Abstract
BACKGROUND
Telomere attrition occurs early in the development of prostatic adenocarcinoma. However, little is known about either telomere status in benign prostatic hyperplasia (BPH), or the spatial and organ-wide distribution of potential telomere aberrations throughout all areas of prostatic glands affected by cancer or BPH.
METHODS
Slot blot titration assay was used to determine telomere DNA content (TC), a proxy for telomere length, in macrodissected tissue consisting of 54 normal samples from 5 disease-free prostates, 128 BPH samples from 4 non-cancerous prostates, and 45 tumor, 73 BPH, and 4 prostatic intraepithelial neoplasia (PIN) samples from 5 cancerous prostates.
RESULTS
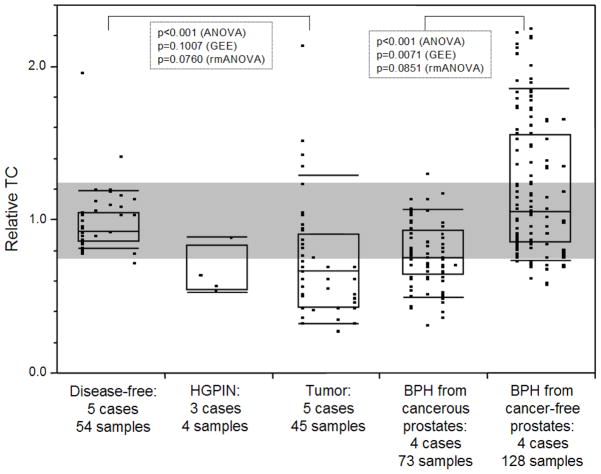
Compared to TC in normal prostate samples (n=54; TC mean=0.98), tumor samples displayed telomere attrition (n=45; TC mean=0.67). TC in PIN samples was similar to tumors. BPH samples from cancerous prostates were similar to TC in tumors and also displayed telomere shortening (n=73; TC mean=0.76), whereas BPH samples from non-cancerous prostates displayed longer telomeres (n=128; TC mean=1.06). In prostates affected by adenocarcinoma, areas of potential telomere attrition occurred in histologically normal tissues through the entire gland. However, three-dimensional zoning revealed a pattern of increasing TC as a function of distance from the primary (index) tumor.
CONCLUSIONS
Spatial distributions of TC in prostate specimens indicate a complex “field effect” with varying contributions from both cancer and BPH. The observation that telomere length variations occur in fields of histologically normal tissues surrounding the tumor is of clinical importance, as it may have implications for the diagnosis and focal therapy of prostate cancer.
Keywords: Telomere alterations, prostate cancer, benign prostatic hyperplasia, field cancerization
Introduction
It has long been recognized that adenocarcinomas of the prostate are characterized by genomic instability, i.e. the amplification, loss or structural rearrangement of critical genes. This is manifested, for example, by allelic imbalance and loss of heterozygousity which appear early in tumorigenesis and persist throughout tumor progression (1,2). The functional consequences of these genetic alterations on downstream targets have been partially elucidated (3), but the molecular mechanisms leading to genomic instability are still largely unknown. However, one potential initiator of genomic instability is telomere dysfunction (4–6). Telomeres are specialized nucleic acid-protein structures that protect the ends of eukaryotic chromosomes. Human telomeres contain 1,000 to 2,000 tandemly repeated copies of the hexanucleotide DNA sequence, TTAGGG (7) and stabilize the ends of chromosomes by masking them from being recognized by the cell as DNA double-strand breaks, thereby preventing degradation and recombination (8). Shortened telomeres, as often seen in cancer cells, may be generated by incomplete replication during DNA synthesis, loss or alterations of factors involved in telomere maintenance, or by DNA damage (4–6,9,10). Critically shortened telomeres trigger successive rounds of breakage-bridge-fusion cycles driving chromosomal instability that normally leads to p53-dependent senescence or apoptosis. However, these mechanisms are often inactivated in cancer cells, and if unchecked, these chromosomal aberrations accumulate and ultimately are lethal. Consequently, activation of the enzyme telomerase to stabilize the telomeric ends is necessary for tumor progression (11,12). In prostate cancer, telomere shortening has been found to occur early in tumorigenesis, as early as in the pre-neoplastic lesions of high grade prostatic intraepithelial neoplasia (HGPIN) (1,2,13,14). In contrast, little is known on the telomere status in benign prostatic hyperplasia (BPH) (15).
Previous studies, including our own, have shown that telomere length has clinical utility as a prognosticator in solid tumors including prostate cancer (16). In particular, we have reported an association between telomere length and clinical outcome parameters after radical prostatectomy such as time of prostate cancer-free survival, and recurrence of and death from prostate cancer (17,18). Remarkably, time of disease-free survival could also be predicted by telomeric status in the histologically normal tissue removed at some distance from the tumor, and comparative telomere length analysis between tumors and their matched histologically normal adjacent tissues revealed a significant correlation (17). However, the distribution of telomere lengths in different prostatic areas, both disease-free and affected by cancerous and non-cancerous alterations, was lacking. In this study, we used our established protocol to measure telomere DNA content (TC), a proxy for telomere length (19), to map the distribution of telomere lengths in prostatic tissues affected by cancerous (adenocarcinoma) and non-cancerous (BPH) disease. Our data show that telomeres in tumor adjacent histologically normal prostatic tissues, as well as in BPH tissues adjacent to prostate adenocarcinomas were shortened. In addition, the extent of organ-wide telomere shortening is a function of distance from the primary (index) tumor. In contrast, telomeres in BPH tissues from non-cancerous prostates showed longer telomeres, potentially indicating different telomere length regulation. Our findings may have clinical implications for prostate cancer assessment in prostate biopsies, and for the efficacy of focal therapy for prostate cancer.
Materials and Methods
Patient specimens and demographics
Five de-identified prostate specimens were obtained from the Cooperative Human Tissue Network (CHTN; Western Division, Nashville TN) in compliance with all Federal laws. These were entirely disease-free and obtained from autopsy cases from individuals who died due to conditions unrelated to cancer. Nine de-identified prostate specimens were obtained through the Department of Pathology at the University of New Mexico Hospital in Albuquerque NM in agreement with all University, State and Federal laws, and as approved by the Institutional Review Board of the University of New Mexico Health Sciences Center. Four prostates (3/4 enucleations) were affected by BPH, but free of adenocarcinoma. Five radical prostatectomies contained adenocarcinoma (5 cases), BPH (4 cases), and/or high grade prostatic intraepithelial neoplasia (HGPIN; 3 cases). Three of the cancerous prostates displayed multiple foci of adenocarcinoma. The median age of the cohort was 55 years with a range of 26–76 years. Gleason scores for the adenocarcinoma samples were 6 or 7, and pathological tumor node metastasis (TNM) stages, assigned according to the American Joint Committee on Cancer, were either pT2 or pT3 (Table I).
Table I.
Description of prostate specimens and demographics. The cohort consisted of 5 disease-free prostates, 5 prostates affected by adenocarcinoma, BPH, and/or HGPIN, and 4 cancer-free prostate specimens affected by BPH only. Specimens were collected at the Department of Pathology at the University of New Mexico Hospital in Albuquerque NM, or obtained from the Cooperative Human Tissue Network (CHTN; Nashville TN).
| Prostate samples | Specimen type | Patient’s age | Clinico-pathologic features | Gleason score1 | TNM stage1 |
|---|---|---|---|---|---|
| Disease-free | |||||
| 1 | Autopsy | 45 | --- | --- | --- |
| 2 | Autopsy | 43 | --- | --- | --- |
| 3 | Autopsy | 26 | --- | --- | --- |
| 4 | Autopsy | 46 | --- | --- | --- |
| 5 | Autopsy | 43 | --- | --- | --- |
| Cancer | |||||
| 1 | Radical prostatectomy | 55 | Multiple tumor foci, presence of BPH | 3+3 | pT2c |
| 2 | Radical prostatectomy | 65 | 0.3cm carcinoma, presence of BPH and HGPIN | 3+3 | pT2a |
| 3 | Radical prostatectomy | 70 | Multiple tumor foci, presence of BPH and HGPIN | 4+3 | pT2c |
| 4 | Radical prostatectomy | 53 | Multiple tumor foci, presence of BPH and HGPIN | 3+3 | pT2c |
| 5 | Radical prostatectomy | 57 | Focal, extra-prostatic extension | 4+3 | pT3a |
| BPH | |||||
| 1 | Enucleation | 69 | --- | --- | --- |
| 2 | Autopsy | 55 | --- | --- | --- |
| 3 | Enucleation | 76 | --- | --- | --- |
| 4 | Enucleation | 59 | --- | --- | --- |
Tumor Nodes Metastasis (TNM) stage was assigned using criteria published by the American Joint Committee on Cancer (http://www.cancerstaging.org/index.html); Gleason scores and TNM stages were determined from the prostatectomy samples.
Tissue processing and histological review
Radical prostatectomy specimens were inked in 4 colors to mark left anterior, left posterior, right anterior, and right posterior surgical margins to allow three-dimensional reconstruction. Specimens were cut into whole cross-sections in axial (transverse) plains as routinely done in our surgical pathology laboratory. Whole cross-sections were cut into 4 quadrants (left anterior, left posterior, right anterior, and right posterior) for smaller specimens or 6 sections (left anterior, left posterior, mid anterior, mid posterior, right anterior, and right posterior) for larger specimens. The exact location of each tissue section was documented in a diagram to allow reconstruction of complete cross-sections at histologic examination. Tissues were fixed in formalin for 4–6 hours and subjected to routine tissue processing for paraffin embedding and histologic examination. Four μm thick sections were stained with hematoxylin and eosin (H&E) and subjected to histopathological review. The H&E-stained sections were digitized into images from the prepared slides and areas with normal histologic appearance, invasive carcinoma, HGPIN, and BPH were identified by the study pathologist (E.G. Fischer). Ten μm thick sections were cut from the paraffin blocks, followed by another 4 μm section, which was stained with H&E. The 10 μm sections were mounted on uncoated glass slides, deparaffinized, rehydrated, and stained with 0.5% toluidine blue to visualize histologic lesions. Tissue samples from the identified areas were removed from the glass slides by macrodissection and analyzed for telomere DNA content as outlined below. The second H&E sections deep to the sample sections were also reviewed by the study pathologist (E.G. Fischer) to confirm presence of the lesions identified on the first H&E section. Digital images of the scanned H&E sections were assembled electronically to reconstruct images in each transverse tissue plain similar to whole mount sections.
Normal autopsy prostates were inked, sectioned, and processed similarly to the radical prostatectomy specimens. No carcinoma, HGPIN or BPH were found at histologic examination. Enucleation specimens were sampled randomly for histologic examination as done in routine surgical pathology practice. Ten μm thick sections were cut, deparaffinized, and sampled in the same fashion. H&E sections bordering both ends of the thick sections were examined to confirm the diagnosis of BPH and exclude the presence of carcinoma or HGPIN.
The above procedures resulted in macrodissected tissue samples isolated from multiple transversal planes from apex to base in all prostate quadrants. The samples consisted of 54 normal samples from the 5 disease-free prostates, 128 BPH samples from the 4 non-cancerous prostates, and 45 tumor (from 5 cases), 73 BPH (from 4 cases), and 4 PIN (from 3 cases) samples from the 5 cancerous prostates (Table I).
Three-dimensional data analysis (zoning)
For further analysis on tissues isolated from the cancerous prostates, a three-dimensional zoning procedure was applied. First, the largest focus of carcinoma was identified in radical prostatectomy specimens. Subsequently, tissue samples were grouped in three-dimensional zones across transverse sections located at increasing distances from the primary tumor. This procedure was individualized for each prostate. Depending on the size of the individual prostate, up to 7 zones of approximately the same width containing an average of 6 tissue samples for the primary (index) tumor area, and 10 tissue samples for the tumor adjacent areas at increasing distances were identified in this manner.
Telomere DNA content (TC) measurements
DNA was isolated from the formalin fixed and paraffin embedded (FFPE) tissue samples using the DNeasy® silica-based spin column extraction kit (Qiagen; Valencia, CA) and the manufacturer’s suggested animal tissue protocol. FFPE samples were treated with xylene and washed with ethanol prior to DNA extraction. DNA concentrations were measured using the Picogreen® dsDNA quantitation assay (Molecular Probes, Eugene, OR) using λ phage DNA as the standard as directed by the manufacturer’s protocol. TC was measured in known DNA masses, typically 5–10 ng, by slot blot titration assay, as previously described (19). TC is expressed as a ratio of the average chemiluminescent signal of replicate DNA samples compared to the value of the internal placental DNA standard at the same genomic DNA concentration. Each individual measurement is typically performed in at least triplicate; coefficients of variation are typically less than 10%. We have previously shown that TC is directly proportional to telomere length determined by Southern blotting (19). However, in contrast to Southern blotting, the TC assay (i) is not affected by TTAGGG sequences outside the telomere, (ii) is not affected by DNA fragmentation to less than 1 kilobase in length, (iii) can be utilized with FFPE, and (iv) can be performed with as little as 5 ng of genomic DNA (19). Thus, the TC assay is well-suited for telomere length analysis on archival specimens.
Statistical methods
Comparisons of TC distributions in cancerous vs. non-cancerous prostates were evaluated using parametric analysis of variance (ANOVA). Post-hoc Dunnett’s and least significant difference (LSD) tests were applied to identify the linking effect between tissues free of, or affected by adenocarcinoma. To take into consideration the repeated TC measurements in multiple samples from the same organs, generalized estimating equations (GEE) and repeated measure ANOVA (rmANOVA) adjusted by type III sum of squares were applied. Student’s t-test was used to compare the average TC values in the zones representing prostatic areas at increasing distance from the primary (index) tumor. SAS 9.2 and JMP® statistical packages (SAS Institute, Cary, NC) were used for all analyses.
Results
Telomere DNA content (TC) in prostatic tissues
TC was measured in 45 tumor, 73 BPH, and 4 HGPIN samples from 5 cancerous prostates, 128 BPH samples from 4 non-cancerous prostates, and 54 normal tissue samples from 5 disease-free prostates (Figure 1). TC values in samples from disease-free prostates were in a tight range with a mean of 0.98; 95% of these normal specimens fell within the range of 0.80–1.25. In contrast, TC in samples from tumor tissues showed a wider range of TC values, with a median of 0.67 and with 73.3% of the measurements falling outside the normal range. TC in HGPIN was similar to the tumor samples, although only 4 samples were available for analysis. TC in BPH samples isolated from prostates with adenocarcinoma was similar to TC in the tumor samples, with a median of 0.76 and with 60.3% of the samples falling outside the normal range. In contrast, BPH samples isolated from prostates that were free of adenocarcinoma displayed a significantly higher median TC of 1.06. Remarkably, 35.2% of TC values were above the normal range, indicating different regulation of telomere maintenance. When comparing the collective group specific means by parametric ANOVA, TC was significantly different between disease-free and tumor cases, and between the two BPH groups (p<0.001). Post-hoc Dunnett’s and LSD tests confirmed the significant difference in TC observed between the tissue groups. However, in our experimental design TC values were measured repeatedly in multiple samples from the same organs which showed partial data clustering for tumor cases and BPH from cancer-free prostates (columns of data points in Figure 1). To account for this potential effect on the comparison of TC between tissue groups, we conducted GEE and repeated measure ANOVA adjusted by type III sum of squares for the samples in each organ. When taking this effect into consideration, the differences in TC between tumor and disease-free samples were less significant (p=0.1007 by GEE and p=0.0760 by repeated ANOVA), and remained significant (p=0.0071 by GEE) and near significant (p=0.0851 by repeated ANOVA) for the comparison between BPH samples from cancer-free prostates and BPH samples from cancerous prostates. Collectively, despite a partial sample effect due to multiple TC measurements in the same organs, the data suggest a differential regulation of telomere maintenance in BPH in cancer-free vs. BPH in adenocarcinoma-affected prostates.
Fig. 1.
TC distributions in disease-free prostates and in prostate affected by adenocarcinoma, HGPIN and BPH. The number of cases and tissue samples is indicated. TC is expressed as a ratio of TC in a placental DNA control. Individual data points are shown as small black squares; vertical columns of data points represent individual cases. The boxes represent group medians (line across middle) and quartiles (25th and 75th percentiles) at its ends. Lines above and below boxes indicate 10th and 90th percentiles, respectively. The gray shaded area indicates 95% of TC measurements in the disease-free normals (0.80–1.25). p values indicate statistical significance between indicated groups, as calculated by parametric analysis of variance (ANOVA), generalized estimating equations (GEE), and repeated measure ANOVA (rmANOVA).
Organ-wide telomere DNA content (TC) in cancerous prostates
To further characterize telomere maintenance in the tissue areas associated with adenocarcinomas in a more refined manner, we determined the distribution of TC at different distances from the primary tumors and across different zones in sequential transverse sections of whole prostates using a heat map that represents deviations from the normal telomere length range. Figure 2 shows a representative spatial visualization of TC distribution in a prostate affected by a peripheral tumor in the right lower quadrant. In these analyses, TC ratios between 0.3 and 2.3 were observed throughout the prostate. As expected, the tumor areas were typically characterized by telomere shortening, while extensive TC variations were observed in different areas adjacent to the primary tumors, similar in distribution to the tumor samples in Figure 1 (not shown). Besides a highly variable pattern of TC distribution, this analysis consistently showed hot spots of reduced TC in areas at substantial distances from the primary tumor (Figure 2). This result demonstrates the presence of telomere shortening in histologically normal prostatic tissues adjacent to prostate adenocarcinomas.
Fig. 2.
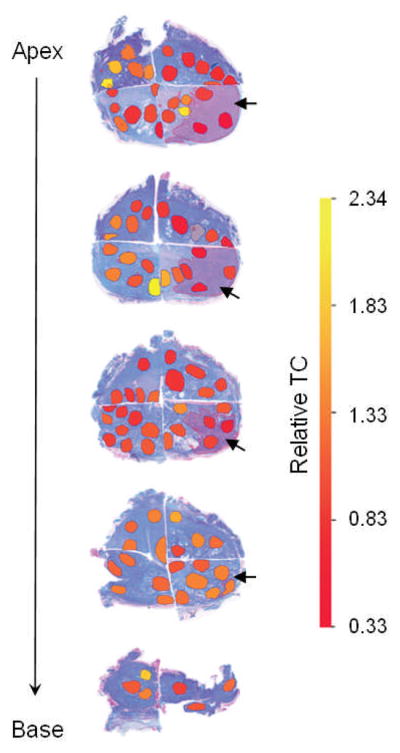
Organ-wide (from apex to base) spatial distribution of TC in transversal sections of a representative prostate affected by adenocarcinoma. The dark shaded areas represent cancerous tissues (indicated by thick arrows); the circumscribed areas represent excised tissues for TC analysis. Relative TC ratios are color coded with red designating low and yellow high TC as indicated by the heat map. Note: Outlier TC measurement (non-colored excision located in upper right quadrant of second section from top) was omitted.
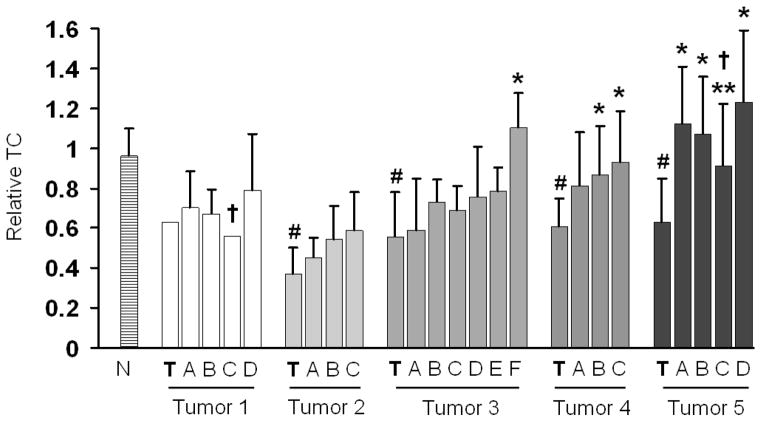
Next, to further reveal possible patterns of organ-wide telomere alterations as a function of the location of the primary tumor, we applied a zoning procedure in which all available tissue samples for a given prostate were grouped in three-dimensional zones across transverse sections and prostatic areas defined at increasing distances from the primary tumor. Depending on the size of the individual prostate, up to 7 zones per prostate were defined with an average number of tissue samples per zone of 6 (for tumor) and 10 (for tumor adjacent). Figure 3 shows the results for this type of analysis for the five prostates affected by adenocarcinoma. Compared to TC in normal disease-free tissues, the zone containing the primary tumor displayed significantly different (p<0.05) telomere shortening in 4/5 prostates. Consistent with the analysis shown in Figure 2, many zones of histologically normal tissue at various distances from the primary tumor displayed telomere shortening, as shown by reduced TC. With some variation, this three-dimensional approach revealed a gradual increase in TC as a function of distance from the primary tumor. In particular, in tumors 4 and 5, TC in almost all the tumor adjacent tissues was significantly different (p<0.05) from the tumor zone, while in tumor 3 this was the case for the farthest zone only. Of further note, in two cancerous prostates (tumors 1 and 3), one of the zones further away from the primary lesion revealed a reduced TC compared to zones closer to and farther from the tumor (Figure 3). In these cases, it could be determined that these zones contained a secondary lesion due to tumor multifocality which was sufficiently sized to affect average TC.
Fig. 3.
Relative TC of zones located at increasing distances from the primary (index) tumor zone (bolded T) for 5 prostates affected by adenocarcinoma. N denotes TC in disease-free normal tissues. Bars denote average TC +/− standard deviation for an average number of tissue samples per zone of 6 (for tumors) and 10 (for tumor adjacent). # denotes statistically significant difference (p<0.05) from disease-free normal tissues (N); * denotes statistically significant difference by student’s t-test (p<0.05) from zone T containing the tumor; † in tumors 1 and 5 denotes the presence of an additional cancerous focus in that zone; ** in tumor 5 denotes statistically significant difference by student’s t-test (p<0.05) from neighboring zone D. Note: The cancerous area in tumor 1 contained only one sample.
Discussion
Although it is accepted that telomere shortening is a characteristic of prostate cancer (13,15), information on the spatial extent and organ-wide distribution of prostate tissue areas harboring cells with short telomeres is missing. In this study, we provide data that indicate the presence of organ-wide telomere length variations in prostates affected by adenocarcinoma and BPH. In particular, using a combination of visual analyses of telomere alterations in bulk tissue areas, and a quantitative assessment using a three-dimensional zoning approach, we have shown the presence of hot spots of shortened telomeres in prostates affected by these conditions. Our data are in part mirrored by earlier studies by Bostwick and colleagues who assessed the occurrence of allelic imbalance in different areas of whole mount prostates harboring multiple foci of PIN and adenocarcinoma. In that study, comparative analyses between PIN and matched cancers had revealed an independent origin (20). In addition, Joshua and colleagues recently assessed telomere length by quantitative fluorescent in situ hybridization (QFISH) in biopsies from a cohort of 68 men diagnosed with HGPIN, but not prostate cancer (21). The QFISH method allows telomere length assessments in FFPE archival material, while providing single cell resolution and intact tissue architecture (22). The authors observed that short telomeres in the surrounding stroma, and possibly HGPIN, were associated with the eventual diagnosis of prostate cancer, suggesting that telomere shortening may drive chromosomal instability and thus, the eventual emergence of prostate cancer (21). Consistent with this view, Vukovic et al. demonstrated that the prostate cancer cell lines with the most complex karyotypes also had the shortest telomeres (23). Our data indicate organ-wide telomere length alterations including tissue areas that are histologically normal with morphologically intact glandular and stromal structures in prostates harboring areas of adenocarcinoma. The data also indicate telomere attrition in BPH adjacent to tumors vs. BPH only. This is partially confirmatory of an earlier study in which marginal telomere shortening (~4%) was observed in BPH samples associated with adenocarcinoma (15). Our findings are in agreement with the concepts of “field cancerization” or “field effect”, a concept that has been used to explain the presence of genetically and/or biochemically altered cells in fields of normal tissues adjacent to primary tumors, including prostate cancers (24,25). In support of this concept, we have recently reported on gene expressional changes in histologically normal human prostatic tissues adjacent to prostate cancers as compared to disease-free tissues (26). The data presented in this study may have important clinical implications.
First, the presence of organ-wide telomere length variations in histologically normal tissue areas of diseased prostates could be indicators of genomic instability and may affect confirmatory diagnosis of prostate cancer at the time of biopsy. It is estimated that up to 2 million prostate biopsies are performed in the United States due to the introduction of screening by serum prostate specific antigen (PSA)(27). However, it has been reported that cancer is missed due to inconspicuous lesions in up to a third of biopsies from patients with subsequently confirmed prostate cancer (false-negatives)(28,29). In the presence of other disease indicators, this greatly impairs informed clinical decision making as it relates to therapeutic intervention. Thus, there is a need for novel, reliable cancer markers that can be used at biopsy, and ideally independent of cancerous histology, i.e. present in histologically normal tissues adjacent to tumors. We have recently reported on the use of TC in prostate biopsies as a predictor of PSA recurrence in patients who underwent prostatectomy (30). In this study, the predictive power of TC was independent of the percentage of tumor content in the biopsy and was thus informative even in the presence of histologically normal tissue. This result is indicative of the potential clinical utility of markers present in “field cancerized” prostatic tissues. In further support of the latter is our finding that BPH tissue isolated from prostates containing adenocarcinoma displayed significantly reduced TC compared to BPH tissue from cancer-free prostates. Accordingly, the presence of a tumor could induce a field of cells with shortened telomeres leading to genomic instability, which is evident even in areas affected by BPH only. These cells may display telomere attrition due to the increased proliferation typically seen in BPH, but are otherwise protected from transformation by the presence of intact cell cycle checkpoints. Our data also suggest a different telomere regulation in pure BPH tissues, as demonstrated by the presence of longer telomeres. While the importance of longer telomeres in this setting is unknown, they could be protective against the development of malignant disease, such as PIN or adenocarcinoma. However, we cannot rule out the possibility that the constitutive telomere length was longer in these patients.
Second, the majority of prostate cancers occur as multiple foci of varying sizes throughout the gland (2,18,31,32). In fact, the rate of unifocal tumors in prostate cancer patients was found to be only 13%–38% (32,33). While the concepts of field cancerization can partially explain prostate cancer multifocality (24,25), a more important implication relates to the use of focal therapy for patients with localized prostate cancer, including high-intensity focused ultrasound, cryosurgery, focal radiotherapy and photochemotherapy (33). The rationale for focal therapy in cases of localized early stage prostate cancer relies on the fact that typically only one focus develops into the primary bulk of cancer while the others remain indolent (20), thereby warranting organ-sparing treatment as successfully applied to other malignancies (e.g. breast, skin, and kidney). Focal therapy has the potential to avoid morbidities associated with prostatectomy and radiotherapy, i.e. impotence, incontinence, bowel dysfunction, inflammation, and others. These efforts are substantiated by the clinical reality of over-treatment with aggressive surgical intervention in the absence of reliable prognostic markers able to identify patients who would benefit from whole prostate gland treatments (34). The data presented in this study show the occurrence of organ-wide telomere length variations in histologically normal tissue in prostatic glands affected by adenocarcinoma, which may indicate areas prone to genomic instability. This adds to the possible concerns about focal therapy, as it may relate to the emergence of secondary tumors and/or disease recurrence initiated by theses field cancerized tissue areas. However, in the future, development of more accurate, foci specific markers of potential tumor regions of the prostate gland could lead to more accurate targeted focal ablative treatment techniques.
Collectively, these data emphasize the need for extended investigations in larger cohorts. This will lead to a better understanding of the mechanisms underlying telomere length variations in histologically normal tumor adjacent prostatic tissues, as well as to assess its potential implications for clinical practice.
Acknowledgments
We would like to thank Cathy Martinez, Myra Zucker, and Kari Rigg in the Department of Pathology at the University of New Mexico Health Sciences Center for skillfully grossing radical prostatectomy specimens and carefully preparing specimen diagrams. We are grateful to Kerry Wiles from the Cooperative Human Tissue Network (CHTN; Western Division, Nashville TN) for the successful procurement of disease-free prostates and annotated reports. We thank Dr. Alan Meeker (The Johns Hopkins Medical Institutions, Department of Pathology, Baltimore MD) for critically reviewing the manuscript and providing constructive comments. This work is supported by National Institutes of Health Grant RR0164880 (to M. Bisoffi and J.K. Griffith), Department of Defense pre-doctoral training award W81XWH-05-0273 (to C.M. Heaphy), University of New Mexico Cancer Center Support Grant NIH/NCI P30CA118110, and the University of New Mexico General Clinical Research Center NIH NCRR GCRC Grant M01-RR00997 (to E.G. Treat).
Abbreviations
- BPH
benign prostatic hyperplasia
- FFPE
formalin-fixed paraffin-embedded
- HGPIN
high grade prostatic intraepithelial neoplasia
- PSA
prostate specific antigen
- TC
telomere DNA content
References
- 1.De Marzo AM, Meeker AK, Zha S, Luo J, Nakayama M, Platz EA, Isaacs WB, Nelson WG. Human prostate cancer precursors and pathobiology. Urology. 2003;62(5 Suppl 1):55–62. doi: 10.1016/j.urology.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 2.Joshua AM, Evans A, Van der Kwast T, Zielenska M, Meeker AK, Chinnaiyan A, Squire JA. Prostatic preneoplasia and beyond. Biochim Biophys Acta. 2008;1785(2):156–181. doi: 10.1016/j.bbcan.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Dong JT. Prevalent mutations in prostate cancer. J Cell Biochem. 2006;97(3):433–447. doi: 10.1002/jcb.20696. [DOI] [PubMed] [Google Scholar]
- 4.Feldser DM, Hackett JA, Greider CW. Telomere dysfunction and the initiation of genome instability. Nat Rev Cancer. 2003;3(8):623–627. doi: 10.1038/nrc1142. [DOI] [PubMed] [Google Scholar]
- 5.Gisselsson D, Jonson T, Petersen A, Strombeck B, Dal Cin P, Hoglund M, Mitelman F, Mertens F, Mandahl N. Telomere dysfunction triggers extensive DNA fragmentation and evolution of complex chromosome abnormalities in human malignant tumors. Proc Natl Acad Sci U S A. 2001;98(22):12683–12688. doi: 10.1073/pnas.211357798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desmaze C, Soria JC, Freulet-Marriere MA, Mathieu N, Sabatier L. Telomere-driven genomic instability in cancer cells. Cancer Lett. 2003;194(2):173–182. doi: 10.1016/s0304-3835(02)00704-8. [DOI] [PubMed] [Google Scholar]
- 7.Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MD, Meyne J, Ratliff RL, Wu JR. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988;85(18):6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maser RS, DePinho RA. Telomeres and the DNA damage response: why the fox is guarding the henhouse. DNA Repair (Amst) 2004;3(8–9):979–988. doi: 10.1016/j.dnarep.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Olovnikov AM. Principle of marginotomy in template synthesis of polynucleotides. Dokl Akad Nauk SSSR. 1971;201(6):1496–1499. [PubMed] [Google Scholar]
- 10.Smogorzewska A, van Steensel B, Bianchi A, Oelmann S, Schaefer MR, Schnapp G, de Lange T. Control of human telomere length by TRF1 and TRF2. Mol Cell Biol. 2000;20(5):1659–1668. doi: 10.1128/mcb.20.5.1659-1668.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiyama E, Gollahon L, Kataoka T, Kuroi K, Yokoyama T, Gazdar AF, Hiyama K, Piatyszek MA, Shay JW. Telomerase activity in human breast tumors. J Natl Cancer Inst. 1996;88(2):116–122. doi: 10.1093/jnci/88.2.116. [DOI] [PubMed] [Google Scholar]
- 12.Lin Y, Miyamoto H, Fujinami K, Uemura H, Hosaka M, Iwasaki Y, Kubota Y. Telomerase activity in human bladder cancer. Clin Cancer Res. 1996;2(6):929–932. [PubMed] [Google Scholar]
- 13.Meeker AK. Telomeres and telomerase in prostatic intraepithelial neoplasia and prostate cancer biology. Urol Oncol. 2006;24(2):122–130. doi: 10.1016/j.urolonc.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Koeneman KS, Pan CX, Jin JK, Pyle JM, 3rd, Flanigan RC, Shankey TV, Diaz MO. Telomerase activity, telomere length, and DNA ploidy in prostatic intraepithelial neoplasia (PIN) J Urol. 1998;160(4):1533–1539. [PubMed] [Google Scholar]
- 15.Sommerfeld HJ, Meeker AK, Piatyszek MA, Bova GS, Shay JW, Coffey DS. Telomerase activity: a prevalent marker of malignant human prostate tissue. Cancer Res. 1996;56(1):218–222. [PubMed] [Google Scholar]
- 16.Bisoffi M, Heaphy CM, Griffith JK. Telomeres: prognostic markers for solid tumors. Int J Cancer. 2006;119(10):2255–2260. doi: 10.1002/ijc.22120. [DOI] [PubMed] [Google Scholar]
- 17.Fordyce CA, Heaphy CM, Joste NE, Smith AY, Hunt WC, Griffith JK. Association between cancer-free survival and telomere DNA content in prostate tumors. J Urol. 2005;173(2):610–614. doi: 10.1097/01.ju.0000143195.49685.ce. [DOI] [PubMed] [Google Scholar]
- 18.Donaldson L, Fordyce C, Gilliland F, Smith A, Feddersen R, Joste N, Moyzis R, Griffith J. Association between outcome and telomere DNA content in prostate cancer. J Urol. 1999;162(5):1788–1792. [PubMed] [Google Scholar]
- 19.Fordyce CA, Heaphy CM, Griffith JK. Chemiluminescent measurement of telomere DNA content in biopsies. Biotechniques. 2002;33(1):144–146. 148. doi: 10.2144/02331md02. [DOI] [PubMed] [Google Scholar]
- 20.Bostwick DG, Shan A, Qian J, Darson M, Maihle NJ, Jenkins RB, Cheng L. Independent origin of multiple foci of prostatic intraepithelial neoplasia: comparison with matched foci of prostate carcinoma. Cancer. 1998;83(9):1995–2002. doi: 10.1002/(sici)1097-0142(19981101)83:9<1995::aid-cncr16>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Joshua AM, Vukovic B, Braude I, Hussein S, Zielenska M, Srigley J, Evans A, Squire JA. Telomere attrition in isolated high-grade prostatic intraepithelial neoplasia and surrounding stroma is predictive of prostate cancer. Neoplasia. 2007;9(1):81–89. doi: 10.1593/neo.06745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meeker AK, Gage WR, Hicks JL, Simon I, Coffman JR, Platz EA, March GE, De Marzo AM. Telomere length assessment in human archival tissues: combined telomere fluorescence in situ hybridization and immunostaining. Am J Pathol. 2002;160(4):1259–1268. doi: 10.1016/S0002-9440(10)62553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vukovic B, Beheshti B, Park P, Lim G, Bayani J, Zielenska M, Squire JA. Correlating breakage-fusion-bridge events with the overall chromosomal instability and in vitro karyotype evolution in prostate cancer. Cytogenet Genome Res. 2007;116(1–2):1–11. doi: 10.1159/000097411. [DOI] [PubMed] [Google Scholar]
- 24.Dakubo GD, Jakupciak JP, Birch-Machin MA, Parr RL. Clinical implications and utility of field cancerization. Cancer Cell Int. 2007;7:2. doi: 10.1186/1475-2867-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nonn L, Ananthanarayanan V, Gann PH. Evidence for field cancerization of the prostate. Prostate. 2009 doi: 10.1002/pros.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haaland CM, Heaphy CM, Butler KS, Fischer EG, Griffith JK, Bisoffi M. Differential gene expression in tumor adjacent histologically normal prostatic tissue indicates field cancerization. International Journal of Oncology. 2009;35:537–546. doi: 10.3892/ijo_00000365. [DOI] [PubMed] [Google Scholar]
- 27.Stamey TA, Caldwell M, McNeal JE, Nolley R, Hemenez M, Downs J. The prostate specific antigen era in the United States is over for prostate cancer: what happened in the last 20 years? J Urol. 2004;172(4 Pt 1):1297–1301. doi: 10.1097/01.ju.0000139993.51181.5d. [DOI] [PubMed] [Google Scholar]
- 28.Rabbani F, Stroumbakis N, Kava BR, Cookson MS, Fair WR. Incidence and clinical significance of false-negative sextant prostate biopsies. J Urol. 1998;159(4):1247–1250. [PubMed] [Google Scholar]
- 29.Stamey TA. Making the most out of six systematic sextant biopsies. Urology. 1995;45(1):2–12. doi: 10.1016/s0090-4295(95)96168-2. [DOI] [PubMed] [Google Scholar]
- 30.Treat EG, Heaphy CM, Massie LW, Bisoffi M, Smith AY, Davis MS, Griffith JK. Telomere DNA Content in Prostate Biopsies Predicts Early Rise in Prostate-specific Antigen After Radical Prostatectomy for Prostate Cancer. Urology. 2009 doi: 10.1016/j.urology.2009.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian J, Wollan P, Bostwick DG. The extent and multicentricity of high-grade prostatic intraepithelial neoplasia in clinically localized prostatic adenocarcinoma. Hum Pathol. 1997;28(2):143–148. doi: 10.1016/s0046-8177(97)90097-6. [DOI] [PubMed] [Google Scholar]
- 32.Wise AM, Stamey TA, McNeal JE, Clayton JL. Morphologic and clinical significance of multifocal prostate cancers in radical prostatectomy specimens. Urology. 2002;60(2):264–269. doi: 10.1016/s0090-4295(02)01728-4. [DOI] [PubMed] [Google Scholar]
- 33.Eggener SE, Scardino PT, Carroll PR, Zelefsky MJ, Sartor O, Hricak H, Wheeler TM, Fine SW, Trachtenberg J, Rubin MA, Ohori M, Kuroiwa K, Rossignol M, Abenhaim L. Focal therapy for localized prostate cancer: a critical appraisal of rationale and modalities. J Urol. 2007;178(6):2260–2267. doi: 10.1016/j.juro.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 34.Fradet Y. Biomarkers in prostate cancer diagnosis and prognosis: beyond prostate-specific antigen. Curr Opin Urol. 2009;19(3):243–246. doi: 10.1097/MOU.0b013e32832a08b5. [DOI] [PubMed] [Google Scholar]