Abstract
We previously reported that three risk factors (RF): initial remission duration <1 year, active B symptoms, and extranodal disease predict outcome in relapsed or refractory Hodgkin lymphoma (HL). Our goal was to improve event-free survival (EFS) for patients with multiple RF and to determine if response to salvage therapy impacted outcome. We conducted a phase II intent-to-treat study of tailored salvage treatment: patients with 0 or 1 RF received standard-dose ifosfamide, carboplatin, and etoposide (ICE); patients with 2 RF received augmented ICE; patients with 3 RF received high-dose ICE with stem cell support. This was followed by evaluation with both computed tomography and functional imaging (FI); those with chemosensitive disease underwent high-dose chemoradiotherapy and autologous stem cell transplantation (ASCT). There was no treatment-related mortality. Compared to historical controls this therapy eliminated the difference in EFS between the 3 prognostic groups. Pre-ASCT FI predicted outcome; 4-year EFS rates was 33% vs. 77% for patients transplanted with positive vs negative FI respectively, p=0.00004, hazard ratio 4.61. Risk-adapted augmentation of salvage treatment in patients with HL is feasible and improves EFS in poorer-risk patients. Our data suggest that normalization of FI pre-ASCT predicts outcome, and should be the goal of salvage treatment.
Keywords: Hodgkin Lymphoma, HSCT, high-dose chemoradiotherapy
INTRODUCTION
Early transplant studies in Hodgkin lymphoma (HL) included many heavily pretreated patients, which influenced the morbidity and mortality of high-dose chemoradiotherapy (HDT) programs.(Bierman, et al 1996, Linch, et al 1993, Schmitz, et al 2002) However, with the use of modern supportive care, transplant-related mortality is <3% in most series. Unfortunately, this only translates to a minimal improvement in 2- and 5-year progression-free survival rates following autologous stem cell transplantation (ASCT), and currently only 40%-50% of patients with chemosensitive relapsed or refractory disease are cured with this approach.(Moskowitz 2004)
For the past 2 decades, we have incorporated accelerated fractionation radiotherapy (RT) either as total lymphoid irradiation (TLI) or as an involved field (IF-RT) into our transplant conditioning regimen. In an initial study conducted from 1986-1993, at Memorial Sloan-Kettering Cancer Center (MSKCC), chemosensitive disease was not required in order to be eligible for ASCT, and despite this, the 10-year survival following ASCT was 45%, with no relapses occurring > 3 years following HDT (Moskowitz et al 2001; Horning et al 1997). Like others, we found a marked survival advantage for patients with chemosensitive disease, and required evidence of chemosensitivity in our subsequent protocols (Josting et al 2002).
From 1994-1998 we utilized uniform salvage therapy (ST) with ifosfamide, carboplatin, and etoposide (ICE), and offered HDT/ASCT only to patients with chemosensitive disease. As analyzed by intent-to-treat, the 5-year event-free survival (EFS) was 55%. Three pre-ST risk factors (RF) predicted for a poorer outcome: extranodal sites of disease (ENS) (P <0.001), initial response duration <1 year (P=0.001), and B symptoms (P<0.001); 5-year EFS rates were 76%, 35%, and 8% for patients with 0-1, 2, and 3 factors, respectively.(Moskowitz, et al 2001) Other investigators have confirmed that these 3 RF have an important prognostic value in the setting of relapsed/refractory HL.(Horning, et al 1997, Josting, et al 2002, Reece, et al 1994)
We utilized a prognostic index based on these 3 RF to develop a risk-adapted, intent-to-treat clinical trial for patients with relapsed or primary refractory HL. This report describes the long-term results of our attempt to determine if further intensification of therapy can improve outcome, particularly for these poorer-risk patients.
PATIENTS AND METHODS
Eligibility Criteria
After obtaining informed consent, 105 consecutive transplant-eligible patients with relapsed or primary refractory HL were enrolled on a prospective MSKCC Institutional Review Board approved protocol, #98-071, between September 1998 and September 2003. All patients were evaluable for outcome.
Each patient's eligibility was reviewed at a multidisciplinary lymphoma staging conference. Disease was staged according to the Cotswold Modification of the Ann Arbor system,(Lister and Crowther 1990) and included a functional imaging (FI) assessment (gallium [67 patients] or 18- fluorodeoxy glucose [FDG] positron emission tomography [PET] scans [38 patients]). FDG-PET scans obtained at MSKCC or outside institutions were reviewed by MSKCC nuclear medicine physicians and presented at the weekly lymphoma staging conference. All pre-ASCT scans that were considered positive were presented alongside baseline scans to verify residual abnormal uptake at sites of previously identified disease. Baseline and interim FDG-PET scans were interpreted visually with correlation to a concurrent or simultaneous computed tomography (CT) scan of the chest, abdomen, and pelvis (when done as integrated PET/CT). Standard uptake values (SUV) were routinely recorded for MSKCC scans, and if they were provided on outside studies. A negative pre-ASCT scan was defined as absence of uptake at any site of positive disease identified in the baseline study, and lack of new functional imaging avid disease. A positive scan for the FDG-PET cohort was defined as any FDG uptake greater than local background activity, with a corresponding abnormality on CT scan.
All patients had a repeat biopsy confirming relapsed or refractory HL before enrolling in this study. We defined primary refractory disease as a patient that progresses during initial therapy or within one month of initial therapy. Both subsets were required to have a repeat biopsy confirming active HL.
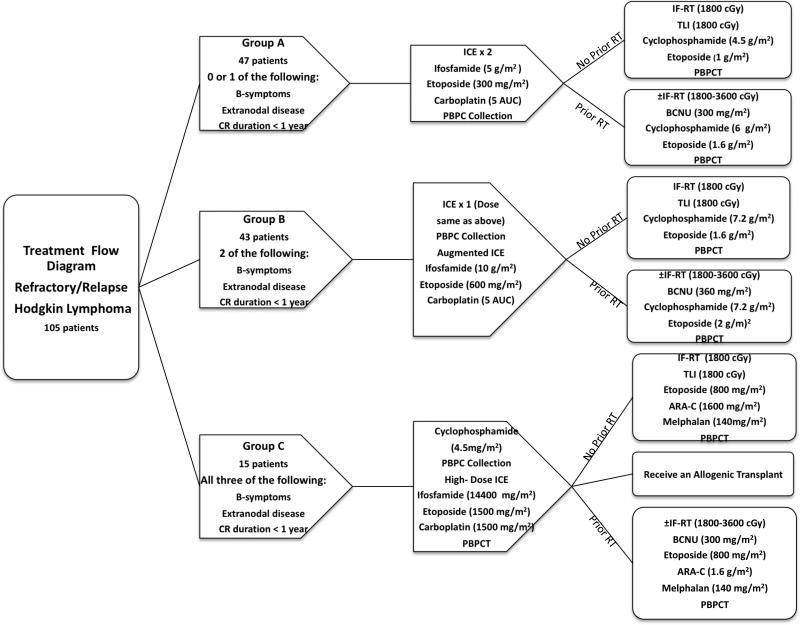
We stratified our patients into 3 risk groups (arms A, B, and C) based on the previously described pre-ST RF, and the treatment for each cohort is described in Figure 1.
Figure 1.
Treatment protocol
Restaging Evaluation
Patients had a repeat CT and gallium or FDG-PET scan after completion of ST. In order to be eligible to undergo HDT/ASCT, patients needed to have a complete, partial or minimal response, defined as follows:
Complete response (CR): No evidence of HL determined clinically, radiologically or pathologically.
Partial Response (PR): 50% or greater decrease in sum of the products of the diameters of each measurable lesion, along with the documented presence of residual disease as determined by CT scan, FI scan, repeat biopsy or a combination thereof.
Minimal Response (MR) : < 50% decrease in size of measurable lesions along with documented presence of residual disease as determined by CT scan, FI scan, repeat biopsy or a combination thereof.
A pre-ASCT FDG-PET positive scan was defined as any FDG uptake greater than local background activity; i.e. mediastinal or para-aortic blood pool. Post-ST imaging information was also used to tailor the IF-RT volume for the conditioning regimen.
Statistical Considerations
Endpoints were overall survival (OS) and EFS. An event was defined as progression of disease, secondary malignancy, or death from any cause. If progression or other cause of treatment failure, including toxicity or secondary malignancy, occurred prior to a patient's death, the earlier date was used for calculation of EFS. OS and EFS curves were generated by the Kaplan-Meier method. Four-year estimates for OS and EFS and the corresponding 95% confidence intervals (CI) are reported for all patients and by risk group. The log-rank test was used to compare OS and EFS by risk group. The stratified log-rank test was used to compare OS and EFS by chemosensitive disease, adjusting for risk group. Associations were considered significant if P was < 0.05. All P values were 2-sided. All analyses were conducted using SAS version 9.1 (SAS Institute, Carey, NC).
RESULTS
Patient Demographics
One hundred and five patients were enrolled in this study: 19 patients (18%) had no RF, 28 (27%) had 1 RF, 43 patients (41%) had 2 RF, and 15 patients (14%) had 3 RF. Patient demographics are listed in Table I. The median age was 31 (range 17-65) years. Forty-eight patients (45%) had primary refractory disease. Of the 57 patients with relapsed disease, 26 (46%) had an initial remission duration of <1 year. Thirty patients (29%) had active B symptoms, and 55 patients (52%) had extranodal disease.
Table I.
Patient/Disease Characteristics at Time of Study Enrollment
| Characteristic | Patients (n=105) | 0-1 RF (n=47) | 2 RF (n=43) | 3 RF (n=15) |
|---|---|---|---|---|
| Median age, years (range) | 31 (17-65) | 33 (22-65) | 31 (17-62) | 25 (18-45) |
| Gender, No. (%) | ||||
| Male | 53 (50) | 25 (53) | 20 (47) | 8 (53) |
| Female | 52 (50) | 22 (47) | 23 (53) | 7 (47) |
| Bulk, cm (No of patients) | ||||
| Median (range) | 4 (0-20) | 3.9 (0-15.7) | 4.6 (0-15) | 4.4 (1.3-20) |
| >5 cm, n (%) | 40 (38) | 14 (30) | 20 (57) | 6 (40) |
| >10 cm, n (%) | 13 (12) | 4 (9) | 7 (16) | 2 (13) |
| Radiotherapy, n (%) | ||||
| Previous RT | 50 (48) | 22 (47) | 20 (47) | 8 (53) |
| Relapse in RT Field | 35 (33) | 13 (28) | 17 (40) | 5 (33) |
| Previous Response | ||||
| Relapse | 57 (54) | 31 (66) | 22 (51) | 4 (27) |
| Refractory | 48 (46) | 16 (34) | 21 (49) | 11 (73) |
RF, risk factors; RT, radiotherapy
Survival Analysis for All Patients
The median follow-up of surviving patients was 7 years, with the last event occurring 44 months after study enrollment. The 4-year EFS and OS, as analyzed by intent-to-treat, was 56% (95% CI, 0.48-0.67) and 72% (95% CI, 0.64-0.81), respectively; outcomes for each risk group are depicted in Table II.
Table II.
Outcome Based Upon Risk Factor Cohort
| Patients | 0-1 RF | 2 RF | 3 RF | ||
|---|---|---|---|---|---|
| Total Patients | 105 | 47 | 43 | 15 | |
| EventFree patients, n. | 31 | 22 | 6 | ||
| Survival patients, n. | 37 | 26 | 7 | ||
| CT | Minor Response | 13 | 11 | 8 | |
| Response to Salvage Therapy | Partial Response | 14 | 12 | 1 | |
| Complete Response | 18 | 14 | 3 | ||
| Progression of Disease | 2 | 5 | 2 | ||
| FI | Positive | 11 | 21 | 9 | |
| Response to Salvage Therapy | Negative | 36 | 22 | 6 | |
RF, risk factors; RT, radiation therapy; CT, computed tomography; FI, functional imaging.
Eleven patients failed ST; 2 were transplanted despite having chemorefractory disease to ICE as both patients subsequently had disease sensitive to MOPP (mechlorethamine, vincristine, prednisone, procarbazine) chemotherapy. Although both are alive and event-free, they are failures of this intent-to-treat approach because they did not have chemosensitive disease to ICE. The remaining 9 patients all died from HL, median survival of these patients was only 6 months. Treatment-related mortality was 0%, and all deaths were secondary to progressive HL.
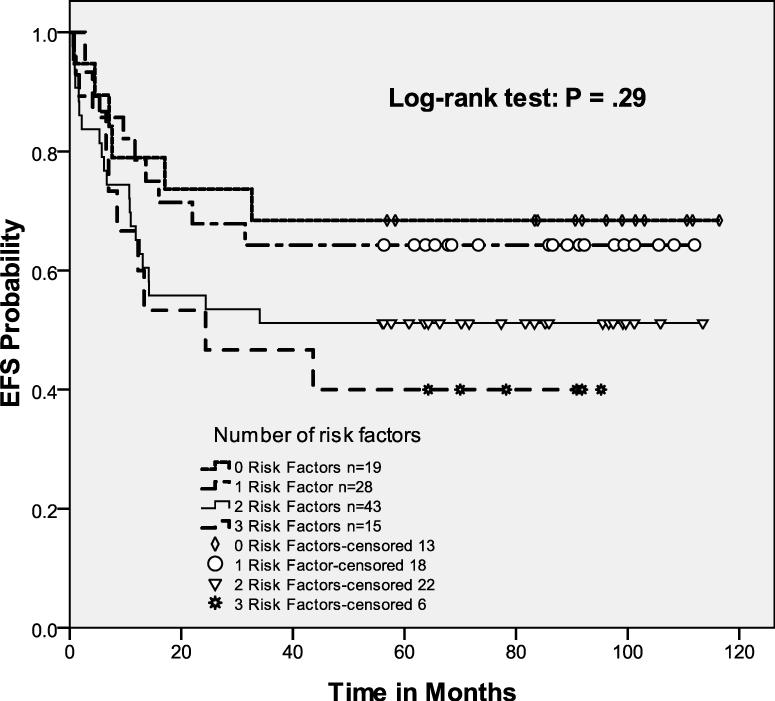
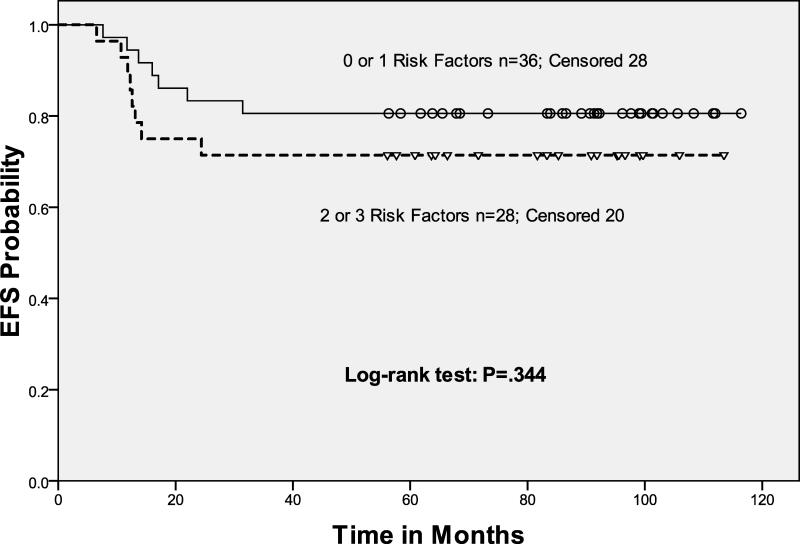
On our previous protocol, all HL patients received 2 cycles of standard-dose ICE followed by HDT/ASCT, and a marked survival difference emerged between the 3 risk groups.(Moskowitz, et al 2001) The risk-adapted approach used in this study eliminated the difference in EFS between the 3 prognostic groups, primarily by improving the outcome for the less favorable patients (Fig 2). As in our previous study, EFS or OS was equivalent for patients with 0 or 1 RF (Table II). However, the intensified approach eliminated the difference in outcome for patients with 2 versus 3 RF. Although the number of patients with 3 RF was relatively small, augmentation of therapy improved their EFS. Of the 15 patients with 3 RF, two had rapid disease progression after the high dose ICE transplant and died prior to additional therapy. Four patients were mobilization failures and could only receive one transplant (3 autologous stem cell transplants and one allotransplant). Seven patients underwent a tandem ASCT and two patients received an ASCT followed by a non-myeloablative allotransplant. Overall, our risk-adapted approach employing augmented salvage chemotherapy separated patients into 2 distinct subgroups: a good risk (0-1 RF) and a poor risk (2-3 RF) group (p=0.07).
Figure 2.
Event-free survival of intent to treat cohort
Survival Analysis for Patients with Chemosensitive Disease
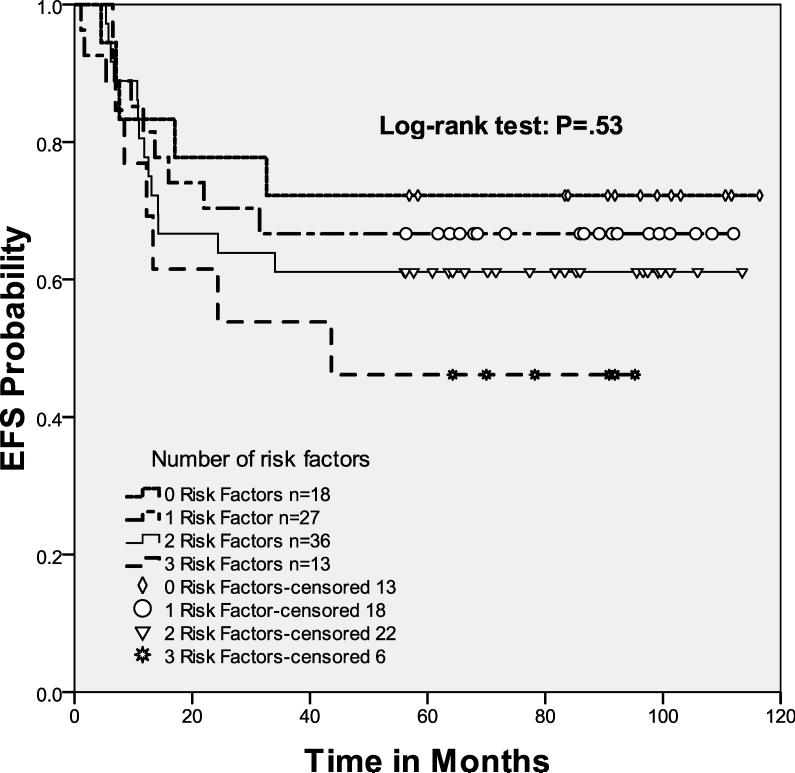
Ninety-four patients (90%) had chemosensitive disease and underwent HDT/ASCT. The 4-year EFS and OS for these patients was 63%, (95% CI, 0.53 - 0.73) and 79%, (95% CI, 0.71 - 0.87), respectively. We saw no difference in EFS and OS for these patients when comparing the 3 different prognostic treatment groups (Fig 3A). For the transplanted patients, there was also no difference in outcome when comparing the good-risk and poor-risk cohorts (p=0.27).
Figure 3.
Event-free survival of chemosensitive patients
3A. Event-free survival of transplanted cohort
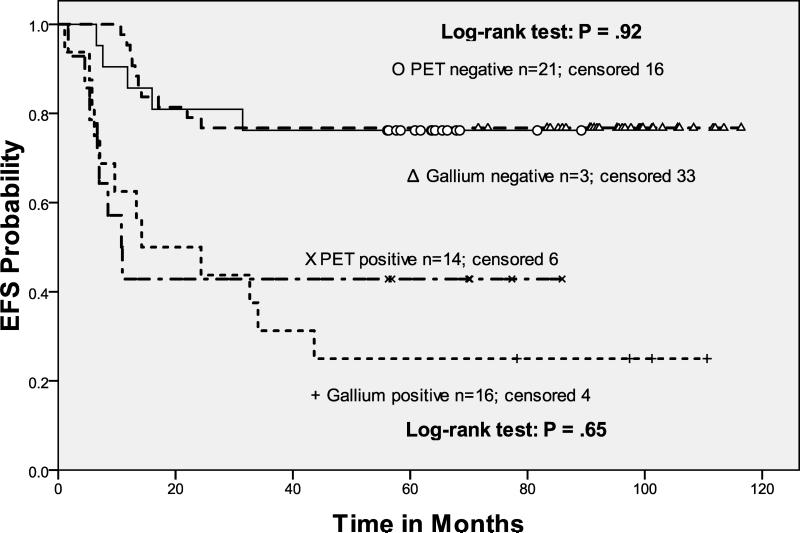
3B. Event-free survival: Pre-transplant functional imaging (Gallium vs. PET)
3C. Event-free survival of transplanted patients with negative pre-ASCT functional imaging
3D. Event-free survival of transplanted patients with positive pre-ASCT functional imaging
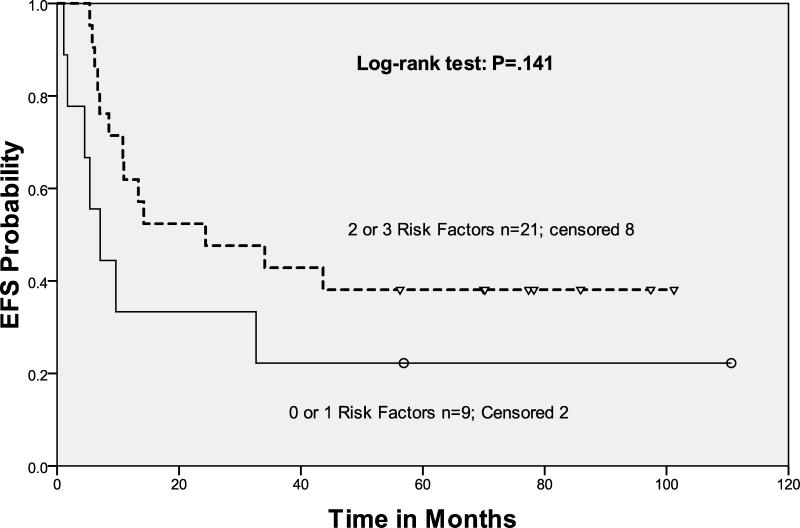
In this study, the response to salvage ICE before ASCT predicted EFS and OS (Table II). Response by gallium or FEG-PET scan were combined in our analysis of FI, because we observed no difference in outcome with respect to the type of FI employed (p=0.9) (Fig 3B). Patients with negative FI pre-ASCT after risk-adapted ICE-based therapy had a statistically significant improvement in both EFS and OS as compared to those with chemosensitive disease (on CT imaging) but persistent abnormalities on FI. In fact, patients with 0, 1, 2, or 3 risk factors all had the same outcome after ASCT provided their pre-ASCT FI test was negative. Not unexpectedly, the patients transplanted with chemosensitive but abnormal FI scans had an unfavorable outcome (Hazard Ratio 4.61), with each of the RF cohorts having similar rates of EFS and OS (Figs 3C, 3D).
Toxicity
The 100-day, post-ASCT treatment-related mortality was 0%. Two patients were transferred to the intensive care unit for septic shock during their transplant admission; both recovered and were subsequently event-free. The only significant non-hematologic toxicity was reversible pneumonitis, which was seen in 10% of transplanted patients, probably due to the radiotherapy and/or carmustine; all responded to a course of corticosteroids. Concerning potential long-term toxicity, there were two patients with coronary artery disease requiring coronary artery bypass grafting. There have been no cases of solid tumors, myelodysplasia or acute leukemia; all surviving patients have blood counts in the normal range.
DISCUSSION
As with aggressive non-Hodgkin lymphoma, it has been established that patients with chemosensitive relapsed and primary refractory HL have a much better outcome than HL transplanted patients with disease refractory to ST.(Colwill, et al 1995, Ferme, et al 2002, Proctor, et al 2003, Yuen, et al 1997) Yet, some patients with chemorefractory but radiosensitive disease may be cured using a HDT/ASCT approach. These data suggest that the quality of the response to ST may be more important than originally suspected.(Diehl, et al 1983, Hoppe 1998, Leigh, et al 1993)
Pre-ST prognostic factors are critical in determining whether a patient is likely to respond to treatment.(Moskowitz 2004, Sweetenham, et al 1999, Sweetenham, et al 1997) In order to improve the response and OS in poorer-risk groups, we developed this risk-adapted study, tailoring ST to a patient's pre-treatment prognostic factors. We showed that augmenting ST in patients with unfavorable disease improved EFS in patients with relapsed or refractory HL, and that patients receiving augmented ST could be divided into a favourable cohort (0-1 RF) and an unfavourable cohort (2-3 RF).
For the past 2 decades, response to ST was determined by CT imaging, and transplant eligibility was based upon CT-defined response.(Lister, et al 1989) However, FI provides an opportunity to redefine the criteria.(Kasamon, et al 2004, Spaepen, et al 2003, Svoboda, et al 2006) HL patients nearly always have residual masses after chemotherapy, and FDG-PET as well as gallium scanning (Hagemeister, et al 1994) is more sensitive and specific than CT in determining residual disease versus fibrosis. Today, imaging with both CT and FDG-PET is required to establish remission status for aggressive lymphoma and for HL. Specifically, a complete remission requires normalization of the FDG-PET.(Cheson, et al 2007, Juweid, et al 2007) Notably, FDGPET negativity may occur in the setting of an incomplete CT-defined response.
In a retrospective analysis of 211 HL patients treated with ASCT, the pre-transplant FI (FDG-PET or gallium scan) correlated with outcome.(Jabbour, et al 2007) FI was positive in only 6 of 110 (5%) CR/complete response unconfirmed (CRu) patients, and in 48 of 86 (56%) PR patients. The 3-year progression-free survival (PFS) was 69% for patients with negative FI versus only 23% for patients with positive FI (P <.0001). Three groups of patients emerged with a 3-year PFS of 76%, 51%, and 27%, respectively, for CR, less than CR with FI negative, and less than CR with positive FI (P <.0001).
In this prospective clinical trial, the quality of response to the patient's pre-assigned ICE-based treatment predicted for outcome following HDT/ASCT. All patients were required to have pre- and post-ST CT scans, as well as FI (initially gallium scans, but since July 2001, FDG-PET). The patients in this trial could undergo HDT/ASCT as long as they achieved at least a minor response on CT or FI after ST. In a Cox regression model, the only factor that predicted for an unfavourable outcome in the transplanted patients was a pre-transplant positive FI scan; the Hazard Ratio for EFS was 4.61. To improve the rates of response to 2nd-line chemotherapy and thus transplant eligibility, we modified the dose of ICE-ST based upon our previously reported 3-factor model (Moskowitz et al 2001). Although patients with favourable disease (0-1 RF) were more likely to respond to ST with normalization of FI, if pre-ASCT FI was still abnormal, the EFS was poor and no different from those with unfavorable disease (2-3 RF) and a persistently positive FI scan. Conversely, augmented ST normalized pre-ASCT FI in patients with unfavorable disease, leading to the same outcome as the favorable patients who normalized their pre-ASCT FI. Both of these results were unanticipated but biologically plausible, given increasing evidence in diffuse large B-cell lymphoma and multiple myeloma that the quality of the response to ST predicts outcome after ASCT.(Alegre, et al 1998, Kaufman and Lonial 2004, Kewalramani, et al 2004)
In summary, this risk-adapted strategy improved EFS in patients with multiple risk factors without an increase in morbidity or treatment-related mortality. This approach attempted to avoid over-treatment of favorable patients, including those with low-risk primary refractory disease, by maintaining treatment intensity for the good-risk group while selectively increasing treatment intensity for poor-risk (2-3 RF) patients. Yet, even with the incremental improvement achieved with this strategy, substantial post-ASCT relapse rate remains. Currently, we hypothesize the need to treat patients until the pre-ASCT FDG-PET scan is negative, and are prospectively studying this in an ongoing risk-adapted study.
Further improvement in EFS for poor-risk patients clearly requires a more novel therapeutic approach than the commonly administered, single high-dose chemotherapy-based ASCT, which excludes radiotherapy. It is possible that poorer-risk patients require additional non-cross-resistant treatment to further cytoreduce disease burden prior to HDT. Treatment possibilities include gemcitabine,(Bartlett, et al 2007) novel antibodies such as anti-CD30,(Ansell, et al 2007) and a higher tightly targeted radiation dose available with modern technologies, such as intensity-modulated radiation therapy (IMRT).(Yahalom 2005) We believe that an international collaboration is required to determine if either tandem transplants (Czyz, et al 2007, Fung, et al 2007) or the use of a non-myeloablative allotransplant(Anderlini and Champlin 2006, Peggs, et al 2007) in lieu of an autotransplant for poor-risk patients can improve outcome.
Acknowledgment
The authors wish to thank Carol Pearce, MFA, and Writer/Editor with the MSKCC Department of Medicine, for her editorial suggestions.
REFERENCES
- Alegre A, Diaz-Mediavilla J, San-Miguel J, Martinez R, Garcia Larana J, Sureda A, Lahuerta JJ, Morales D, Blade J, Caballero D, De la Rubia J, Escudero A, Diez-Martin JL, Hernandez-Navarro F, Rifon J, Odriozola J, Brunet S, De la Serna J, Besalduch J, Vidal MJ, Solano C, Leon A, Sanchez JJ, Martinez-Chamorro C, Fernandez-Ranada JM. Autologous peripheral blood stem cell transplantation for multiple myeloma: a report of 259 cases from the Spanish Registry. Spanish Registry for Transplant in MM (Grupo Espanol de Trasplante Hematopoyetico-GETH) and PETHEMA. Bone Marrow Transplant. 1998;21:133–140. doi: 10.1038/sj.bmt.1701062. [DOI] [PubMed] [Google Scholar]
- Anderlini P, Champlin RE. Reduced intensity conditioning for allogeneic stem cell transplantation in relapsed and refractory Hodgkin lymphoma: where do we stand? Biol Blood Marrow Transplant. 2006;12:599–602. doi: 10.1016/j.bbmt.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Ansell SM, Horwitz SM, Engert A, Khan KD, Lin T, Strair R, Keler T, Graziano R, Blanset D, Yellin M, Fischkoff S, Assad A, Borchmann P. Phase I/II study of an anti-CD30 monoclonal antibody (MDX-060) in Hodgkin's lymphoma and anaplastic large-cell lymphoma. J Clin Oncol. 2007;25:2764–2769. doi: 10.1200/JCO.2006.07.8972. [DOI] [PubMed] [Google Scholar]
- Bartlett NL, Niedzwiecki D, Johnson JL, Friedberg JW, Johnson KB, van Besien K, Zelenetz AD, Cheson BD, Canellos GP. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin's lymphoma: CALGB 59804. Ann Oncol. 2007;18:1071–1079. doi: 10.1093/annonc/mdm090. [DOI] [PubMed] [Google Scholar]
- Bierman PJ, Anderson JR, Freeman MB, Vose JM, Kessinger A, Bishop MR, Armitage JO. High-dose chemotherapy followed by autologous hematopoietic rescue for Hodgkin's disease patients following first relapse after chemotherapy. Ann Oncol. 1996;7:151–156. doi: 10.1093/oxfordjournals.annonc.a010542. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- Colwill R, Crump M, Couture F, Danish R, Stewart AK, Sutton DM, Scott JG, Sutcliffe SB, Brandwein JM, Keating A. Mini-BEAM as salvage therapy for relapsed or refractory Hodgkin's disease before intensive therapy and autologous bone marrow transplantation. J Clin Oncol. 1995;13:396–402. doi: 10.1200/JCO.1995.13.2.396. [DOI] [PubMed] [Google Scholar]
- Czyz J, Dziadziuszko R, Knopinska-Posluszny W, Hellmann A, Kachel L, Holowiecki J, Czyz A, Komarnicki M, Osowiecki M, Walewski J, Jurczak W, Skotnicki A. Two autologous transplants in the treatment of patients with Hodgkin's lymphoma: analysis of prognostic factors and comparison with a single procedure. Leuk Lymphoma. 2007;48:535–541. doi: 10.1080/10428190601158621. [DOI] [PubMed] [Google Scholar]
- Diehl LF, Perry DJ, Terebelo H, Baldwin PE, Hurwitz M, Kimball DB, Dorn RV. Radiation as salvage therapy for patients with Hodgkin's disease relapsing after MOPP (mechlorethamine, vincristine, prednisone, and procarbazine) chemotherapy. Cancer Treat Rep. 1983;67:827–829. [PubMed] [Google Scholar]
- Ferme C, Mounier N, Divine M, Brice P, Stamatoullas A, Reman O, Voillat L, Jaubert J, Lederlin P, Colin P, Berger F, Salles G. Intensive salvage therapy with high-dose chemotherapy for patients with advanced Hodgkin's disease in relapse or failure after initial chemotherapy: results of the Groupe d'Etudes des Lymphomes de l'Adulte H89 Trial. J Clin Oncol. 2002;20:467–475. doi: 10.1200/JCO.2002.20.2.467. [DOI] [PubMed] [Google Scholar]
- Fung HC, Stiff P, Schriber J, Toor A, Smith E, Rodriguez T, Krishnan A, Molina A, Smith D, Ivers B, Kogut N, Popplewell L, Rodriguez R, Somlo G, Forman SJ, Nademanee A. Tandem autologous stem cell transplantation for patients with primary refractory or poor risk recurrent Hodgkin lymphoma. Biol Blood Marrow Transplant. 2007;13:594–600. doi: 10.1016/j.bbmt.2007.01.072. [DOI] [PubMed] [Google Scholar]
- Hagemeister FB, Purugganan R, Podoloff DA, Hess M, Rodriguez MA, McLaughlin P, Swan F, Jr., Romaguera JE, Cabanillas F. The gallium scan predicts relapse in patients with Hodgkin's disease treated with combined modality therapy. Ann Oncol. 1994;5(Suppl 2):59–63. doi: 10.1093/annonc/5.suppl_2.s59. [DOI] [PubMed] [Google Scholar]
- Hoppe RT. Radiation therapy as a component of high-dose salvage strategies in Hodgkin's disease. Ann Oncol. 1998;9(Suppl 5):S87–90. doi: 10.1093/annonc/9.suppl_5.s87. [DOI] [PubMed] [Google Scholar]
- Horning SJ, Chao NJ, Negrin RS, Hoppe RT, Long GD, Hu WW, Wong RM, Brown BW, Blume KG. High-dose therapy and autologous hematopoietic progenitor cell transplantation for recurrent or refractory Hodgkin's disease: analysis of the Stanford University results and prognostic indices. Blood. 1997;89:801–813. [PubMed] [Google Scholar]
- Jabbour E, Hosing C, Ayers G, Nunez R, Anderlini P, Pro B, Khouri I, Younes A, Hagemeister F, Kwak L, Fayad L. Pretransplant positive positron emission tomography/gallium scans predict poor outcome in patients with recurrent/refractory Hodgkin lymphoma. Cancer. 2007;109:2481–2489. doi: 10.1002/cncr.22714. [DOI] [PubMed] [Google Scholar]
- Josting A, Franklin J, May M, Koch P, Beykirch MK, Heinz J, Rudolph C, Diehl V, Engert A. New prognostic score based on treatment outcome of patients with relapsed Hodgkin's lymphoma registered in the database of the German Hodgkin's lymphoma study group. J Clin Oncol. 2002;20:221–230. doi: 10.1200/JCO.2002.20.1.221. [DOI] [PubMed] [Google Scholar]
- Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, Wiseman GA, Kostakoglu L, Scheidhauer K, Buck A, Naumann R, Spaepen K, Hicks RJ, Weber WA, Reske SN, Schwaiger M, Schwartz LH, Zijlstra JM, Siegel BA, Cheson BD. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–578. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- Kasamon YL, Wahl RL, Swinnen LJ. FDG PET and high-dose therapy for aggressive lymphomas: toward a risk-adapted strategy. Curr Opin Oncol. 2004;16:100–105. doi: 10.1097/00001622-200403000-00003. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Lonial S. Multiple myeloma: the role of transplant and novel treatment strategies. Semin Oncol. 2004;31:99–105. doi: 10.1053/j.seminoncol.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Kewalramani T, Zelenetz AD, Nimer SD, Portlock C, Straus D, Noy A, O'Connor O, Filippa DA, Teruya-Feldstein J, Gencarelli A, Qin J, Waxman A, Yahalom J, Moskowitz CH. Rituximab and ICE as second-line therapy before autologous stem cell transplantation for relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2004;103:3684–3688. doi: 10.1182/blood-2003-11-3911. [DOI] [PubMed] [Google Scholar]
- Leigh BR, Fox KA, Mack CF, Baier M, Miller TP, Cassady JR. Radiation therapy salvage of Hodgkin's disease following chemotherapy failure. Int J Radiat Oncol Biol Phys. 1993;27:855–862. doi: 10.1016/0360-3016(93)90460-d. [DOI] [PubMed] [Google Scholar]
- Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, Chopra R, Milligan D, Hudson GV. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin's disease: results of a BNLI randomised trial. Lancet. 1993;341:1051–1054. doi: 10.1016/0140-6736(93)92411-l. [DOI] [PubMed] [Google Scholar]
- Lister TA, Crowther D. Staging for Hodgkin's disease. Semin Oncol. 1990;17:696–703. [PubMed] [Google Scholar]
- Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, Rosenberg SA, Coltman CA, Tubiana M. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol. 1989;7:1630–1636. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- Moskowitz C. An update on the management of relapsed and primary refractory Hodgkin's disease. Semin Oncol. 2004;31:54–59. doi: 10.1053/j.seminoncol.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Moskowitz CH, Nimer SD, Zelenetz AD, Trippett T, Hedrick EE, Filippa DA, Louie D, Gonzales M, Walits J, Coady-Lyons N, Qin J, Frank R, Bertino JR, Goy A, Noy A, O'Brien JP, Straus D, Portlock CS, Yahalom J. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001;97:616–623. doi: 10.1182/blood.v97.3.616. [DOI] [PubMed] [Google Scholar]
- Peggs KS, Sureda A, Qian W, Caballero D, Hunter A, Urbano-Ispizua A, Cavet J, Ribera JM, Parker A, Canales M, Mahendra P, Garcia-Conde J, Milligan D, Sanz G, Thomson K, Arranz R, Goldstone AH, Alvarez I, Linch DC, Sierra J, Mackinnon S. Reduced-intensity conditioning for allogeneic haematopoietic stem cell transplantation in relapsed and refractory Hodgkin lymphoma: impact of alemtuzumab and donor lymphocyte infusions on long-term outcomes. Br J Haematol. 2007;139:70–80. doi: 10.1111/j.1365-2141.2007.06759.x. [DOI] [PubMed] [Google Scholar]
- Proctor SJ, Jackson GH, Lennard A, Angus B, Wood K, Lucraft HL, White J, Windebank K, Taylor PR. Strategic approach to the management of Hodgkin's disease incorporating salvage therapy with high-dose ifosfamide, etoposide and epirubicin: a Northern Region Lymphoma Group study (UK). Ann Oncol. 2003;14(Suppl 1):i47–50. doi: 10.1093/annonc/mdg710. [DOI] [PubMed] [Google Scholar]
- Reece DE, Connors JM, Spinelli JJ, Barnett MJ, Fairey RN, Klingemann HG, Nantel SH, O'Reilly S, Shepherd JD, Sutherland HJ. Intensive therapy with cyclophosphamide, carmustine, etoposide +/− cisplatin, and autologous bone marrow transplantation for Hodgkin's disease in first relapse after combination chemotherapy. Blood. 1994;83:1193–1199. [PubMed] [Google Scholar]
- Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, Boissevain F, Zschaber R, Muller P, Kirchner H, Lohri A, Decker S, Koch B, Hasenclever D, Goldstone AH, Diehl V. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. 2002;359:2065–2071. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- Spaepen K, Stroobants S, Dupont P, Vandenberghe P, Maertens J, Bormans G, Thomas J, Balzarini J, De Wolf-Peeters C, Mortelmans L, Verhoef G. Prognostic value of pretransplantation positron emission tomography using fluorine 18-fluorodeoxyglucose in patients with aggressive lymphoma treated with high-dose chemotherapy and stem cell transplantation. Blood. 2003;102:53–59. doi: 10.1182/blood-2002-12-3842. [DOI] [PubMed] [Google Scholar]
- Svoboda J, Andreadis C, Elstrom R, Chong EA, Downs LH, Berkowitz A, Luger SM, Porter DL, Nasta S, Tsai D, Loren AW, Siegel DL, Glatstein E, Alavi A, Stadtmauer EA, Schuster SJ. Prognostic value of FDG-PET scan imaging in lymphoma patients undergoing autologous stem cell transplantation. Bone Marrow Transplant. 2006;38:211–216. doi: 10.1038/sj.bmt.1705416. [DOI] [PubMed] [Google Scholar]
- Sweetenham JW, Taghipour G, Milligan D, Blystad AK, Caballero D, Fassas A, Goldstone AH. High-dose therapy and autologous stem cell rescue for patients with Hodgkin's disease in first relapse after chemotherapy: results from the EBMT. Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1997;20:745–752. doi: 10.1038/sj.bmt.1700963. [DOI] [PubMed] [Google Scholar]
- Sweetenham JW, Carella AM, Taghipour G, Cunningham D, Marcus R, Della Volpe A, Linch DC, Schmitz N, Goldstone AH. High-dose therapy and autologous stem-cell transplantation for adult patients with Hodgkin's disease who do not enter remission after induction chemotherapy: results in 175 patients reported to the European Group for Blood and Marrow Transplantation. Lymphoma Working Party. J Clin Oncol. 1999;17:3101–3109. doi: 10.1200/JCO.1999.17.10.3101. [DOI] [PubMed] [Google Scholar]
- Yahalom J. Transformation in the use of radiation therapy of Hodgkin lymphoma: new concepts and indications lead to modern field design and are assisted by PET imaging and intensity modulated radiation therapy (IMRT). Eur J Haematol Suppl. 2005:90–97. doi: 10.1111/j.1600-0609.2005.00461.x. [DOI] [PubMed] [Google Scholar]
- Yuen AR, Rosenberg SA, Hoppe RT, Halpern JD, Horning SJ. Comparison between conventional salvage therapy and high-dose therapy with autografting for recurrent or refractory Hodgkin's disease. Blood. 1997;89:814–822. [PubMed] [Google Scholar]