Abstract
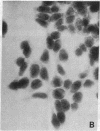
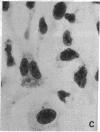
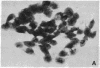
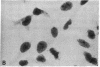
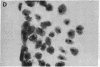
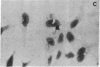
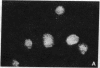
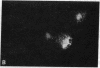
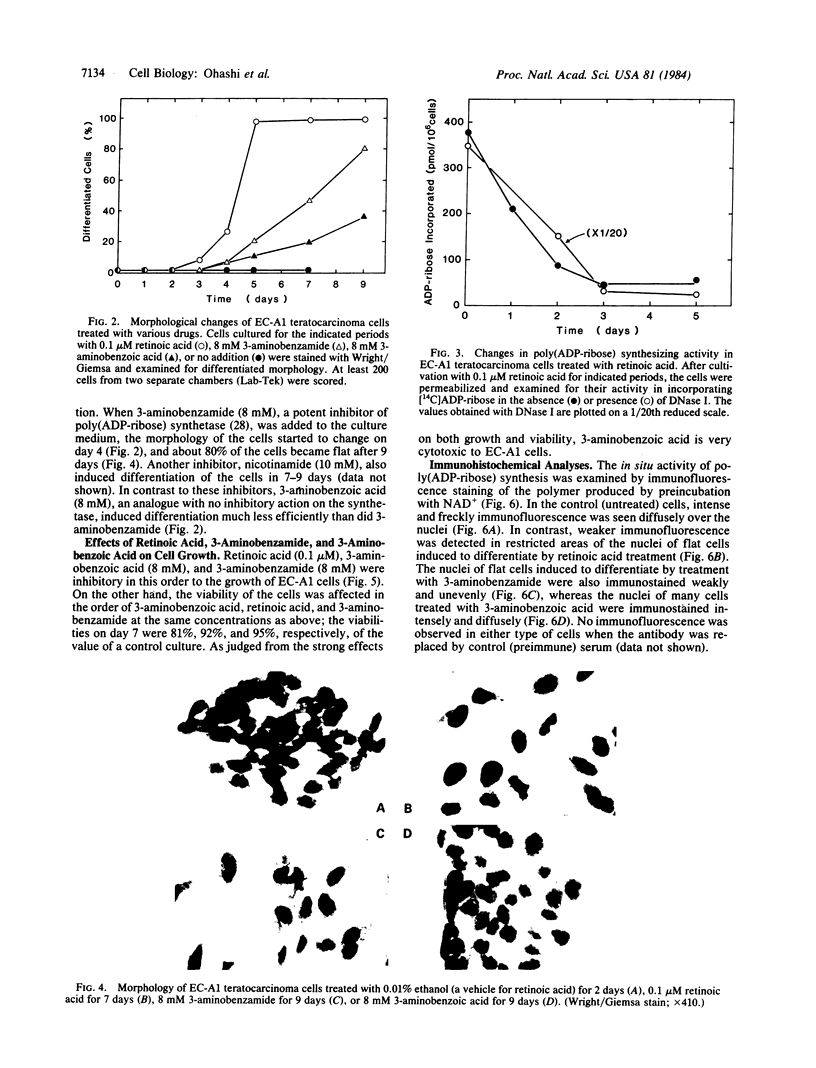
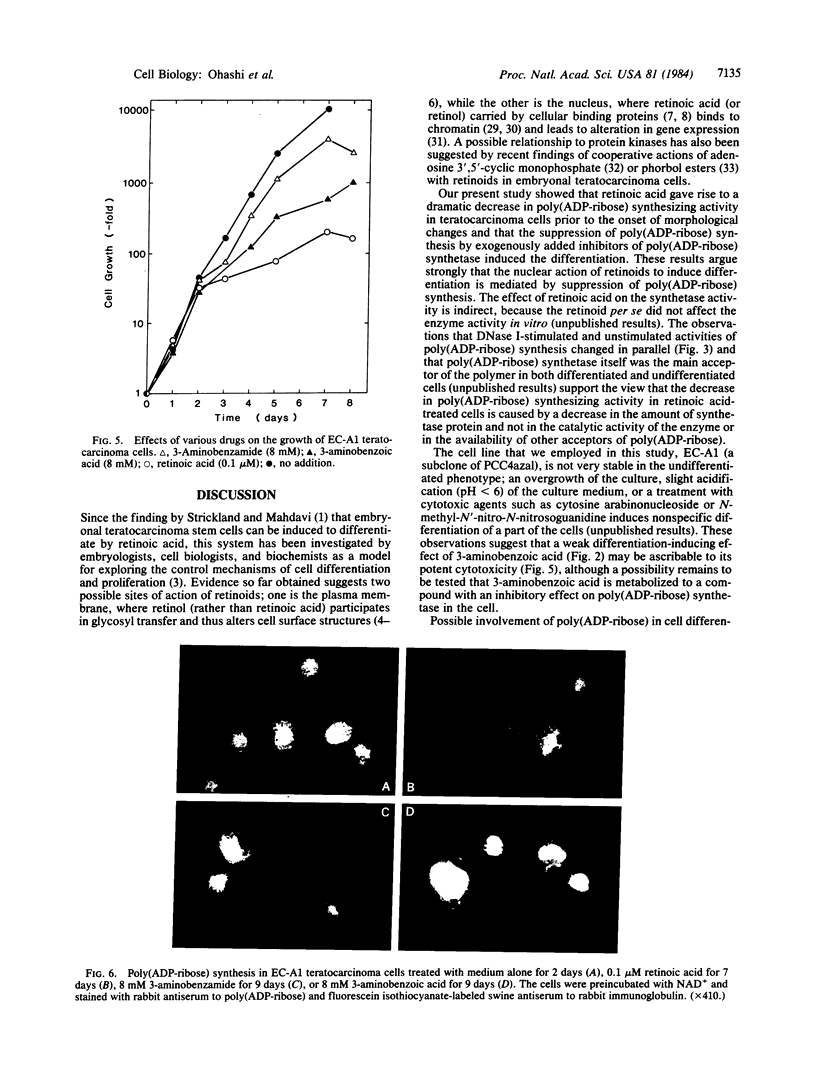
Poly(ADP-ribose) synthesizing activity in mouse teratocarcinoma EC-A1 cells decreased markedly during differentiation induced by retinoic acid; the activities assayed in permeabilized cells decreased to 25% and 10% of the activity of control (uninduced cells) 2 and 3 days, respectively, after the addition of 0.1 microM retinoic acid to the culture medium. This change preceded changes in morphology and DNA synthesis, which became prominent after 4 days. The decrease in poly(ADP-ribose) synthesizing activity appeared to be caused by a diminution of the synthetase protein and not by a decrease in its catalytic activity, because the full activity disclosed by DNase I treatment decreased in parallel, albeit at about 20 times higher levels. When 8 mM 3-aminobenzamide or 10 mM nicotinamide, specific inhibitors of poly(ADP-ribose) synthetase, was added to the culture medium, the cells underwent differentiation after 7-9 days. An analogue, 3-aminobenzoic acid, which is not inhibitory to the synthetase, induced differentiation much less efficiently than did 3-aminobenzamide, and the effect of 3-aminobenzoic acid appeared to be ascribable to its potent cytotoxicity. Immunohistochemical analysis using anti-poly(ADP-ribose) antibody confirmed the marked reduction in poly(ADP-ribose) synthesizing activity in nuclei of the cells treated with retinoic acid or 3-aminobenzamide but not with 3-aminobenzoic acid. These results suggest that a decrease in poly(ADP-ribose) synthesis triggers differentiation of teratocarcinoma cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger N. A., Weber G., Kaichi A. S. Characterization and comparison of poly(adenosine dephosphoribose) synthesis and DNA synthesis in nucleotide-permeable cells. Biochim Biophys Acta. 1978 Jun 22;519(1):87–104. doi: 10.1016/0005-2787(78)90064-3. [DOI] [PubMed] [Google Scholar]
- Caplan A. I., Niedergang C., Okazaki H., Mandel P. Poly(ADPRibose) levels as a function of chick limb mesenchymal cell development as studied in vitro and in vivo. Dev Biol. 1979 Sep;72(1):102–109. doi: 10.1016/0012-1606(79)90101-5. [DOI] [PubMed] [Google Scholar]
- Caplan A. I., Rosenberg M. J. Interrelationship between poly (ADP-Rib) synthesis, intracellular NAD levels, and muscle or cartilage differentiation from mesodermal cells of embryonic chick limb. Proc Natl Acad Sci U S A. 1975 May;72(5):1852–1857. doi: 10.1073/pnas.72.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chytil F., Ong D. E. Cellular retinol- and retinoic acid-binding proteins in vitamin A action. Fed Proc. 1979 Oct;38(11):2510–2514. [PubMed] [Google Scholar]
- De Luca L. M. The direct involvement of vitamin A in glycosyl transfer reactions of mammalian membranes. Vitam Horm. 1977;35:1–57. doi: 10.1016/s0083-6729(08)60520-8. [DOI] [PubMed] [Google Scholar]
- Farzaneh F., Zalin R., Brill D., Shall S. DNA strand breaks and ADP-ribosyl transferase activation during cell differentiation. Nature. 1982 Nov 25;300(5890):362–366. doi: 10.1038/300362a0. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Regulation of terminal differentiation of cultured human keratinocytes by vitamin A. Cell. 1981 Sep;25(3):617–625. doi: 10.1016/0092-8674(81)90169-0. [DOI] [PubMed] [Google Scholar]
- Gautsch J. W. Embryonal carcinoma stem cells lack a function required for virus replication. Nature. 1980 May 8;285(5760):110–112. doi: 10.1038/285110a0. [DOI] [PubMed] [Google Scholar]
- Hirai K., Ueda K., Hayaishi O. Aberration of poly(adenosine diphosphate-ribose) metabolism in human colon adenomatous polyps and cancers. Cancer Res. 1983 Jul;43(7):3441–3446. [PubMed] [Google Scholar]
- Ikai K., Danno K., Imamura S., Ueda K. Immunohistochemical demonstration of poly(adenosine diphosphate-ribose) synthesis in human skin. J Dermatol. 1982 Apr;9(2):125–129. doi: 10.1111/j.1346-8138.1982.tb02613.x. [DOI] [PubMed] [Google Scholar]
- Ikai K., Ueda K., Fukushima M., Nakamura T., Hayaishi O. Poly(ADP-ribose) synthesis, a marker of granulocyte differentiation. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3682–3685. doi: 10.1073/pnas.77.6.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikai K., Ueda K., Hayaishi O. Immunohistochemical demonstration of poly(adenosine diphosphate-ribose) in nuclei of various rat tissues. J Histochem Cytochem. 1980 Jul;28(7):670–676. doi: 10.1177/28.7.6993553. [DOI] [PubMed] [Google Scholar]
- Jakob H., Boon T., Gaillard J., Nicolas J., Jacob F. Tératocarcinome de la spuris: isolement, culture et propriétés de cellules a potentialités multiples. Ann Microbiol (Paris) 1973 Oct;124(3):269–282. [PubMed] [Google Scholar]
- Jetten A. M., De Luca L. M. Induction of differentiation of embryonal carcinoma cells by retinol: possible mechanisms. Biochem Biophys Res Commun. 1983 Jul 29;114(2):593–599. doi: 10.1016/0006-291x(83)90821-5. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Jetten M. E. Possible role of retinoic acid binding protein in retinoid stimulation of embryonal carcinoma cell differentiation. Nature. 1979 Mar 8;278(5700):180–182. doi: 10.1038/278180a0. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Jetten M. E., Sherman M. I. Stimulation of differentiation of several murine embryonal carcinoma cell lines by retinoic acid. Exp Cell Res. 1979 Dec;124(2):381–391. doi: 10.1016/0014-4827(79)90213-1. [DOI] [PubMed] [Google Scholar]
- Johnstone A. P., Williams G. T. Role of DNA breaks and ADP-ribosyl transferase activity in eukaryotic differentiation demonstrated in human lymphocytes. Nature. 1982 Nov 25;300(5890):368–370. doi: 10.1038/300368a0. [DOI] [PubMed] [Google Scholar]
- Jones P., Benedict W., Strickland S., Reich E. Fibrin overlay methods for the detection of single transformed cells and colonies of transformed cells. Cell. 1975 Jul;5(3):323–329. doi: 10.1016/0092-8674(75)90108-7. [DOI] [PubMed] [Google Scholar]
- Kanai M., Miwa M., Kondo T., Tanaka Y., Nakayasu M., Sugimura T. Involvement of poly (ADP-ribose) metabolism in induction of differentiation of HL-60 promyelocytic leukemia cells. Biochem Biophys Res Commun. 1982 Mar 30;105(2):404–411. doi: 10.1016/0006-291x(82)91448-6. [DOI] [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Characterization of cytosolic calcium-activated phospholipid-dependent protein kinase activity in embryonal carcinoma cells. Effect of retinoc acid-induced differentiation of F9 cells to parietal endoderm. J Biol Chem. 1983 Aug 10;258(15):9178–9183. [PubMed] [Google Scholar]
- Liau G., Ong D. E., Chytil F. Interaction of the retinol/cellular retinol-binding protein complex with isolated nuclei and nuclear components. J Cell Biol. 1981 Oct;91(1):63–68. doi: 10.1083/jcb.91.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa M., Oda K., Segawa K., Tanaka M., Irie S. Cell density-dependent increase in chromatin-associated ADP-ribosyltransferase activity in simian virus 40-transformed cells. Arch Biochem Biophys. 1977 May;181(1):313–321. doi: 10.1016/0003-9861(77)90510-0. [DOI] [PubMed] [Google Scholar]
- Morioka K., Tanaka K., Nokuo T., Ishizawa M., Ono T. Erythroid differentiation and poly(ADP-ribose) synthesis in Friend leukemia cells. Gan. 1979 Feb;70(1):37–46. [PubMed] [Google Scholar]
- Nakao Y., Matsuda S., Kimoto H., Matsui T., Kobayashi N., Kishihara M., Fujita T., Watanabe S., Ueda K., Ito Y. Paradoxical anti-leukemic effects of plant-derived tumor promoters on a human thymic lymphoblast cell line. Int J Cancer. 1982 Dec 15;30(6):687–695. doi: 10.1002/ijc.2910300603. [DOI] [PubMed] [Google Scholar]
- Nishio A., Nakanishi S., Doull J., Uyeki E. M. Enhanced chondrocytic differentiation in chick limb bud cell cultures by inhibitors of poly(ADP-ribose) synthetase. Biochem Biophys Res Commun. 1983 Mar 16;111(2):750–759. doi: 10.1016/0006-291x(83)90368-6. [DOI] [PubMed] [Google Scholar]
- Niwa O., Yokota Y., Ishida H., Sugahara T. Independent mechanisms involved in suppression of the Moloney leukemia virus genome during differentiation of murine teratocarcinoma cells. Cell. 1983 Apr;32(4):1105–1113. doi: 10.1016/0092-8674(83)90294-5. [DOI] [PubMed] [Google Scholar]
- Nolan N. L., Butt T. R., Wong M., Lambrianidou A., Smulson M. E. Characterization of poly(ADP-ribose)--histone H1 complex formation in purified polynucleosomes and chromatin. Eur J Biochem. 1980 Dec;113(1):15–25. doi: 10.1111/j.1432-1033.1980.tb06133.x. [DOI] [PubMed] [Google Scholar]
- Ohashi Y., Ueda K., Kawaichi M., Hayaishi O. Activation of DNA ligase by poly(ADP-ribose) in chromatin. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3604–3607. doi: 10.1073/pnas.80.12.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Edson C. M., Fukushima M., Ueda K., Hayaishi O. Purification and properties of poly(adenosine diphosphate ribose) synthetase. J Biol Chem. 1977 Oct 25;252(20):7000–7005. [PubMed] [Google Scholar]
- Pekala P. H., Lane M. D., Watkins P. A., Moss J. On the mechanism of preadipocyte differentiation. Masking of poly(ADP-ribose) synthetase activity during differentiation of 3T3-L1 preadipocytes. J Biol Chem. 1981 May 25;256(10):4871–4876. [PubMed] [Google Scholar]
- Poirier G. G., de Murcia G., Jongstra-Bilen J., Niedergang C., Mandel P. Poly(ADP-ribosyl)ation of polynucleosomes causes relaxation of chromatin structure. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3423–3427. doi: 10.1073/pnas.79.11.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell M. R., Whish W. J. Novel inhibitors of poly(ADP-ribose) synthetase. Biochem J. 1980 Mar 1;185(3):775–777. doi: 10.1042/bj1850775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Role of retinoids in differentiation and carcinogenesis. Cancer Res. 1983 Jul;43(7):3034–3040. [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Takase S., Ong D. E., Chytil F. Cellular retinol-binding protein allows specific interaction of retinol with the nucleus in vitro. Proc Natl Acad Sci U S A. 1979 May;76(5):2204–2208. doi: 10.1073/pnas.76.5.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada M., Fujiki H., Marks P. A., Sugimura T. Induction of erythroid differentiation of murine erythroleukemia cells by nicotinamide and related compounds. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6411–6414. doi: 10.1073/pnas.76.12.6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf G., Kiorpes T. C., Masushige S., Schreiber J. B., Smith M. J., Anderson R. S. Recent evidence for the participation of vitamin A in glycoprotein synthesis. Fed Proc. 1979 Oct;38(11):2540–2543. [PubMed] [Google Scholar]