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon BS, Yoo SJ, Lee JE, You S, Lee HT. and Yoon HS. (2006). Enhanced differentiation of human embryonic stem cells into cardiomyocytes by combining hanging drop culture and 5-azacytidine treatment. Differentiation 74:149–159 [DOI] [PubMed] [Google Scholar]
- 15.Schuldiner M, Eiges R, Eden A, Yanuka O, Itskovitz-Eldor J, Goldstein RS. and Benvenisty N. (2001). Induced neuronal differentiation of human embryonic stem cells. Brain Res 913:201–205 [DOI] [PubMed] [Google Scholar]
- 16.Zhang SC, Werning M, Duncan ID, Brüstle O. and Thomson JA. (2001). In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol 19:1129–1133 [DOI] [PubMed] [Google Scholar]
- 17.D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK. and Baetge EE. (2006). Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 24:1392–1401 [DOI] [PubMed] [Google Scholar]
- 18.Van Hoof D, D'Amour KA. and German MS. (2009). Derivation of insulin-producing cells from human embryonic stem cells. Stem Cell Res 3:73–87 [DOI] [PubMed] [Google Scholar]
- 19.Zhang D, Jiang W, Liu M, Sui X, Yin X, Chen S, Shi Y. and Deng H. (2009). Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res 19:429–438 [DOI] [PubMed] [Google Scholar]
- 20.Kunisada Y, Tsubooka-Yamazoe N, Shoji M. and Hosoya M. (2012). Small molecules induce efficient differentiation into insulin-producing cells from human induced pluripotent stem cells. Stem Cell Res 8:274–284 [DOI] [PubMed] [Google Scholar]
- 21.Jiang J, Au M, Lu K, Eshpeter A, Korbutt G, Fisk G. and Majumdar AS. (2007). Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells 25:1940–1953 [DOI] [PubMed] [Google Scholar]
- 22.Cho YM, Lim JM, Yoo DH, Kim JH, Chung SS, Park SG, Kim TH, Oh SK, Choi YM, et al. (2008). Betacellulin and nicotinamide sustain PDX1 expression and induce pancreatic beta-cell differentiation in human embryonic stem cells. Biochem Biophys Res Commun 366:129–134 [DOI] [PubMed] [Google Scholar]
- 23.Cheng EH, Chen W, Chang SY, Huang JJ, Huang CC, Huang LS, Liu CH. and Lee MS. (2008). Blastocoel volume is related to successful establishment of human embryonic stem cell lines. Reprod Biomed Online 17:436–444 [DOI] [PubMed] [Google Scholar]
- 24.D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E. and Baetge EE. (2005). Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol 23:1534–1541 [DOI] [PubMed] [Google Scholar]
- 25.Villa-Diaz LG, Ross AM, Lahann J. and Krebsbach PH. (2013). Concise review: the evolution of human pluripotent stem cell culture: from feeder cells to synthetic coatings. Stem Cells 31:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villa-Diaz LG, Nandivada H, Ding J, Nogueira-de-Souza NC, Krebsbach PH, O'Shea KS, Lahann J. and Smith GD. (2010). Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nat Biotechnol 28:581–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Gautam A, Yang J, Qiu L, Melkoumian Z, Weber J, Telukuntla L, Srivastava R, Whiteley EM. and Brandenberger R. (2013). Differentiation of oligodendrocyte progenitor cells from human embryonic stem cells on vitronectin-derived synthetic peptide acrylate surface. Stem Cells Dev 22:1497–1505 [DOI] [PubMed] [Google Scholar]
- 28.Jin S, Yao H, Weber JL, Melkoumian ZK. and Ye K. (2012). A synthetic, xeno-free peptide surface for expansion and directed differentiation of human induced pluripotent stem cells. PLoS One 7:e50880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frandsen U, Porneki AD, Floridon C, Abdallah BM. and Kassem M. (2007). Activin B mediated induction of Pdx1 in human embryonic stem cell derived embryoid bodies. Biochem Biophys Res Commun 362:568–574 [DOI] [PubMed] [Google Scholar]
- 30.Borowiak M, Maehr R, Chen S, Chen AE, Tang W, Fox JL, Schreiber SL. and Melton DA. (2009). Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell 4:348–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips BW, Hentze H, Rust WL, Chen QP, Chipperfield H, Tan EK, Abraham S, Sadasivam A, Soong PL, et al. (2007). Directed differentiation of human embryonic stem cells into the pancreatic endocrine lineage. Stem Cells Dev 16:561–578 [DOI] [PubMed] [Google Scholar]
- 32.Shim JH, Kim SE, Woo DH, Kim SK, Oh CH, McKay R. and Kim JH. (2007). Directed differentiation of human embryonic stem cells towards a pancreatic cell fate. Diabetologia 50:1228–1238 [DOI] [PubMed] [Google Scholar]
- 33.Segev H, Fishman B, Ziskind A, Shulham M. and Itskovitz-Eldor J. (2004). Differentiation of human embryonic stem cells into insulin-producing clusters. Stem Cells 22:265–274 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.