Abstract
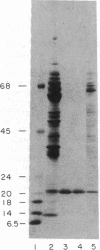
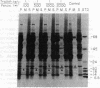
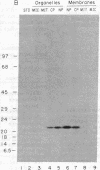
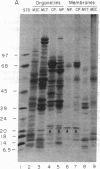
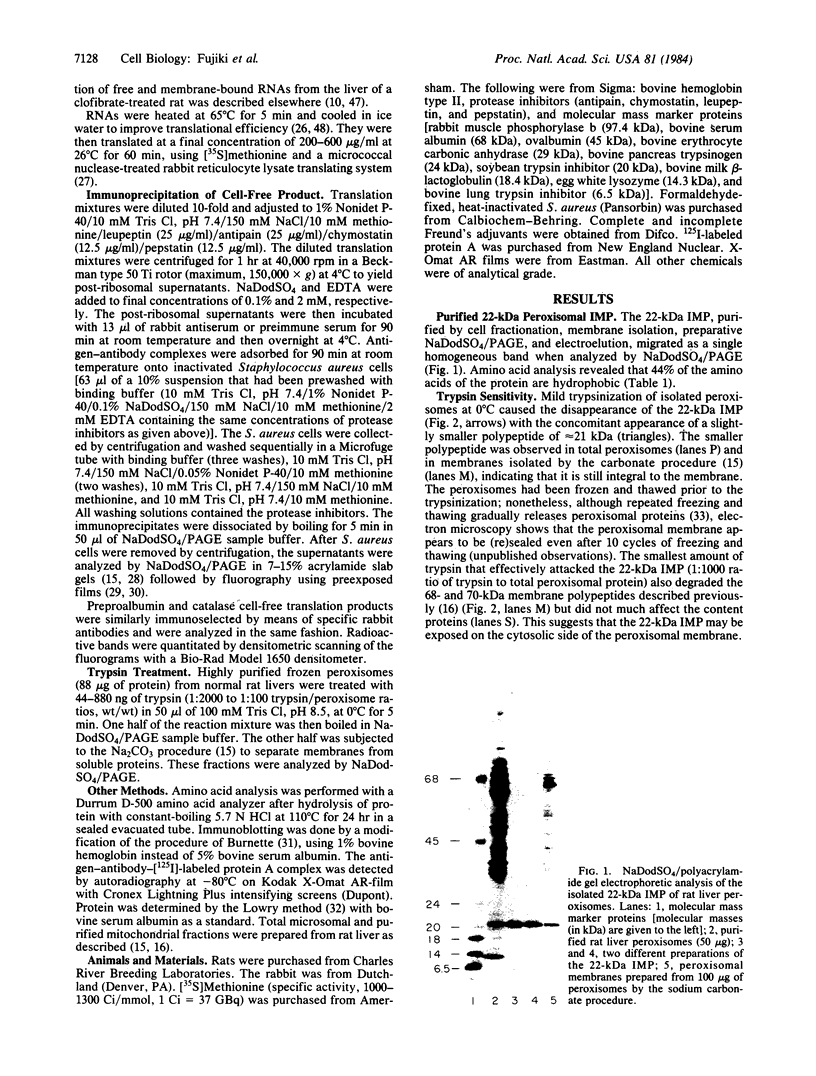
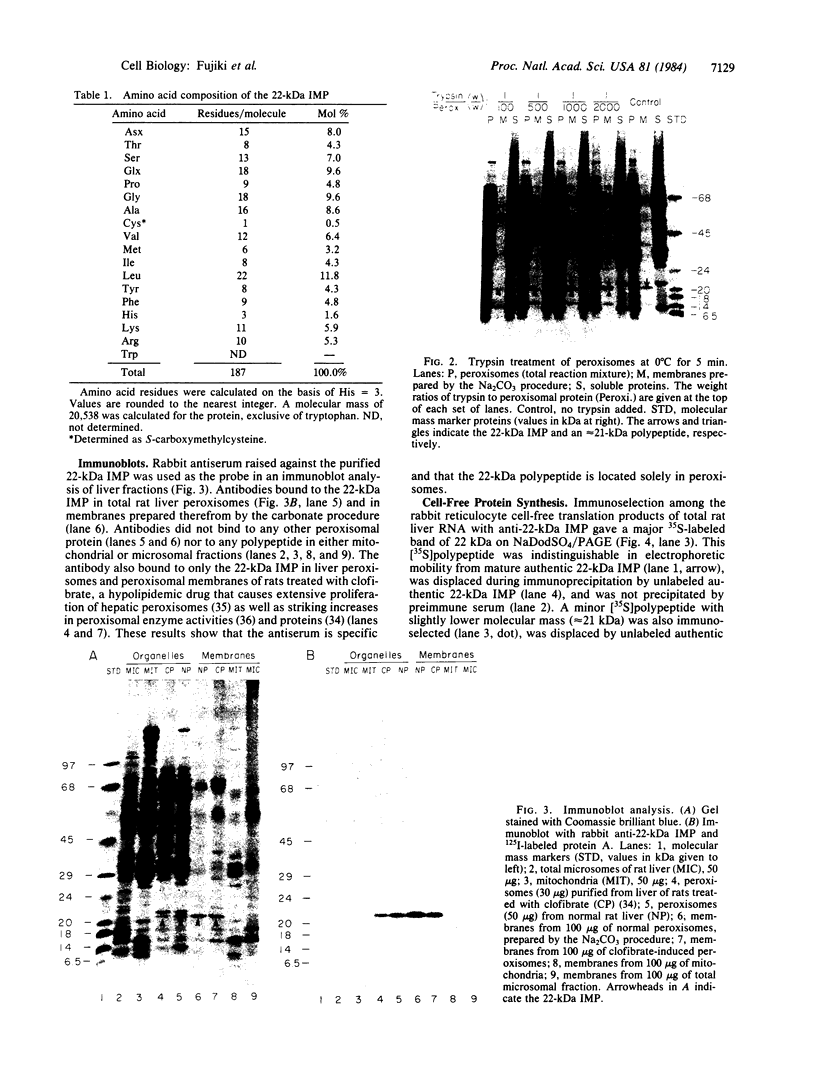
The manner of synthesis and assembly of the peroxisomal membrane proteins is unknown. Understanding these processes is essential to an understanding of the formation of the organelle. We have investigated the biogenesis of the previously identified major 21.7-kDa integral peroxisomal membrane polypeptide [Fujiki, Y., Fowler, S., Shio, H., Hubbard, A. L. & Lazarow, P. B. (1982) J. Cell Biol. 93, 103-110]. This protein was purified to apparent homogeneity and used to elicit a rabbit antiserum. In immunoblotting analysis, antibody bound only to the 22-kDa membrane polypeptide present exclusively in peroxisomal membranes. Total rat liver RNA was translated in a nuclease-treated rabbit reticulocyte cell-free protein-synthesizing system. The in vitro translation product, isolated by means of the antibody and Staphylococcus aureus cells, comigrated with the mature 22-kDa polypeptide in NaDodSO4/PAGE. Analysis of the translation products of RNAs from free and membrane-bound polysomes indicated that the mRNA for the 22-kDa membrane polypeptide is found predominantly in free polysomes. The results imply post-translational insertion of the membrane polypeptide into the peroxisomal membrane without proteolytic processing and suggest that peroxisomes, like mitochondria and chloroplasts, form by fission from preexisting organelles.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Borgese N., Gaetani S. Site of synthesis of rat liver NADH--cytochrome b5 reductase, an integral membrane protein. FEBS Lett. 1980 Apr 7;112(2):216–220. doi: 10.1016/0014-5793(80)80183-9. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Colman D. R., Kreibich G., Frey A. B., Sabatini D. D. Synthesis and incorporation of myelin polypeptides into CNS myelin. J Cell Biol. 1982 Nov;95(2 Pt 1):598–608. doi: 10.1083/jcb.95.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y., Fowler S., Shio H., Hubbard A. L., Lazarow P. B. Polypeptide and phospholipid composition of the membrane of rat liver peroxisomes: comparison with endoplasmic reticulum and mitochondrial membranes. J Cell Biol. 1982 Apr;93(1):103–110. doi: 10.1083/jcb.93.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta S., Hashimoto T., Miura S., Mori M., Tatibana M. Cell-free synthesis of the enzymes of peroxisomal beta-oxidation. Biochem Biophys Res Commun. 1982 Mar 30;105(2):639–646. doi: 10.1016/0006-291x(82)91482-6. [DOI] [PubMed] [Google Scholar]
- Goldman B. M., Blobel G. Biogenesis of peroxisomes: intracellular site of synthesis of catalase and uricase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5066–5070. doi: 10.1073/pnas.75.10.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudie R. B., Horne C. H., Wilkinson P. C. A simple method for producing antibody specific to a single selected diffusible antigen. Lancet. 1966 Dec 3;2(7475):1224–1226. doi: 10.1016/s0140-6736(66)92305-1. [DOI] [PubMed] [Google Scholar]
- HIGASHI T., PETERS T., Jr STUDIES ON RAT LIVER CATALASE. II. INCORPORATION OF 14-C-LEUCINE INTO CATALASE OF LIVER CELL FRACTIONS IN VIVO. J Biol Chem. 1963 Dec;238:3952–3954. [PubMed] [Google Scholar]
- Hess R., Stäubli W., Riess W. Nature of the hepatomegalic effect produced by ethyl-chlorophenoxy-isobutyrate in the rat. Nature. 1965 Nov 27;208(5013):856–858. doi: 10.1038/208856a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow P. B., Fujiki Y., Mortensen R., Hashimoto T. Identification of beta-oxidation enzymes among peroxisomal polypeptides. Increase in Coomassie blue-stainable protein after clofibrate treatment. FEBS Lett. 1982 Dec 27;150(2):307–310. doi: 10.1016/0014-5793(82)80757-6. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., de Duve C. The synthesis and turnover of rat liver of rat liver peroxisomes. IV. Biochemical pathway of catalase synthesis. J Cell Biol. 1973 Nov;59(2 Pt 1):491–506. doi: 10.1083/jcb.59.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow P. B., de Duve C. The synthesis and turnover of rat liver peroxisomes. V. Intracellular pathway of catalase synthesis. J Cell Biol. 1973 Nov;59(2 Pt 1):507–524. doi: 10.1083/jcb.59.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton F., Poole B., Lazarow P. B., De Duve C. The synthesis and turnover of rat liver peroxisomes. I. Fractionation of peroxisome proteins. J Cell Biol. 1969 May;41(2):521–535. doi: 10.1083/jcb.41.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara K., Blobel G., Sato R. In vitro synthesis and integration into mitochondria of porin, a major protein of the outer mitochondrial membrane of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7102–7106. doi: 10.1073/pnas.79.23.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen R. M., Rachubinski R. A., Fujiki Y., Lazarow P. B. Heating RNA before cell-free translation is essential for the efficient and reproducible synthesis of several peroxisomal proteins. Biochem J. 1984 Oct 15;223(2):547–550. doi: 10.1042/bj2230547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Frey A. B., Guenthner T. M., Oesch F., Sabatini D. D., Kreibich G. Studies on the biosynthesis of microsomal membrane proteins. Site of synthesis and mode of insertion of cytochrome b5, cytochrome b5 reductase, cytochrome P-450 reductase and epoxide hydrolase. Eur J Biochem. 1982 Feb;122(2):393–402. doi: 10.1111/j.1432-1033.1982.tb05894.x. [DOI] [PubMed] [Google Scholar]
- Ozasa H., Miyazawa S., Osumi T. Biosynthesis of carnitine octanoyltransferase and carnitine palmitoyltransferase. J Biochem. 1983 Aug;94(2):543–549. doi: 10.1093/oxfordjournals.jbchem.a134385. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rachubinski R. A., Verma D. P., Bergeron J. J. Synthesis of rat liver microsomal cytochrome b5 by free ribosomes. J Cell Biol. 1980 Mar;84(3):705–716. doi: 10.1083/jcb.84.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey J. C., Steele W. J. A procedure for the quantitative recovery of homogeneous populations of undegraded free and bound polysomes from rat liver. Biochemistry. 1976 Apr 20;15(8):1704–1712. doi: 10.1021/bi00653a018. [DOI] [PubMed] [Google Scholar]
- Raymond Y., Shore G. C. The precursor for carbamyl phosphate synthetase is transported to mitochondria via a cytosolic route. J Biol Chem. 1979 Oct 10;254(19):9335–9338. [PubMed] [Google Scholar]
- Redman C. M., Grab D. J., Irukulla R. The intracellular pathway of newly formed rat liver catalase. Arch Biochem Biophys. 1972 Oct;152(2):496–501. doi: 10.1016/0003-9861(72)90244-5. [DOI] [PubMed] [Google Scholar]
- Roa M., Blobel G. Biosynthesis of peroxisomal enzymes in the methylotrophic yeast Hansenula polymorpha. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6872–6876. doi: 10.1073/pnas.80.22.6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbi M., Lazarow P. B. Peptide mapping of peroxisomal catalase and its precursor. Comparison to the primary wheat germ translation product. J Biol Chem. 1982 Jan 25;257(2):964–970. [PubMed] [Google Scholar]
- Robbi M., Lazarow P. B. Synthesis of catalase in two cell-free protein-synthesizing systems and in rat liver. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4344–4348. doi: 10.1073/pnas.75.9.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagara Y., Ito A. In vitro synthesis of monoamine oxidase of rat liver outer mitochondrial membrane. Biochem Biophys Res Commun. 1982 Dec 31;109(4):1102–1107. doi: 10.1016/0006-291x(82)91890-3. [DOI] [PubMed] [Google Scholar]
- Shore G. C., Carignan P., Raymond Y. In vitro synthesis of a putative precursor to the mitochondrial enzyme, carbamyl phosphate synthetase. J Biol Chem. 1979 May 10;254(9):3141–3144. [PubMed] [Google Scholar]
- Steck T. L., Yu J. Selective solubilization of proteins from red blood cell membranes by protein perturbants. J Supramol Struct. 1973;1(3):220–232. doi: 10.1002/jss.400010307. [DOI] [PubMed] [Google Scholar]
- Tobe T., Higashi T. Studies on rat liver catalase. XI. Site of synthesis and segregation by stripped ER membranes. J Biochem. 1980 Nov;88(5):1341–1347. doi: 10.1093/oxfordjournals.jbchem.a133102. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Yamada T., Tanaka A., Horikawa S., Numa S., Fukui S. Cell-free translation and regulation of Candida tropicalis catalase messenger RNA. Eur J Biochem. 1982 Dec 15;129(2):251–255. doi: 10.1111/j.1432-1033.1982.tb07046.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman R., Paluch U., Sprinzl M., Neupert W. Cell-free synthesis of the mitochondrial ADP/ATP carrier protein of Neurospora crassa. Eur J Biochem. 1979 Sep;99(2):247–252. doi: 10.1111/j.1432-1033.1979.tb13251.x. [DOI] [PubMed] [Google Scholar]