Abstract
Wound healing is a dynamic process that relies on coordinated signaling molecules to succeed. Heparin-binding epidermal growth factor (EGF)-like growth factor (HB-EGF) is proven to accelerate healing, however precise control over its application is necessary to reduce side effects and achieve desired therapeutic benefit. To achieve effective growth factor delivery we designed a bioactive heparin-based coacervate. In vitro, HB-EGF released from the coacervate delivery system displayed enhanced bioactivity and promoted human keratinocyte migration while preserving cell proliferative capability. In a mouse excisional full-thickness wound model, controlled release of HB-EGF within the wound significantly accelerated wound closure more effectively than an equal dosage of free HB-EGF. Healing was induced by rapid re-epithelialization, granulation tissue formation, and accompanied by angiogenesis. Consistent with in vitro results, wounds treated with HB-EGF coacervate exhibited enhanced migration of keratinocytes with retained proliferative potential, forming a confluent layer for regained barrier function within 7 days. Collectively, these results suggest that coacervate-based controlled release of HB-EGF may serve as a new therapy to accelerate healing of cutaneous wounds.
Keywords: wound healing, coacervate, heparin, polycation, growth factors
INTRODUCTION
Dermal injury remains one of the most prevalent and economically burdensome healthcare issues in the world. In the US alone there are more than 100 million acute wounds annually, including surgical incisions, trauma, and burns [1]. Current wound management requires frequent dressing changes and patients are at constant risk of infection until the skin regains its barrier function. In chronic wounds, a variety of underlying detriments, including pressure, insufficient blood flow, and edema, prevents healing from occurring without proactive treatment [2]. Current treatment options are limited, costly, and inefficient. As a result, the development of new therapeutics is necessary to satisfy the clinical need.
Growth factor therapies hold tremendous potential to address the shortcomings of current wound care modalities [3, 4]. Growth factors act as critical extracellular cues that orchestrate the wound healing process; supply of exogenous growth factors may therefore induce faster re-epithelialization, leading to reduced risk of infection and shorter inpatient stays. In cases of chronic wounds, growth factors may provide the necessary stimuli to induce wound closure that is otherwise unlikely to occur [5]. Several important growth factors involved in the wound healing process are from the epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and transforming growth factor-β (TGF-β) families. Heparin-binding EGF-like growth factor (HB-EGF), although less studied, is present in both human and porcine wound fluid [6, 7], and is a potent stimulator of keratinocyte proliferation and migration [8, 9]. HB-EGF has been shown to have mitogenic effects on fibroblasts [6, 10], and may therefore be involved in the formation of granulation tissues. Lastly, HB-EGF also plays a role in angiogenesis during wound healing [11, 12]. Clearly, HB-EGF acts as a versatile regulator of wound healing and would be highly beneficial as a regenerative therapeutic.
Provision of exogenous growth factors can accelerate wound healing, however they must be applied in a sustained and localized fashion to be effective. This is due to short growth factor half-lives, rapid dilution in the body, and undesirable effects at high systemic levels [13]. Our group has developed a controlled delivery system based on intact heparin, a highly-sulfated glycosaminoglycan with similar functionality to the extracellular matrix (ECM)-derived proteoglycan, heparan sulfate. Heparin and heparan sulfate bind many growth factors with high affinity, extend their half-lives by protection from proteolytic degradation, and increase their bioactivity [14, 15]. To keep the water-soluble heparin:growth factor complex localized to the site of application, a synthetic polycation, poly(ethylene argininylaspartate diglyceride) (PEAD), was designed to interact with heparin through polyvalent charge attraction, forming a growth factor-containing coacervate [16]. In this simple delivery system, incorporated growth factors are separated from the aqueous environment in the oil phase of the coacervate, further protecting them from degradation. This delivery platform is capable of controlling the release of heparin-binding growth factors and has been used to successfully deliver FGF2 to stimulate therapeutic angiogenesis [17]. Here we report the utility of this platform in controlled delivery of HB-EGF for wound healing.
MATERIALS & METHODS
HB-EGF coacervate preparation
PEAD was synthesized as previously described [18, 19]. Heparin USP from porcine intestine (Scientific Protein Labs, Waunakee, WI) and PEAD were each dissolved at 10 mg ml−1 in sterile saline and 0.22 μm filter-sterilized. Heparin was initially combined with recombinant human HB-EGF (R&D Systems, Minneapolis, MN). PEAD was then added (50:10:1 mass ratio of PEAD:heparin:HB-EGF) which immediately precipitated the complex out of solution to form the HB-EGF coacervate, visible as a turbid solution.
Fluorescent imaging of the coacervate
A minimal amount of heparin (less than 1% by weight) in the coacervate was replaced with fluorescein (Aldrich Chemicals, Milwaukee, WI) by dissolving it in the heparin solution. The coacervate was centrifuged at 12,100 g for 10 min and the supernatant with unbound fluorescein was aspirated and replaced with deionized water. The coacervate was loaded to a glass chamber slide and imaged by an Olympus Fluoview FV100 confocal microscope (Olympus, Tokyo, Japan).
HB-EGF release profile
200μl HB-EGF coacervate containing 1 μg HB-EGF was pelleted by centrifugation at 12,100 g for 10 min and placed at 37 C. On day 0, 1, 4, 7, and 10 the supernatant was aspirated and stored at −80 C, and 200μl fresh saline was replaced to cover the pellet. The amount of released HB-EGF in three separate fractions per timepoint was determined by ELISA (R&D Systems, Minneapolis, MN).
In vitro scratch wound assay with primary human keratinocytes
1x106 normal human epidermal keratinocytes (NHEK, Lonza, Basel, Switzerland) at passage 3 were seeded per well in a 24-well plate and cultured in full keratinocyte growth media, KGM-Gold (Lonza, Basel, Switzerland), until confluency. A 10 μl pipette tip was used to scratch the cell layer and wells were washed once with DPBS to remove debris. Cells were cultured at 37 C in KGM-Gold, without bovine pituitary extract (BPE) or epidermal growth factor (EGF) supplements, containing 100 ng ml−1 free HB-EGF or HB-EGF coacervate, or no HB-EGF as control. Photographs were taken of in vitro wounds in three wells per group with an Eclipse Ti inverted microscope (Nikon, Tokyo, Japan) at the beginning of the experiment and every 6 hours for one day. Scratch width was measured using NIS Elements Analysis software (Nikon, Tokyo, Japan).
In vitro proliferation of primary human keratinocytes
2x105 passage 3 NHEK were seeded per well in a 24-well plate and cultured at 37 C in KGM-Gold media, without BPE or EGF supplements, containing either 10 ng ml−1 or 1 ng ml−1 free HB-EGF or HB-EGF coacervate, or no HB-EGF as control. After 4 days cell lysates from three wells per group were prepared by the addition of 0.1 % Triton X-100 (Sigma, St. Louis, MO) followed by 3 freeze-thaw cycles. Double-stranded DNA (dsDNA) content of cell lysates was measured by Quant-iT dsDNA high-sensitivity assay kit (Invitrogen, Carlsbad, CA).
Murine wound model and wound closure analysis
6–7 week old C57BL6/J mice (Jackson Labs, Bar Harbor, ME) were anesthesized with isoflurane and the dorsal area was shaved. Two 0.5 mm-thick silicone donut-shaped splints (OD=16 mm, ID=8 mm) were fixed on either side of the dorsal midline, approximately 2.5 cm from the ears, with 6-0 Prolene suture. Identical full-thickness wounds were made using a 5 mm round skin biopsy punch, centered within each splint. 1 μg free HB-EGF, as an HB-EGF coacervate, vehicle alone, or saline was applied to wounds (n=7 animals/ 14 wounds per group) by sterile pipet as a 10 μl solution. Two wounds were made on each mouse to increase sample size and both wounds received the same treatment to avoid cross-contamination. Polyskin II sterile hydrocolloid bandages (Tyco Healthcare, Mansfield, MA) were applied followed by Coban self-adhesive wrap (3M, St. Paul, MN) around the midsection to deter chewing of the splints. On days 7, 10, 14, and 17 only the wrap was removed and wounds were digitally photographed through the clear hydrocolloid bandage. Wound area was measured by blinded operator using a fine-resolution computer mouse to trace the wound margin in MetaMorph Image Analysis Software (Molecular Devices, Sunnyvale, CA). Suture and wraps were replaced as necessary to keep the splints fixed. On day 7, two animals per group were sacrificed and wounds and surrounding were tissue collected for histological evaluation. Remaining animals were sacrificed on day 17. University of Pittsburgh Institutional Animal Care and Use Committee (IACUC) approval was obtained prior to beginning all animal studies.
Histological analysis
Wound and surrounding tissue was collected using a 7 mm skin biopsy punch and then bisected through the midline of the wound using a razor blade. Both halves were immediately embedded in optimal cutting temperature (OCT) medium (Sakura Finetek, Torrance, CA), frozen, and sequentially sectioned at 5 μm using a MICROM HM525 cryomicrotome (Thermo Fisher Scientific, Waltham, MA). Only sections from the same area of the wound were compared histologically. Skin sections were stained with hematoxylin and eosin (H&E) for standard evaluation and with Masson’s trichrome staining (MTS) for evaluation of collagen content, counterstained with hematoxylin. All histological analyses were performed on at least 4 wounds per group per timepoint, and images presented are representative of all replicates.
Immunohistochemical analysis
Wound sections were fixed and stained using a rabbit polyclonal anti-wide spectrum cytokeratin antibody (1:75, Abcam, Cambridge, MA) with an IHC staining kit (Santa Cruz Biotech, Santa Cruz, CA), then counterstained with hematoxylin. Day 7 sections were dual-stained using rabbit polyclonal anti-Ki-67 (1:500, Abcam, Cambridge, MA) and rabbit anti-integrin β4 (1:200, Abcam, Cambridge, MA) followed by goat anti-rabbit IgG Alexa Fluor 488 and Alexa Fluor 594-conjugated secondary antibodies (1:200, Invitrogen, Carlsbad, CA), respectively, and counterstained with DAPI. Day 17 skin sections were dual-stained with purified anti-mouse CD31 (1:50, BioLegend, San Diego, CA) followed by Alexa Fluor 588-conjugated secondary antibody (1:200, Invitrogen, Carlsbad, CA), and FITC-conjugated mouse anti-α-smooth muscle actin (α-SMA, 1:500, Sigma, St. Louis, MI). All images were taken using an Eclipse Ti inverted microscope (Nikon, Tokyo, Japan).
Statistical analysis
Statistical analysis was performed using SPSS 16.0 software (SPSS Inc, Chicago, IL). Statistical differences were detected using analysis of variance (ANOVA) followed by Bonferroni correction as post-hoc testing. Differences were considered significant at P<0.05.
RESULTS
Characterization of HB-EGF coacervate
PEAD contains two functional groups per repeating unit that carry positive charge (Fig. 1a). The coacervate forms immediately upon addition of PEAD to the heparin:HB-EGF complex in PBS, visible macroscopically as a turbid solution. Fluorescein carries negative charges and integrates into the coacervate via Coulombic forces. Fluorescent imaging of the coacervate with embedded fluorescein revealed spherical droplet with diameters ranging from 10–500 nm (Fig. 1b). The release kinetics of HB-EGF from the coacervate were determined in vitro. Less than 8% HB-EGF was detected in the supernatant after pelleting the coacervate (day 0 of release assay), indicating a loading efficiency of greater than 92%. No initial burst release of HB-EGF was observed on day 1, and release thereafter was approximately linear at 15 ng day−1 (Fig. 1c).
Figure 1. The coacervate controls the release of HB-EGF in a steady fashion.
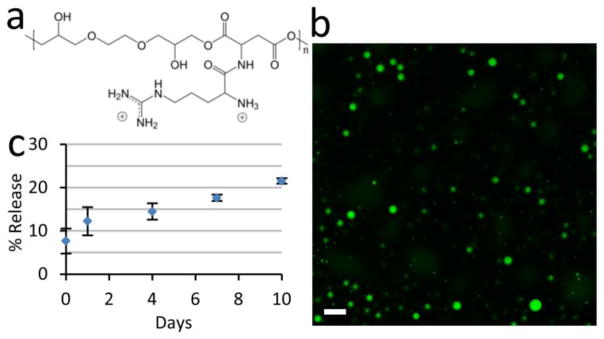
(a) Chemical structure of the PEAD. The backbone of PEAD contains aspartic acid and ethylene glycol diglyceride, connected by biodegradable ester bonds. Arginine is conjugated to provide two cationic charges per repeating unit, giving PEAD strong affinity for polyanions. (b) Confocal fluorescent imaging of fluorescein-labeled coacervate shows spherical morphology with droplet diameters of 10–500 nm. Bar = 1 μm. (c) In vitro release profile of HB-EGF from the coacervate into saline over 10 days. Bars indicate means ± SD.
HB-EGF stimulates keratinocyte migration
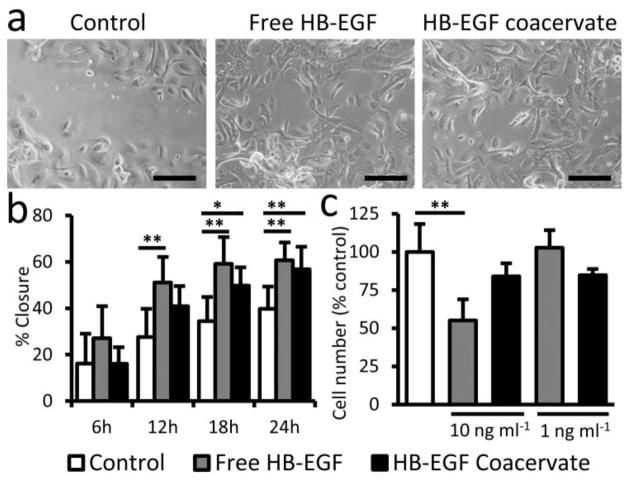
The effect of free and coacervate HB-EGF on the migratory capacity of primary human keratinocytes was determined by their ability to induce in vitro wound closure in a scratch assay. The closure of wounds made in confluent cell monolayers was tracked over 24 hours (Fig. 2a). It is important to mention that cell proliferation may also affect closure rate in this assay, but the short timeframe limits its contribution. Though not statistically significant, the effect of bolus addition of free HB-EGF is evident 6 hours after wounding (Fig. 2b). By 12 hours post-wounding, free HB-EGF significantly improved closure of scratch wounds compared to the control. Beginning 18 hours post-wounding, HB-EGF coacervate also significantly improved closure compared to the control. Scratch wound closure in free and coacervate HB-EGF groups was statistically the same at all timepoints. It is important to note that although the same amount of HB-EGF was added to both groups (100 ng ml−1), in the controlled delivery case it is mostly incorporated in the coacervate so the concentration of free growth factor in the medium is much lower.
Figure 2. HB-EGF coacervate stimulates primary human keratinocyte migration without inhibiting proliferation in vitro.
(a) Representative images of scratch wounds on confluent keratinocyte monolayers after 24 h of culture in media supplemented with saline (Control), 100 ng ml−1 HB-EGF free or in the coacervate. Bars = 200 μm. (b) Quantification of percent closure of scratch wounds over time. Bars indicate means ± SD. *P<0.05; **P<0.01. (c) Quantification of cell number after 4d culture of keratinocytes in media supplemented with saline (Control), 1 ng ml−1 or 10 ng ml−1 HB-EGF free or in the coacervate. Bars indicate means ± SD. **P<0.01.
Controlled release of HB-EGF from the coacervate does not inhibit keratinocyte proliferation
Primary human keratinocytes were cultured in the presence of free or coacervate HB-EGF in the culture media. Cell proliferation over 4 days culture was analyzed by DNA quantification. At high HB-EGF concentration (10 ng ml−1), free growth factor reduced cell proliferation compared to control while cell proliferation in the presence of coacervate with the same amount of growth factor had no statistically significant change (Fig. 2c). This is consistent with the premise of controlled release reducing the burst effect. At lower concentration (1 ng ml−1) however, neither free nor coacervate HB-EGF had any significant effect on cell number.
HB-EGF coacervate accelerates mouse wound closure
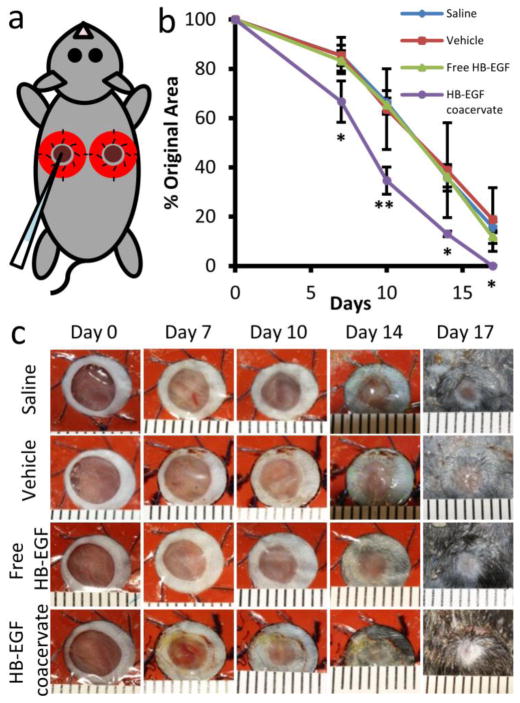
The effects of free and coacervate HB-EGF on wound closure were examined in a splinted mouse full-thickness excisional wound model. A modified skin biopsy punch wounding procedure led to a more uniform wound size than procedures requiring scissors. To mimic human healing, splints were used to avoid skin contraction, thus making re-epithelialization the major factor in wound healing. The wounds were treated immediately with saline, vehicle (PEAD:heparin complex), 1μg HB-EGF free or in the coacervate (Fig. 3a). Wound area was tracked over 17 days and animals were sacrificed on days 7 and 17 for histological analysis. Accelerated closure of wounds treated with HB-EGF coacervate was evident by day 7 and continued throughout the test period compared to all other groups (Fig. 3b-c). Only this treatment induced complete wound closure by day 17. No significant difference existed between the other three groups at any time point. Histological analysis after 7 days showed a thick cell-rich granulation region in HB-EGF coacervate-treated wounds with high collagen content, revealed by Masson’s trichrome staining (MTS) (Fig. 4). Immunohistochemical detection of cytokeratin shows a thick stratum corneum on day 7 that was remodeled to identical structure to the surrounding normal epidermis by day 17 (Fig. 4). Signs of returning dermal appendages were also observed by day 17 in HB-EGF coacervate-treated wounds. Other groups showed thin and inconsistent granulation regions which stained less strongly with MTS. Positive cytokeratin staining was observed in all groups in dermal appendages such as hair follicles and sebaceous glands where epithelial cells also reside [20].
Figure 3. HB-EGF coacervate accelerates healing of full-thickness excisional wounds.
(a) Silicone donut-shaped splints, fixed with suture, surrounded the wounds and forced healing to occur by re-epithelialization. Wounds were treated immediately with 10 μl of group-specific solution by sterile pipet. (b) Wound closure over time, measured as percent of original area. Fourteen wounds were averaged for day 7 timepoint after which two animals were sacrificed per group for histology; the remaining 10 wounds per group were photographed until sacrifice on day 17. Bars indicate means ± SD. *P<0.05; **P<0.01. (c) Representative photographs of wounds treated with saline, vehicle (PEAD:heparin complex), 1 μg HB-EGF free or in the coacervate. Ruler units are mm.
Figure 4. HB-EGF coacervate accelerates wound re-epithelialization and increases collagen content of granulation tissue.
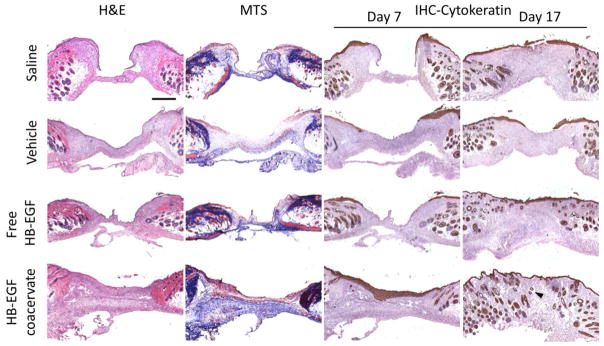
Wound sections stained with hematoxylin and eosin (H&E) for general observation, Masson’s trichrome (MTS) for collagen, and immunohistochemically for cytokeratin (IHC-Cytokeratin) as a marker of epithelial cells. Representative light microscopy images of sections from the center of day 7 wounds are presented for all three staining methods, and of day 17 wound sections stained for IHC-Cytokeratin. Arrows indicate newly formed dermal appendages present within the granulation tissue. Bar = 500 μm.
HB-EGF coacervate increases keratinocyte proliferative and migratory potential in vivo
To further investigate the effect of HB-EGF on the behavior of keratinocytes after wounding, day 7 wound sections were immunostained for proliferation and migration. The former was reflected by Ki-67, a marker of cell proliferation [21]. The latter was observed by staining of β4 integrins, which heterodimerize with α6 subunits on the surface of epithelial cells and play a key role in cell motility, especially during wound healing [22–24]. Both markers were localized almost exclusively to the epidermis (Fig. 5a). In the unwounded skin, normal epidermal stratification was accurately reflected with β4 integrin -positive cells overlying cells positive for Ki-67; however the majority of cells at the wound margin were double-positive. Saline and vehicle groups showed minimal cell migration beyond the proliferating cells at the wound margin. Free HB-EGF induced migration of non-proliferating (Ki-67-negative) cells, consistent with the inhibited proliferation observed in vitro. In contrast, keratinocytes in wounds treated by HB-EGF coacervate exhibited co-localization of both markers well beyond the wound margin, indicating that the migratory cells still retain their proliferative capacity. Only HB-EGF coacervate-treated wounds showed a complete layer of epithelial (β4 integrin-positive) cells across the entire wound length on day 7.
Figure 5. HB-EGF induces migration of epithelial cells with retained proliferative capability, and increases angiogenesis in wounds.
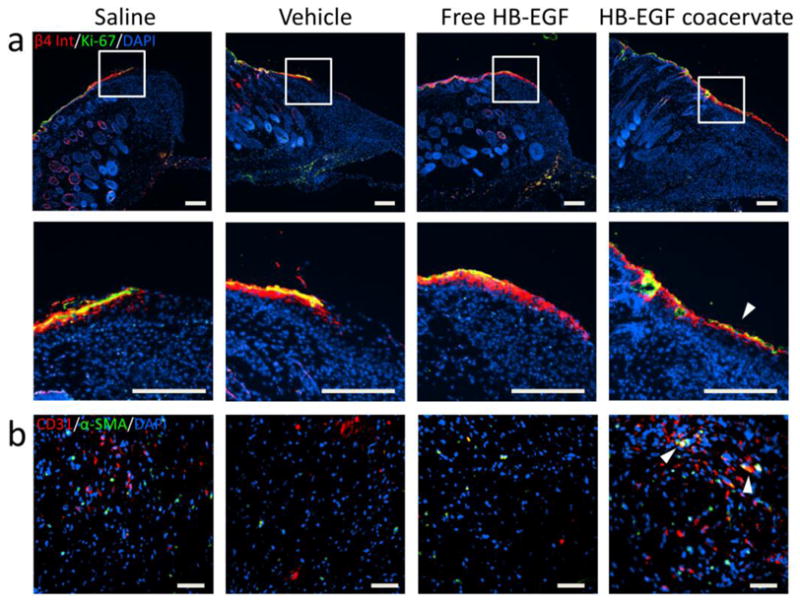
(a) Immunofluorescent staining of day 7 wound sections for β4 integrin (red) and Ki-67 (green) with DAPI (blue) nuclear staining. High magnification images of the wound margin reveal epithelial cells with retained proliferation potential (co-localization of markers; red + green = yellow). Arrow indicates significant migration of these dual-positive cells. Bars = 200 μm. (b) Immunofluorescent staining of day 17 wound sections for CD31 (red) and α-smooth muscle actin (green) with DAPI (blue) nuclear staining. Representative images of granulation tissue near the wound margin show endothelial cell and mural cell infiltration from the adjacent dermis. Arrows indicate co-localization of cells (red + green = yellow) as potential nascent vessels. Bars = 50 μm.
Angiogenesis accompanies faster healing
The vascularization of day 17 wounds was examined with immunofluorescent staining for endothelial cells (CD31+) and mural cells (α-smooth muscle actin+). At the base of the granulation region near the wound margins, endothelial cells were observed in the HB-EGF coacervate-treated wounds (Fig. 5b). Additionally, mural cells were observed co-localizing with endothelial cells in the HB-EGF coacervate group, indicating early blood vessel formation not present in other groups.
DISCUSSION
Many delivery systems have been developed to provide controlled spatio-temporal release of EGF, including sponges [25], hydrogels [26], polymer pellets [27], electrospun nanofibers [28], and microspheres [29]. However, clinical translation of these systems has been deterred by setbacks such as poor loading efficiency, large initial burst release, and low bioactivity of the released growth factors [30]. It is likely that these challenges can be overcome by examining the integral role the ECM plays in sequestering, protecting, coordinating, and enhancing growth factor activity. Thus, a particularly effective approach to design biomimetic growth factor delivery systems is to incorporate ECM components such as hyaluronic acid [31], collagen [32], and vitronectin [33]. These systems utilize controlled release while also mimicking the natural extracellular environment of regenerating tissue [34].
Of similar structure to ECM-derived heparan sulfate proteoglycans, heparin is also used in many delivery systems [35]. In addition to protecting growth factors from proteolytic degradation, heparin potentiates the activity of some factors by facilitating their interaction with cell receptors [36, 37]. Delivering growth factors pre-bound to heparin also prevents their rapid sequestration by the endogenous ECM. This may be especially important in a cutaneous wound setting where growth factors are required at the wound margin to stimulate re-epithelialization and are less useful if immobilized elsewhere in the wound [38]. In support of this notion is the finding that adding heparanase, which cleaves heparan sulfate thereby liberating bound growth factors, enhances wound healing and angiogenesis [39].
Our delivery approach is one of a handful of systems that utilize intact, native heparin, thereby fully retaining its natural functionality. Others have incorporated intact heparin by conjugation to fibrin matrices via bi-domain peptides [40], to peptide amphiphiles [41], or within dendrimer-crosslinked collagen gels [42]. Our delivery system employs a polycation that is a simplistic mimetic of the heparin-binding domain of the fibroblast growth factor receptor [43]. This bio-inspired ternary complex is stable, held together by polyvalent ionic interactions. Thus heparin imparts significant bioactivity to our delivery system, which PEAD keeps localized to the application site in a simple, unobtrusive way. This work represents, to the best of our knowledge, the first use of a bio-active controlled release system to deliver HB-EGF in a pre-clinical animal model.
HB-EGF release from the coacervate, governed by degradation of PEAD and dissociation of the delivery complex, was slow and sustained over at least 10 days in vitro with a steady release rate of 15 ng per day. We expect that release is accelerated in vivo, driven by additional enzymatic activity in the wound bed. Degradation of the coacervate in vivo is catalyzed by esterases and heparinases, however we do not observe any toxic effects of PEAD or its degradation products, locally or systemically [18]. Indeed, the vehicle did not inhibit healing in the present study, showing no difference from the control. The viscous liquid-like properties of a coacervate, not too different from wound fluid, also make it highly attractive for use in a wound healing therapeutic. It is prepared in water and then simply applied topically in this work. The coacervate can also be injected [17] or could be incorporated into bandages.
The effect of HB-EGF on epithelial cell proliferation and migration was examined individually using primary human keratinocytes. HB-EGF significantly increased keratinocyte migratory capacity but not proliferation, in accordance with previous reports [8]. HB-EGF coacervate consistently produced results similar to that of the free growth factor group; however, our release study shows that only 12% and 15% HB-EGF was released by the end of the 1 day migration study and 4 day proliferation study, respectively. This indicates an enhanced bioactivity of HB-EGF released from the coacervate. This is consistent with potentiated activity of fibroblast growth factor (FGF) released from this delivery system [16]. Though not statistically significant, a slight advantage of free growth factor over coacervate was evident in the scratch migration assay, particularly during the first 12 hours. Interestingly, the difference in means between these two groups gradually decreased over the course of the experiment, reflecting the increasing concentration of HB-EGF in the medium in the coacervate group. At high concentrations, free HB-EGF inhibited cell proliferation, as previously reported [7]. However, using the coacervate to maintain a low, stable concentration of free HB-EGF avoided this inhibition.
Splinted excisional wound models in rodents force healing to occur by re-epithelialization as in humans, rather than by contraction as in most loose-skinned mammals [44]. Thus they provide an ideal first validation tool for the potential of HB-EGF coacervate to accelerate wound closure in vivo. We found that controlled delivery is essential to effective HB-EGF therapy, as free growth factor healed wounds at the same rate as controls at a dosage of 1 μg. HB-EGF coacervate significantly accelerated wound closure over 17 days by comprehensive healing which included expedited re-epithelialization, improved granulation tissue formation, and angiogenesis.
Endogenous HB-EGF expression is normally low but is upregulated in cutaneous wounds [7]. It is produced by multiple cells and in the setting of dermal injury acts primarily as a potent stimulator of re-epithelialization [8]. The presence of exogenous HB-EGF delivered by the coacervate accelerated re-epithelialization, as evident by a complete keratinized layer with regained barrier function 7 days after administration. We also observed keratinocytes well beyond the wound margin which stained positive for β4 integrin co-localized with Ki-67 protein, expressed exclusively in nuclei of cells in active cell cycle. In agreement with our in vitro results, these migrating keratinocytes continued to proliferate as long as the soluble HB-EGF concentration was maintained below threshold levels by the coacervate. Conversely, free HB-EGF stimulated cell migration beyond that of the controls, however proliferation was restricted to near the wound margin and ultimately led to no difference in wound closure rate.
In the first week of acute wound healing, dermal fibroblasts enter the wound bed, proliferate, and gradually replace the provisional fibrin- and fibronectin-rich matrix with a collagenous one, also containing proteoglycans. This collagen matrix, primarily type I and III, serves as inductive scaffolding for infiltrating stromal cells and provides strength and structure to the new tissue as remodeling occurs [45, 46]. HB-EGF may act as a fibroblast mitogen and indeed, we observed that granulation tissue in HB-EGF coacervate-treated wounds had high collagen content 7 days after injury. This likely resulted from both direct effects on dermal fibroblasts and indirect effects such as activation of paracrine signaling by epithelial cells [47]. The presence of dermal appendages within the granulation region at day 17 further indicates that HB-EGF coacervate-treated wounds were at a later stage of healing compared to other groups.
Angiogenesis also plays a critical role in the wound healing process. Endothelial sprouts derived from adjacent tissue invade the provisional wound matrix and support the formation of granulation tissue [46]. HB-EGF may have direct mitogenic and motogenic effects on endothelial cells [11] and vascular smooth muscle cells [10], or may influence the angiogenic process by initiating paracrine signaling by keratinocytes and fibroblasts in the wound bed. HB-EGF may also be involved through the stimulation of matrix metalloproteinases (MMPs), which are critical in the initial stages of angiogenesis [48]. Our results indicate that controlled release of HB-EGF to the wound seemed to induce endothelial cell infiltration and signs of nascent blood vessels, not observed in other groups. The exact mechanisms by which HB-EGF affects angiogenic response require further investigation.
CONCLUSIONS
This study assesses the benefit of a controlled release approach to the utility of HB-EGF therapy for wound healing. We demonstrated that sustained coacervate-based delivery of HB-EGF significantly accelerated wound closure within 17 days, while free growth factor application had no effect on closure rate compared to controls. Keratinocyte migratory capacity was significantly increased and proliferative capacity was maintained by HB-EGF coacervate. This led to accelerated wound re-epithelialization which was accompanied by healthy granulation tissue formation and angiogenesis. These results suggest that coacervate-based controlled delivery of HB-EGF can induce fast and comprehensive healing of cutaneous wounds.
Acknowledgments
The authors would like to thank Scientific Protein Laboratories LLC for their kind donation of the clinical-grade heparin and Dr. Patricia Hebda for providing the NHEK cells. This work was supported by the National Science Foundation (NSF Award DMR-1005766).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Franz MG, Robson MC, Steed DL, Barbul A, Brem H, Cooper DM, Leaper D, Milner SM, Payne WG, Wachtel TL, Wiersema-Bryant L. Guidelines to aid healing of acute wounds by decreasing impediments of healing. Wound Repair Regen. 2008;16:723–748. doi: 10.1111/j.1524-475X.2008.00427.x. [DOI] [PubMed] [Google Scholar]
- 2.Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol. 2008;58:185–206. doi: 10.1016/j.jaad.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 3.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 4.Behm B, Babilas P, Landthaler M, Schreml S. Cytokines, chemokines and growth factors in wound healing. J Eur Acad Dermatol Venereol. 2011;26:812–820. doi: 10.1111/j.1468-3083.2011.04415.x. [DOI] [PubMed] [Google Scholar]
- 5.Goldman R. Growth factors and chronic wound healing: past, present, and future. Adv Skin Wound Care. 2004;17:24–35. doi: 10.1097/00129334-200401000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Marikovsky M, Breuing K, Liu PY, Eriksson E, Higashiyama S, Farber P, Abraham J, Klagsbrun M. Appearance of heparin-binding EGF-like growth factor in wound fluid as a response to injury. Proc Natl Acad Sci U S A. 1993;90:3889–3893. doi: 10.1073/pnas.90.9.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoll SW, Rittie L, Johnson JL, Elder JT. Heparin-Binding EGF-Like Growth Factor Promotes Epithelial-Mesenchymal Transition in Human Keratinocytes. J Invest Dermatol. 2012;132:2148–2157. doi: 10.1038/jid.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shirakata Y, Kimura R, Nanba D, Iwamoto R, Tokumaru S, Morimoto C, Yokota K, Nakamura M, Sayama K, Mekada E, Higashiyama S, Hashimoto K. Heparin-binding EGF-like growth factor accelerates keratinocyte migration and skin wound healing. J Cell Sci. 2005;118:2363–2370. doi: 10.1242/jcs.02346. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto K, Higashiyama S, Asada H, Hashimura E, Kobayashi T, Sudo K, Nakagawa T, Damm D, Yoshikawa K, Taniguchi N. Heparin-binding epidermal growth factor-like growth factor is an autocrine growth factor for human keratinocytes. J Biol Chem. 1994;269:20060–20066. [PubMed] [Google Scholar]
- 10.Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251:936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- 11.Mehta VB, Zhou Y, Radulescu A, Besner GE. HB-EGF stimulates eNOS expression and nitric oxide production and promotes eNOS dependent angiogenesis. Growth Factors. 2008;26:301–315. doi: 10.1080/08977190802393596. [DOI] [PubMed] [Google Scholar]
- 12.Abramovitch R, Neeman M, Reich R, Stein I, Keshet E, Abraham J, Solomon A, Marikovsky M. Intercellular communication between vascular smooth muscle and endothelial cells mediated by heparin-binding epidermal growth factor-like growth factor and vascular endothelial growth factor. FEBS Lett. 1998;425:441–447. doi: 10.1016/s0014-5793(98)00283-x. [DOI] [PubMed] [Google Scholar]
- 13.Chen RR, Mooney DJ. Polymeric growth factor delivery strategies for tissue engineering. Pharm Res. 2003;20:1103–1112. doi: 10.1023/a:1025034925152. [DOI] [PubMed] [Google Scholar]
- 14.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 15.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu H, Johnson NR, Mason NS, Wang Y. A [polycation:heparin] complex releases growth factors with enhanced bioactivity. J Control Release. 2011;150:157–163. doi: 10.1016/j.jconrel.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 17.Chu H, Gao J, Chen CW, Huard J, Wang Y. Injectable fibroblast growth factor-2 coacervate for persistent angiogenesis. Proc Natl Acad Sci U S A. 2011;108:13444–13449. doi: 10.1073/pnas.1110121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu H, Gao J, Wang Y. Design, synthesis, and biocompatibility of an arginine-based polyester. Biotechnol Prog. 2012;28:257–264. doi: 10.1002/btpr.728. [DOI] [PubMed] [Google Scholar]
- 19.Chu H, Johnson NR, Mason NS, Wang Y. A [polycation:heparin] complex releases growth factors with enhanced bioactivity. J Control Release. 2011;150:157–163. doi: 10.1016/j.jconrel.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 20.Cribbs RK, Harding PA, Luquette MH, Besner GE. Endogenous production of heparin-binding EGF-like growth factor during murine partial-thickness burn wound healing. J Burn Care Rehabil. 2002;23:116–125. doi: 10.1097/00004630-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Sehgal BU, DeBiase PJ, Matzno S, Chew TL, Claiborne JN, Hopkinson SB, Russell A, Marinkovich MP, Jones JC. Integrin beta4 regulates migratory behavior of keratinocytes by determining laminin-332 organization. J Biol Chem. 2006;281:35487–35498. doi: 10.1074/jbc.M606317200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikolopoulos SN, Blaikie P, Yoshioka T, Guo W, Puri C, Tacchetti C, Giancotti FG. Targeted deletion of the integrin beta4 signaling domain suppresses laminin-5-dependent nuclear entry of mitogen-activated protein kinases and NF-kappaB, causing defects in epidermal growth and migration. Mol Cell Biol. 2005;25:6090–6102. doi: 10.1128/MCB.25.14.6090-6102.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell AJ, Fincher EF, Millman L, Smith R, Vela V, Waterman EA, Dey CN, Guide S, Weaver VM, Marinkovich MP. Alpha 6 beta 4 integrin regulates keratinocyte chemotaxis through differential GTPase activation and antagonism of alpha 3 beta 1 integrin. J Cell Sci. 2003;116:3543–3556. doi: 10.1242/jcs.00663. [DOI] [PubMed] [Google Scholar]
- 25.Buckley A, Davidson JM, Kamerath CD, Wolt TB, Woodward SC. Sustained release of epidermal growth factor accelerates wound repair. Proc Natl Acad Sci U S A. 1985;82:7340–7344. doi: 10.1073/pnas.82.21.7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Draye JP, Delaey B, Van de Voorde A, Van Den Bulcke A, Bogdanov B, Schacht E. In vitro release characteristics of bioactive molecules from dextran dialdehyde cross-linked gelatin hydrogel films. Biomaterials. 1998;19:99–107. doi: 10.1016/s0142-9612(97)00164-6. [DOI] [PubMed] [Google Scholar]
- 27.Cribbs RK, Luquette MH, Besner GE. Acceleration of partial-thickness burn wound healing with topical application of heparin-binding EGF-like growth factor (HB-EGF) J Burn Care Rehabil. 1998;19:95–101. doi: 10.1097/00004630-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Choi JS, Leong KW, Yoo HS. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF) Biomaterials. 2008;29:587–596. doi: 10.1016/j.biomaterials.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Ulubayram K, Nur Cakar A, Korkusuz P, Ertan C, Hasirci N. EGF containing gelatin-based wound dressings. Biomaterials. 2001;22:1345–1356. doi: 10.1016/s0142-9612(00)00287-8. [DOI] [PubMed] [Google Scholar]
- 30.Hardwicke J, Schmaljohann D, Boyce D, Thomas D. Epidermal growth factor therapy and wound healing--past, present and future perspectives. Surgeon. 2008;6:172–177. doi: 10.1016/s1479-666x(08)80114-x. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto Y, Kuroyanagi Y. Development of a wound dressing composed of hyaluronic acid sponge containing arginine and epidermal growth factor. J Biomater Sci Polym Ed. 2010;21:715–726. doi: 10.1163/156856209X435844. [DOI] [PubMed] [Google Scholar]
- 32.Yang CH. Evaluation of the release rate of bioactive recombinant human epidermal growth factor from crosslinking collagen sponges. J Mater Sci-Mater M. 2008;19:1433–1440. doi: 10.1007/s10856-007-3249-5. [DOI] [PubMed] [Google Scholar]
- 33.Xie Y, Upton Z, Richards S, Rizzi SC, Leavesley DI. Hyaluronic acid: Evaluation as a potential delivery vehicle for vitronectin:growth factor complexes in wound healing applications. J Control Release. 2011;153:225–232. doi: 10.1016/j.jconrel.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 34.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 35.Koria P. Delivery of growth factors for tissue regeneration and wound healing. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2012;26:163–175. doi: 10.2165/11631850-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 36.Mohammadi M, Olsen SK, Goetz R. A protein canyon in the FGF-FGF receptor dimer selects from an a la carte menu of heparan sulfate motifs. Curr Opin Struct Biol. 2005;15:506–516. doi: 10.1016/j.sbi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 38.Falanga V, Eaglstein WH. The “trap” hypothesis of venous ulceration. Lancet. 1993;341:1006–1008. doi: 10.1016/0140-6736(93)91085-z. [DOI] [PubMed] [Google Scholar]
- 39.Zcharia E, Zilka R, Yaar A, Yacoby-Zeevi O, Zetser A, Metzger S, Sarid R, Naggi A, Casu B, Ilan N, Vlodavsky I, Abramovitch R. Heparanase accelerates wound angiogenesis and wound healing in mouse and rat models. Faseb J. 2005;19:211–221. doi: 10.1096/fj.04-1970com. [DOI] [PubMed] [Google Scholar]
- 40.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000;65:389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 41.Rajangam K, Behanna HA, Hui MJ, Han X, Hulvat JF, Lomasney JW, Stupp SI. Heparin binding nanostructures to promote growth of blood vessels. Nano Lett. 2006;6:2086–2090. doi: 10.1021/nl0613555. [DOI] [PubMed] [Google Scholar]
- 42.Princz MA, Sheardown H. Heparin-modified dendrimer crosslinked collagen matrices for the delivery of heparin-binding epidermal growth factor. J BIOMED MATER RES-A. 2012;100:1929–1937. doi: 10.1002/jbm.a.34128. [DOI] [PubMed] [Google Scholar]
- 43.Zern BJ, Chu H, Wang Y. Control growth factor release using a self-assembled [polycation:heparin] complex. PLoS One. 2010;5:e11017. doi: 10.1371/journal.pone.0011017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galiano RD, Michaels Jt, Dobryansky M, Levine JP, Gurtner GC. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004;12:485–492. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- 45.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 46.Monaco JL, Lawrence WT. Acute wound healing an overview. Clin Plast Surg. 2003;30:1–12. doi: 10.1016/s0094-1298(02)00070-6. [DOI] [PubMed] [Google Scholar]
- 47.Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127:998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- 48.Ongusaha PP, Kwak JC, Zwible AJ, Macip S, Higashiyama S, Taniguchi N, Fang L, Lee SW. HB-EGF is a potent inducer of tumor growth and angiogenesis. Cancer Res. 2004;64:5283–5290. doi: 10.1158/0008-5472.CAN-04-0925. [DOI] [PubMed] [Google Scholar]