Abstract
Strategies for bone regeneration are undergoing a paradigm shift, moving away from the replication of end-stage bone tissue and instead aiming to recapture the initial events of fracture repair. Although this is known to resemble endochondral bone formation, chondrogenic cell types with favorable proliferative and hypertrophic differentiation properties are lacking. Recent advances in cellular reprogramming have allowed the creation of alternative cell populations with specific properties through the forced expression of transcription factors. Herein, we investigated the in vitro hypertrophic differentiation and in vivo tissue formation capacity of induced chondrogenic cells (iChon cells) obtained through direct reprogramming. In vitro hypertrophic differentiation was detected in iChon cells that contained a doxycycline-inducible expression system for Klf4, cMyc, and Sox9. Furthermore, endochondral bone formation was detected after implantation in nude mice. The bone tissue was derived entirely from host origin, whereas cartilage tissue contained cells from both host and donor. The results obtained highlight the promise of cellular reprogramming for the creation of functional skeletal cells that can be used for novel bone healing strategies.
Introduction
The regenerative capacity of bone is impaired when a fracture exceeds a critical size. This deficiency has triggered the development of novel strategies to improve bone healing. Currently, stem cell–based approaches are being investigated for their regenerative potential in bone tissue engineering. However, progress in the field is being hampered by the low bone tissue formation capacities in vivo from available cell populations (Roberts et al., 2011). The lack of adequate bone tissue formation has been attributed to several factors, including the failure of recapitulating native tissue formation processes (Lenas et al., 2009a; Lenas et al., 2009b). Skeletal development and repair is often preceded by cartilage formation and subsequent hypertrophic differentiation, a process known as endochondral ossification (EO) (Shapiro, 2008). The applicability of mimicking this process is currently being investigated for bone healing and repair (Scotti et al., 2013). Our research group has previously demonstrated that hypertrophic differentiation of ATDC5, a clonal murine chondrogenic cell line, allows EO in vivo (Weiss et al., 2012). Despite this, ATDC5s are derived from murine teratocarcinomas, hence an equivalent human cell population does not exist, thus limiting any clinical translation.
Recent advances in cellular reprogramming have allowed the creation of alternative cell types through the forced expression of transcription factors (TFs) that define the target cell fate. Indeed, Takahashi et al. were the first to report that through the use of a combination of TFs, including Oct4, Sox2, cMyc, and Klf4, the cell state could be reprogrammed from a mature somatic cell (fibroblast) to a pluripotent state similar to that of the embryonic stem cell (ESC), although termed induced pluripotent stem cells (iPSCs) (Takahashi et al., 2007; Takahashi and Yamanaka 2006). This technology has been exploited further for direct reprogramming of fibroblasts to other functional adult somatic cells. Indeed, it has previously been shown that direct reprogramming of fibroblasts to functional neurons using combinations of TFs is possible (Vierbuchen et al., 2010). Within this study, a combination of three TFs, namely Ascl1, Brn2 (also called Pou3f2), and Myt1l rapidly and efficiently convert embryonic and postnatal fibroblasts into functional neurons in vitro. Additionally, recent reports describe direct reprogramming of fibroblasts to functional mesoderm-derived cells, such as cardiomyocytes (Ieda et al., 2010) and cartilage-forming cells (Hiramatsu et al., 2011; Ishii et al., 2012) by incorporating various TFs, which regulate cell fate.
All of these reports provide us with proof of principle that the state of a cell is not irreversible. On the contrary, a cell can be reprogrammed to the desired phenotype through the forced expression of the correct TFs. This reprogramming strategy is currently being investigated to overcome technical difficulties in differentiation protocols for pluripotent stem cells, which often exhibit low efficiency. The removal of contaminating residual pluripotent stem cells or partially differentiated cell populations in these protocols is necessary for clinical applications because these contaminating cells are able to form teratomas in vivo. The use of targeted reprogramming may act as alternative strategy for obtaining the desired cell type and thus overcome this issue. Additionally, direct reprogrammed cells may serve as an alternative to adult stem cells, including mesenchymal stem cells (MSCs). Adult stem cell populations are often contaminated with different cell types, such as fibroblasts and cells at different stages of differentiation. This heterogeneity results in nonsynchronized cell behavior and potentially disrupts tissue formation. Moreover, adult stem cells often exhibit a decrease in cell proliferation and differentiation potential upon culturing (Banfi et al., 2000).
In this study, we aimed to develop novel endochondral bone formation strategies using chondrocytes, which are obtained through direct reprogramming. Two induced chondrogenic cell populations (iChon cells) were investigated for their in vitro hypertrophic differentiation and in vivo tissue formation capacity. iChon cells were obtained by transducing postnatal mouse dermal fibroblasts with either constitutive (iChonCon) or doxycycline-inducible (iChonInd) human Klf4, cMyc, and Sox9. Both cell types undergo chondrogenic differentiation in vitro, as detected by an increase in collagen type II expression and glycosaminoglycan (GAG) deposition; however, hypertrophic differentiation (upregulation of collagen type X) was only detected in iChonInd. To investigate whether these in vitro findings could be translated to the in vivo setting, both cell types were seeded onto orthopedic bone void filler (CopiOs™) and subsequently assessed in an ectopic nude mice model. Cartilage tissue formation was only detected in implants that were seeded with iChonCon cells; however, no bone was detected. Interestingly, predifferentiation of iChonInd prior to ectopic implantation resulted in formation of hypertrophic-like cartilage islands, surrounded by bone, indicating an endochondral process. Our results emphasize the promise of cellular reprogramming for the creation of functional skeletal cell types that are capable of in vivo tissue formation. Indeed, iChonInd cells are able to trigger EO in vivo and thus may have applications in bone regeneration strategies.
Materials and Methods
Cell culture
iChon cells were created as previously described (Hiramatsu et al., 2011). Briefly, dermal fibroblasts were isolated from Col11a2-βgeo (to produce iChonCon) and Col11a2–puromycin (to produce iChonInd) mice and were transduced with viruses carrying constitutive (retrovirus; pMXs) or doxycycline-inducible (lentivirus; pLe6Δ-Ptight) human Sox9, Klf4, and cMyc, respectively. Subsequently, clonogenic cell populations were obtained using either G418 (pMXs) or puromycin (pLe6Δ-Ptight) antibiotic selection and cloning rings. The cells carrying the constitutive transgenes (iChonCon) were transduced with pMXs-green fluorescent protein (GFP), hence were GFP positive, whereas the iChonInd cells were chondrogenic and GFP positive in the presence of doxycycline.
iChon cells were expanded in growth medium consisting of Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% sodium pyruvate, 1% antibiotics, 500 μg/mL G418, or 1 μg/mL puromycin. iChonInd also received 1 μg/mL doxycycline to induce transgene expression. ATDC5s and dermal fibroblasts (DF) were cultured as previously described (Hiramatsu et al., 2011; Weiss et al., 2012), as a positive and negative control, respectively. Cells were grown in a humidified incubator at 37°C and 5% CO2. All components, with the exception of doxycycline (Sigma), were purchased from Invitrogen.
Chondrogenic differentiation
Cells were chondrogenically differentiated in micromasses. Briefly, cells were trypsinized and resuspended at a concentration of 2×107 cells/mL. Droplets of 10 μL (2×105 cells) were then added to the center of each well of a 24-well plate and incubated for 2 h to allow cell attachment. Finally, 500 μL of basal medium (BM), consisting of DMEM/Ham's nutrient mixture (DMEM/F12; Invitrogen) supplemented with 5% FBS, 1% antibiotics, 10 μg/mL human transferrin (Sigma), and 30 nM sodium selenite (Sigma), was added to each well. Micromasses were allowed to mature for 24 h prior to chondrogenic treatment. In the case of iChonInd cultures, doxycycline was withdrawn 24 h following the initiation of chondrogenic differentiation.
Differentiation was carried out by stimulating cells for 7 days using chondrogenic medium (CM), consisting of BM supplemented with the following chondrogenic factors: 1× insulin-transferrin-selenite (ITS; Gibco), 10 ng/mL transforming growth factor-β1 (TGF-β1; R&D Systems), 10 nM dexamethasone (DEX; Sigma), and 50 μg/mL ascorbic acid (AA; Sigma). Following 7 days of culture, the cells were stimulated to enter hypertrophy using hypertrophic medium (HM): alpha modified Eagle medium (αMEM; Invitrogen) supplemented with 5% FBS, 1% antibiotics, 1×ITS, 50 μg/ml AA, 10 nM DEX, 7 mM β-glycerolphosphate (Sigma), and 50 nM thyroxine (Sigma). During the 21-day period of chondrogenic differentiation (Diff), the medium was changed every other day. Micromasses cultured for up to 21 days in BM were used as controls.
Extracellular matrix analysis and quantification
Extracellular matrix deposition and mineralization were analyzed after 21 days of differentiation. Briefly, Alcian Blue and Alizarin Red stainings were performed by fixing the micromasses with ice-cold methanol for 1 h at 4°C. Afterward, 1 mL of each dye [1% Alizarin Red (Sigma) pH 4.1 in water; 0.1% Alcian Blue in 0.1 M HCl (Fluka)] was added to each well and incubated for 1 h at room temperature. Subsequently, micromasses were washed with water until clear of any dye. For quantitative analysis, Alizarin Red and Alcian Blue were destained using 10% cetylpyridinium chloride (Sigma) and 6 M guanidine hydrochloride (Merck), respectively. Absorbance was measured with the ICN Titertek Plus MS 212 plate reader (Tecan) at 570 nm for Alizarin Red and 620 nm for Alcian Blue.
DNA quantification
DNA was collected by lysing each micromass with RLT Buffer (Qiagen) supplemented with 10 μL/mL β-mercaptoethanol (Sigma). Each sample was diluted 1:10 prior to quantification. DNA was quantified by using the Quant-iT™ dsDNA HS Assay (Invitrogen) kit as previously described (Chen et al., 2012).
Gene expression analysis
RNA extraction and cDNA synthesis was carried out at each time point using the RNeasy mini kit (Qiagen) and cDNA Synthesis Kit (Fermentas), respectively. Gene expression analysis was carried out using a SYBR® Green PCR Master Mix (Applied Biosystems) based qPCR reaction on the Rotor-Gene 6000 system (Corbett). Quantification of gene expression was calculated using the 2−ΔCT method (Livak and Schmittgen, 2001). All experiments were carried according to each manufacturer's protocol. All primer sequences are detailed in Table S1 (Supplementary Data are available at www.liebertpub.com/cell/).
In vivo tissue formation
Growth medium–expanded iChon cells were trypsinized and resuspended at a concentration of 2×106 cells/mL in phosphate-buffered saline (PBS; Lonza). iChon cells were labeled with CM-DiI (Invitrogen) for 5 min at 37°C and subsequently incubated for 15 min at 4°C. Cells were then washed twice in PBS before being resuspended at 1×108 cells/mL. A 20-μL amount of each cell suspension was seeded on a cylindrical CopiOs™ (Zimmer) scaffold (21 mm3, 3 mm×3 mm). The cells were allowed to attach for 1 h before adding 5 mL of growth medium. Following overnight incubation at 37°C, the scaffolds were implanted at the cervical region of NMRI nu/nu mice. To stimulate EO from iChonInd, scaffolds seeded with this cell type were chondrogenically predifferentiated for 21 days (as described above) prior to implantation. Eight weeks later, all scaffolds were collected and histologically processed. All animal experimental procedures were approved by the local ethical committee for animal research (KU Leuven). The animals were housed according to the guidelines provided by the Animalium Leuven (KU Leuven).
Histological processing and analysis
Explants were fixed in 4% paraformaldehyde (PFA) for 2 h at room temperature before decalcification using EDTA/PBS (pH 7.5) for 2 weeks. Subsequently, explants were embedded in paraffin, and 6-μm histological sections were made.
All sections were deparaffinized in Histoclear™ (Laborimpex, Brussels, Belgium) followed by methanol and rinsed with water before performing histological stainings. Sections were stained with Hematoxylin & Eosin (H&E), Toluidine Blue, and tartrate-resistant acid phosphatase (TRAP), as described previously (Weiss et al., 2012). Immunostaining for green fluorescent protein (GFP) was carried out by blocking endogenous peroxidase with 3% hydrogen peroxidase for 2×5 min, followed by antigen retrieval with 0.02% pepsin (Sigma) in 0.02 M HCl for 11 min at 37°C. Sections were then fixed with 4% PFA followed by blocking with 2% goat serum, before being incubated at 4°C overnight with mouse anti-GFP antibody (1:100, ABGent). Subsequently, sections were incubated with horseradish peroxidase–conjugated goat anti-mouse antibody (1:100, Jackson) for 1 h before staining with the diaminobenzidine (DAB)+substrate chromogen kit (Dako). For quantification, each staining was analyzed by using ImageJ software (National Institutes of Health).
Statistical analysis
All experiments were carried out in triplicate to assess statistical significance, with the exception of in vivo studies (n=4). Data represented in each graph are depicted as mean±standard error of the mean. Statistical significance was calculated in Excel (Microsoft) using the unpaired two-tailed t-test. All graphs were generated using Prism software (Graphpad). Statistical significance is represented on each graph as follows: (*) p<0.05, (**) p<0.01, (***) p<0.001, and (****) p<0.0001.
Results
iChon cells are able to undergo chondrogenic differentiation in vitro
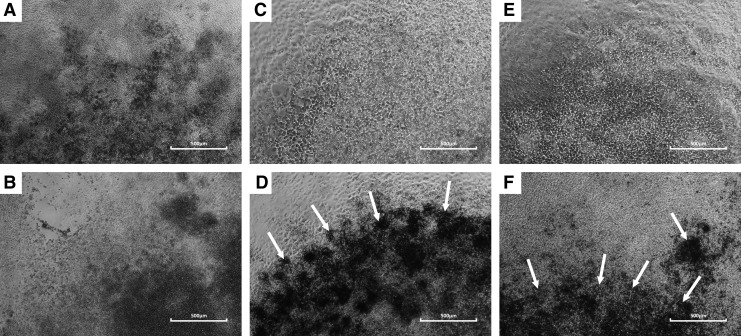
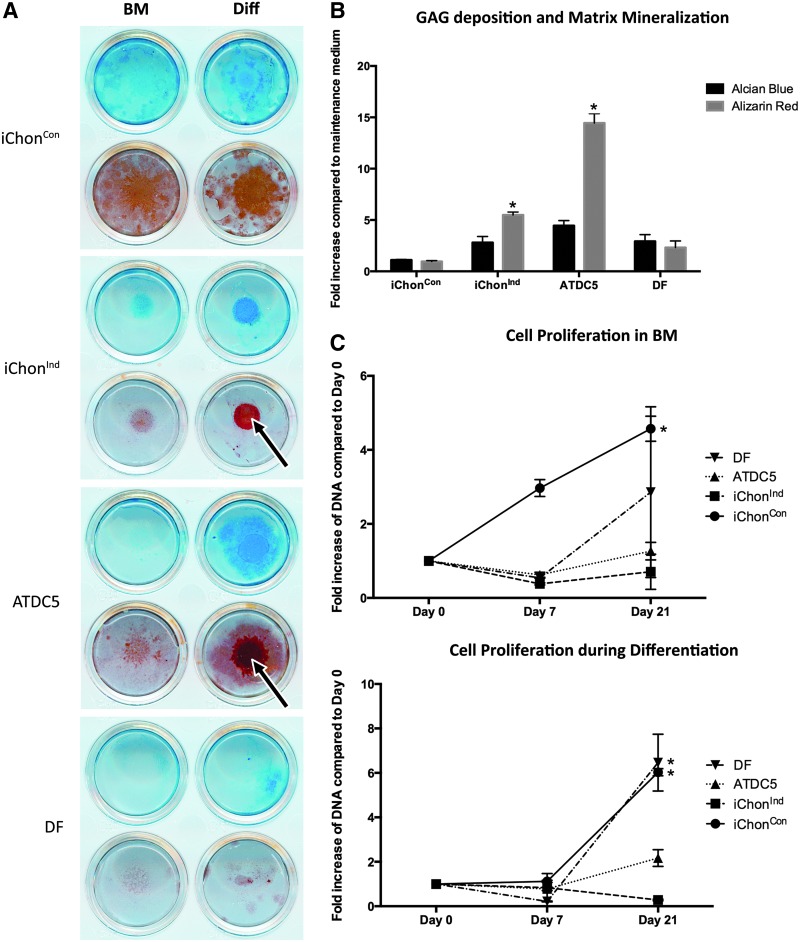
iChon cells exhibited a polygonal shape and were morphologically similar to the ATDC5 chondrogenic cell line (Fig. S1). Upon differentiation, dark areas, suggestive of mineral deposition, became visible in iChonInd and ATDC5 cultures (Fig. 1); however, these were absent in all other cell populations, or in iChonInd and ATDC5 when cultured in BM. Alcian Blue staining revealed an increase in GAG deposition in iChonInd and ATDC5 cultures (2.4- and 4.45-fold when compared to control conditions, respectively). Moreover, upon analysis of mineral deposition by Alizarin Red staining, an increase was detected in both iChonInd (5.50-fold) and ATDC5 (14.44-fold) cultures (Fig. 2). Cell proliferation, measured by quantifying DNA content of different cultures, indicated consistent cell proliferation in iChonCon at every time point when cultured in maintenance or differentiation conditions. Conversely, DNA content had a tendency to decrease at day 7 in ATDC5 and iChonInd when cultured under maintenance conditions. However, at day 21 a trend for an increase in DNA content was detected for both cell types. Similarly, a decrease in DNA content was detected at day 7 in iChonInd and ATDC5s when cultured in differentiation conditions. However at day 21, DNA content for iChonInd continued to decrease whereas an increase was detected in ATDC5 (Fig. 2).
FIG. 1.
In vitro mineralization of iChonInd and ATDC5. Following 21 days of in vitro differentiation, dark regions (white arrows) became visible in iChonInd (D) and ATDC5 (F) micromasses. No distinct dark regions were visible in iChonCon (A), iChonInd (C), and ATDC5 (E) cultured in BM or in iChonCon (B) cultured in differentiation medium.
FIG. 2.
Chondrogenic differentiation of iChon cells in vitro. (A) GAG deposition and mineralized matrix was detected following differentiation in iChonInd and ATDC5 cells. (B) Quantification of dye incorporation revealed significant increase in matrix mineralization in iChonInd and ATDC5 upon differentiation. Significance compared to DF is shown as (*) p<0.05. (C) Cell proliferation of iChonCon was higher than all other cell populations when cultured in BM; however, the profile was more similar during chondrogenic differentiation (Diff). Chondrogenic differentiation was carried out by culturing each cell population in CM for 7 days followed by 14 days in HM. Significance between subsequent time points within a cell line is shown as (*) p<0.05.
iChonInd cells are able to undergo hypertrophic differentiation in vitro
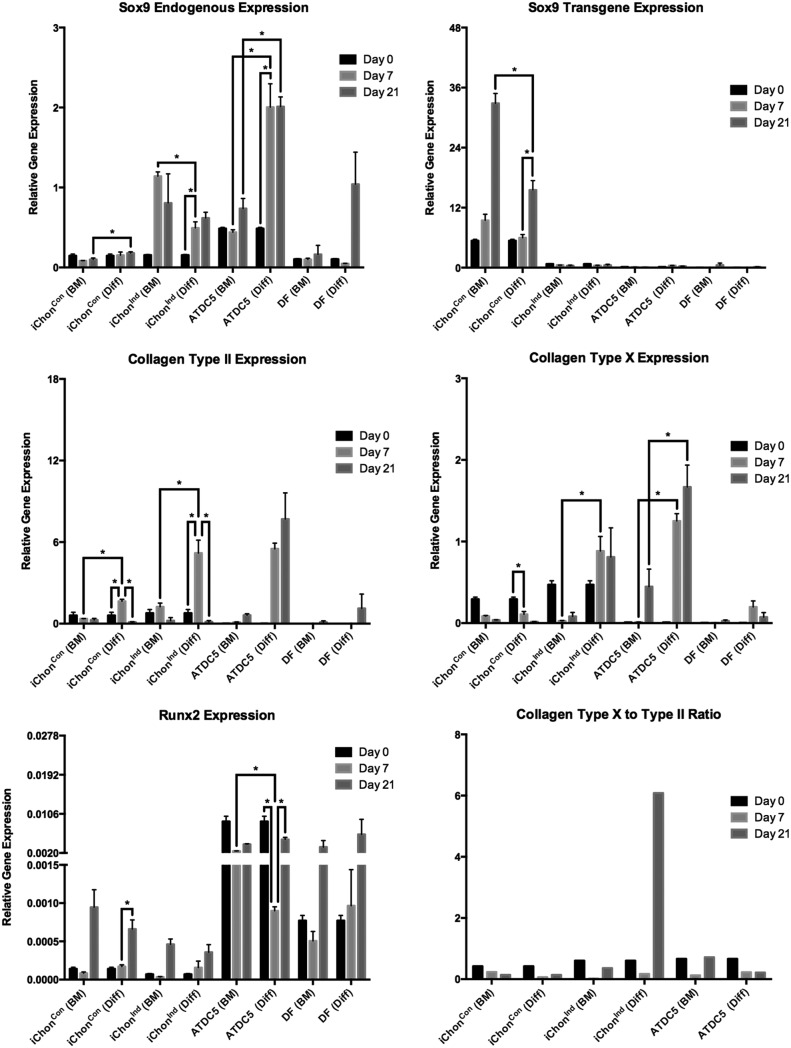
Upon chondrogenic differentiation, increasing levels of endogenous mouse Sox9 (mSox9) were detected in iChonInd. mSox9 expression significantly increased from 3.2-fold at day 7 to a maximum of 4-fold at day 21. Interestingly, mSox9 was expressed at higher levels by iChonInd when cultured in maintenance conditions (7.4-fold increase at day 7); however, the expression profile did not indicate chondrogenic differentiation. This may be a result of cellular condensation because no upregulation in the downstream differentiation markers including collagen type II and X could be detected. Sox9 expression levels were increased in ATDC5s during differentiation, with a significant increase from 4.11-fold to a maximum of 4.13-fold detected at days 7 and 21, respectively. The human Sox9 (hSox9) transgene levels ceased in iChonInd during differentiation, while remaining highly and increasingly expressed in iChonCon. An increase of 1.75-fold at day 7 and 6.09-fold at day 21 was detected in iChonCon upon culturing in basal medium, whereas an increase of 1.11-fold and 2.88-fold was detected upon differentiation at day 7 and day 21, respectively. Collagen type II, indicative for chondrogenic differentiation, was increased by 6.68-fold in iChonInd and 421.13-fold in ATDC5 cells at day 7, whereas its level decreased by 5.84-fold in iChonInd at day 21.
ATDC5 maintained elevated levels of collagen type II throughout the differentiation. Collagen type X, a marker for hypertrophic chondrocytes, was increased by 1.8-fold in iChonInd at day 7, whereas its expression was increased by 143.45-fold in ATDC5. The collagen type X to collagen type II ratio (CCR) was calculated for every cell population and culture condition to evaluate hypertrophic differentiation. The CCR indicates that iChonInd cells were able to enter hypertrophy, whereas iChonCon or ATDC5 maintained high levels of collagen type II and thus may be considered as nonhypertrophic or less hypertrophic. Despite apparent hypertrophic differentiation by iChonInd, only low levels of Runx2 were detected when compared to ATDC5. However, a trend toward increased Runx2 expression in iChonInd was present at day 7 and 21, respectively (Fig. 3).
FIG. 3.
Gene expression and hypertrophic differentiation analysis. Endogenous Sox9 levels increased upon chondrogenic differentiation (Diff) in iChonInd and ATDC5, whereas transgene Sox9 levels ceased. Collagen type II increased in iChonInd and ATDC5 after 7 days of chondrogenic treatment. The expression of collagen type II decreased in iChonInd during hypertrophic differentiation, while remaining high in ATDC5. Collagen type X increased in iChonInd and ATDC5 when cells were cultured in hypertrophic medium. Similarly, an increase in Runx2 upon differentiation was detected in iChonInd. The CCR indicates hypertrophic differentiation of iChonInd. Micromasses cultured for up to 21 days in BM were used as controls.
iChonCon are able to form stable cartilage on CopiOs™ in vivo
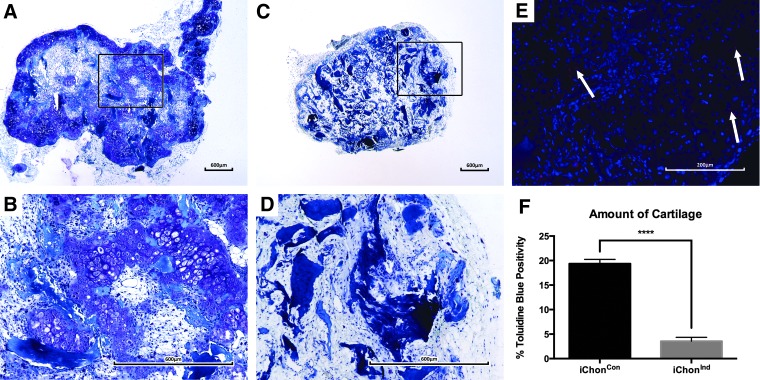
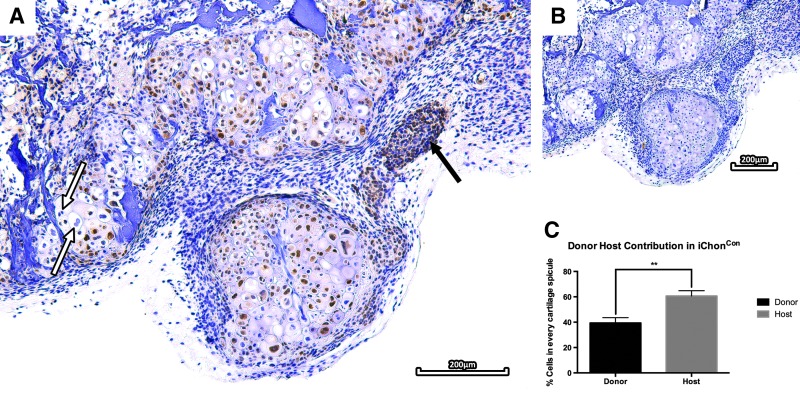
To evaluate the tissue formation capacities of the iChon cells, cells were seeded on a commercially available bone void filler (CopiOs™). These scaffolds were then implanted subcutaneously in nude mice for 8 weeks. Upon scaffold explantation and histological processing, sections were evaluated for tissue formation. Scaffolds seeded with iChonCon contained cartilaginous tissues at the outer areas, which were positively stained with Toluidine Blue (19.37%±0.87% of the entire scaffold surface area). CM-DiI labeling revealed the presence of donor cells in cartilage tissues throughout the implant (Fig. 4). GFP immunostaining was carried out to assess the host/donor contribution to the formed tissue. The staining revealed that cartilaginous tissues contained chondrocytes derived from both the host (60.64%±4.2%) and donor (39.36%±4.2%), indicating active cross talk. However, despite chondrogenic differentiation of iChonCon in vivo, a subset of the cells was unable to form cartilage and remained fibroblastic following implantation. Additionally, the presence of enlarged (pre)-hypertrophic-like chondrocytes, which were mostly derived from host origin, were detected in these scaffolds; however, no bone formation was observed (Fig. 5).
FIG. 4.
Toluidine Blue staining and CM-DiI labeling of iChon cells. Implants seeded with iChonCon cells were able to form stable cartilage in vivo (A, B). No cartilage formation was detected in scaffolds containing iChonInd (C, D). CM-DiI labeling indicated the presence of donor cells in iChonCon implants upon explantation (E). Quantification of Toluidine Blue staining revealed that implants seeded with iChonCon contained 19.37%±0.87% cartilage, whereas only 3.56%±0.77% (p=2.6×10−14) positive staining was detected in iChonInd (F).
Fig. 5.
GFP immunostaining of implants seeded with iChonCon. GFP immunostaining revealed the presence of donor cells throughout the implants. However, a subset of donor cells was unable to form cartilage and remained fibroblastic (black arrow). Interestingly, chondrocytes contained within the cartilage tissue did not stain for GFP and hence are thought to be host derived (white arrow) (A). IgG control (B). Staining quantification revealed presence of both donor (39.36%±4.2%) and host (60.64%±4.2%) cells throughout each cartilaginous spicule (p=0.0012).
Scaffolds seeded with iChonInd did not form cartilage or bone tissue (Fig. 4); however, the presence of donor cells could be detected by CM-DiI throughout each implant (data not shown). Upon staining quantification, 3.56%±0.77% Toluidine Blue positivity was detected throughout these implants and is likely to have been caused by background from the scaffold matrix (Fig. 4).
Predifferentiation of iChonInd triggers EO in vivo
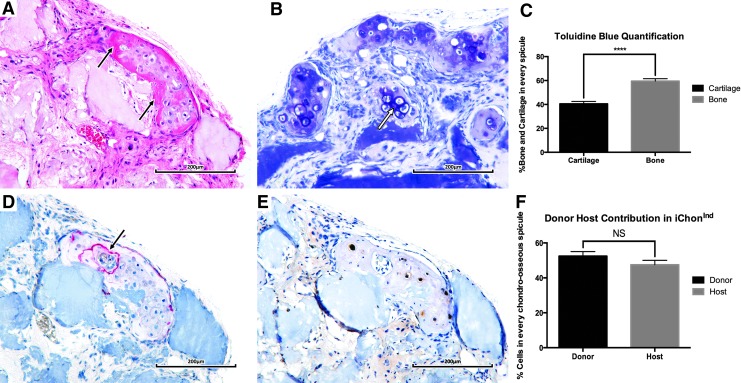
Although in vitro hypertrophic differentiation of iChonInd was achieved, no cartilage or bone formation was detected after implantation. This indicates that the cells were not truly committed to the chondrogenic lineage without external stimuli. Therefore, scaffolds containing iChonInd cells were predifferentiated for 21 days prior to implantation. Upon explantation, bone formation was detected in regions surrounding cartilage tissue, which was positively stained with Toluidine Blue. Each chondro–osseous spicule contained 40.52%±1.99% cartilage and 59.48%±1.99% bone. GFP immunostaining revealed that the cartilage tissue contained cells derived from both host (52.5%±2.57%) and donor (47.5%±2.57%) origin. The staining also suggested that the bone tissue was derived from the host. However, transdifferentiation of iChonInd cannot be ruled out, as in this setting CollXI controls GFP expression, which is not present in osteoblasts. Nevertheless, transdifferentiation is unlikely because iChonInd cells were unable to undergo osteogenic differentiation when cultured in vitro (data not shown). TRAP positivity was found between the chondro–osseous interface, indicating active resorption of iChonInd tissue by osteoclasts. The presence of blood vessels was detected in close proximity to these cartilage/bone islands. (Fig. 6).
FIG. 6.
Scaffolds seeded with iChonInd are able to trigger EO in vivo. H&E-staining revealed the presence of bone tissue surrounding each cartilage island (black arrow) (A). Bone tissue was found to be preferentially formed surrounding Toluidine Blue–positive hypertrophic chondrocytes (white arrow) (B). Quantification of the Toluidine Blue staining revealed that each chondro–osseous spicule contained 40.52%±1.99% cartilage and 59.48%±1.99% bone (p=1.79×10−8) (C). TRAP staining revealed osteoclastic activity in each spicule, preferentially at the chondro–osseous interface (black arrow) (D). GFP immunostaining suggested presence of both donor (52.49%±2.57%) and host cells (47.51%±2.57%) in the cartilaginous region (NS, not significant, p=0.18), while bone tissue was thought to be completely host derived (E, F).
Discussion
Bone development and repair is often preceded by the formation of temporal cartilage tissue, called the fracture callus, which undergoes maturation and hypertrophy before finally being remodeled to bone. During this EO process, skeletal progenitors condense and differentiate into chondrocytes while upregulating Sox9 and collagen type II. Upon maturation, chondrocytes enlarge, become hypertrophic, and express Runx2 and collagen type X. Hypertrophic chondrocytes deposit a calcified matrix, which is resorbed by osteoclasts and replaced by bone. As cartilage formation is often required for successful fracture healing, we investigated the hypertrophic differentiation and tissue formation capacities of iChon cells in this study.
iChon cells were able to undergo chondrogenic differentiation and deposited a GAG-rich matrix that was positively stained with Alcian Blue. However, upon hypertrophic treatment, only iChonInd and ATDC5 cells were able to mineralize their matrix in vitro. Interestingly, no matrix mineralization was observed in iChonCon cultures, despite a similar approach being used for the creation of these cells. This can be explained as iChonCon have a constitutively active cytomegalovirus (CMV) promoter that drives the expression of Sox9. Indeed, it has previously been shown that Sox9 is able to directly repress the osteogenic glycoprotein SPP1, which is necessary for matrix mineralization. Peacock et al. have shown that primary chondrocytes lacking Sox9 displayed elevated SPP1 levels and thereby an enhanced mineralization in culture (Peacock et al., 2011). Moreover, mice haploinsufficient in Sox9 show premature skeletal mineralization, leading to stature shortening, whereas the overall skeletal morphology was preserved (Bi et al., 2001).
These results are in agreement with our in vitro findings, because iChonInd cells, which lost the constitutive transgene expression upon differentiation, were able to undergo matrix mineralization and subsequent hypertrophy. Indeed, these cells were able to express Runx2 and collagen type X, the main markers for hypertrophic differentiation. However, only low levels of Runx2 were detected upon differentiation as compared to ATDC5 controls. Proliferation of iChonCon increased steadily upon culture, while it decreased in iChonInd cells during hypertrophic differentiation.
The continued proliferation of iChonCon can be attributed to the continuous expression of cMyc. Indeed, cMyc has been previously shown to alter cell proliferation by preventing cell cycle exit (Amati et al., 1998). Because high transgene Sox9 levels were detected in iChonCon, it is probable that cMyc is also expressed, hence explaining the high proliferative behavior. The decrease in proliferation in iChonInd can be attributed to the presence of CaP extracellular matrix mineralization upon differentiation. Indeed, it has been previously reported that upon hypertrophic differentiation, chondrocytes activate an apoptosis program as a result of phosphate stimuli and hence contribute to cartilage remodeling during EO (Miedlich et al., 2010).
Surprisingly, higher levels of Runx2 were detected in iChonCon cells when compared to iChonInd, whereas no collagen type X expression was detected. The absence of hypertrophic differentiation could potentially be attributed to the inhibitory effects of Sox9 on the transcriptional activities of Runx2 during differentiation. Indeed, Zhou et al. have previously reported that Sox9 was able to bind to Runx2, thereby inhibiting its transcriptional activities (Zhou et al., 2006). Furthermore, Bapx1, one of the downstream targets of Sox9, has been shown to repress Runx2 and inhibit chondrocyte maturation (Provot et al., 2006; Yamashita et al., 2009).
Interestingly, although iChonCon and iChonInd are created through the forced expression of identical TFs, both responded differently to exogenous stimuli as a result of differences in the promoter that was used to drive transgene expression. Indeed, similar to our findings, the choice of the promoter system for driving transgene expression is known to affect the transcriptional program and epigenetic signature in iPSCs (Sommer et al., 2012). iPSC clones that lost their transgene expression, were remarkably more similar to their native counterparts (ESCs), whereas others that contained a continuous expression of transgenes prevented them from acquiring the native transcriptional profile (Sommer et al., 2012). Hence, the choice of promoter for driving transgene expression may affect cellular response and cellular phenotype.
When comparing the hypertrophic differentiation kinetics of iChonInd and ATDC5, iChonInd cultures were able to recapitulate a similar gene expression profile as observed during embryonic endochondral bone development. Indeed, collagen type II expression increased upon chondrogenic differentiation while its expression ceased during hypertrophic differentiation. Conversely, ATDC5 maintained elevated levels of collagen type II during differentiation. This differential response upon differentiation could potentially be attributed to the origin of ATDC cells. ATDC cells are derived from teratocarcinomas and thus may display alterations in the genetic profile when compared to native chondroblasts/chondrocytes. Moreover, unsynchronized behavior of ATDC5s upon differentiation has been previously reported (Kondo et al., 2007). These observations make ATDC cells unlikely as a source for synchronized tissue formation.
Upon analysis of the tissue formation capacity for each iChon cell population, it was found that scaffolds containing iChonCon were able to form stable cartilage containing cells derived from both host and donor. This indicates that implanted cells were able to recruit circulating progenitors for tissue formation, as previously reported for other bone/cartilage-forming cell types (Khosla and Eghbali-Fatourechi, 2006; Mifune et al., 2008; Roberts et al., 2011). However, a subset of donor cells were unable to undergo chondrogenic differentiation and remained fibroblastic. This could potentially be explained by transgene silencing upon implantation. Interestingly, hypertrophic-like chondrocytes were detected and were mostly derived from host origin. However, no bone formation was detected in these implants. It is likely that this is a direct result of constitutive expression of Sox9. Indeed, constitutive expression of Sox9 is known to maintain a stable cartilaginous phenotype, caused by preventing vascularization during EO (Hattori et al., 2010). This would potentially negate the recruitment of osteoblast precursors, which have previously been shown to migrate along invading blood vessels during EO (Maes et al., 2010).
Additionally, the presence of parathyroid hormone–related protein (PTHrP) signaling in maintaining a stable cartilaginous phenotype through Sox9 may also play a role (Huang et al., 2001). PTHrP is secreted by articular chondrocytes, which do not undergo endochondral remodeling during healthy tissue homeostasis (Fischer et al., 2010). However, its expression together with Sox9 was found to be altered in osteoarthritic conditions, whereby articular cartilage initiates an EO program leading to joint dysfunction (Cox et al., 2013; Kim and Im, 2011). These studies confirm that constitutive expression of Sox9 is required for the formation of stable cartilage in vivo. Moreover, the donor cells were able to induce a stable cartilaginous phenotype of host cells, possibly as a result of paracrine PTHrP-mediated signaling.
Scaffolds containing unstimulated iChonInd failed to induce cartilage or bone formation in vivo. Possibly as a result of losing transgene expression upon implantation, as the presence of donor cells was detected within the scaffold (data not shown). However, following in vitro differentiation of iChonInd towards hypertrophy prior to implantation, EO at the edges of the scaffolds was observed. It is likely that this bone tissue is host-derived, whereas the cartilage is comprised of cells derived from both host and donor. Despite absence of donor contribution to bone formation, we can conclude that iChonInd cells are able to trigger EO. Indeed, the results that have been published on whether hypertrophic chondrocytes are able to transdifferentiate to osteoblasts during EO are conflicting (Miedlich et al., 2010; Roach, 1992). Moreover, it has previously been reported that hypertrophic chondrocytes activate an apoptosis program while expressing vascular endothelial growth factor to stimulate blood vessel ingrowth (Adams and Shapiro, 2002; Gerber et al., 1999). This process was found to be necessary for hypertrophic cartilage remodeling and trabecular bone formation. The presence of blood vessels together with osteoclasts near the bone–cartilage interface in our study supports this hypothesis, and may be attributed to paracrine vascular endothelial growth factor (VEGF) signaling during bone formation. The absence of osteoblast transdifferentiation from iChonInd is therefore unsurprising. The in vivo data presented implies that predifferentiation of iChonInd allows the cells to form a cartilaginous intermediate prior to implantation, which was not reliant on the transgene expression. Following exposure to the in vivo environment, the cartilaginous template could trigger the recruitment of host cells to allow the endochondral process to occur. Furthermore, bone formation was observed in scaffold-free implanted iChonInd micromasses, indicating that in vivo bone formation was not triggered through the presence of calcium phosphate in each scaffold (data not shown).
In summary, this study shows that direct reprogrammed chondrogenic cell populations are able to stimulate EO in vivo and may provide additional opportunities for cell engineering that can be applied to bone regeneration strategies. Furthermore, future translation to human cell populations may also allow disease modeling to assess the efficiency of therapeutics aimed at conditions that result in nonunions, such as congenital tibial pseudoarthrosis.
Supplementary Material
Acknowledgments
This work was supported by the Research Foundation–Flanders (grant no. G096211N) and the Stem Cell Institute of Leuven–KU Leuven. S.R. is a postdoctoral fellow of the Research Foundation–Flanders (FWO).
Author Disclosure Statement
The authors declare that no conflicting financial interests exist.
References
- Adams C.S., and Shapiro I.M. (2002). The fate of the terminally differentiated chondrocyte: Evidence for microenvironmental regulation of chondrocyte apoptosis. Crit. Rev. Oral Biol. Med. 13, 465–473 [DOI] [PubMed] [Google Scholar]
- Amati B., Alevizopoulos K., and Vlach J. (1998). Myc and the cell cycle. Front. Biosci. 3, d250–d268 [DOI] [PubMed] [Google Scholar]
- Banfi A., Muraglia A., Dozin B., Mastrogiacomo M., Cancedda R., and Quarto R. (2000). Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp. Hematol. 28, 707–715 [DOI] [PubMed] [Google Scholar]
- Bi W., Huang W., Whitworth D.J., Deng J.M., Zhang Z., Behringer R.R., and de Crombrugghe B. (2001). Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc. Natl. Acad. Sci. USA 98, 6698–6703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Sonnaert M., Roberts S.J., Luyten F.P., and Schrooten J. (2012). Validation of a PicoGreen-based DNA quantification integrated in an RNA extraction method for two-dimensional and three-dimensional cell cultures. Tissue Eng. Part C Methods 18, 444–452 [DOI] [PubMed] [Google Scholar]
- Cox L.G., van Donkelaar C.C., van Rietbergen B., Emans P.J., and Ito K. (2013). Alterations to the subchondral bone architecture during osteoarthritis: Bone adaptation vs endochondral bone formation. Osteoarthritis Cartilage 21, 331–338 [DOI] [PubMed] [Google Scholar]
- Fischer J., Dickhut A., Rickert M., and Richter W. (2010). Human articular chondrocytes secrete parathyroid hormone-related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum. 62, 2696–2706 [DOI] [PubMed] [Google Scholar]
- Gerber H.P., Vu T.H., Ryan A.M., Kowalski J., Werb Z., and Ferrara N. (1999). VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 5, 623–628 [DOI] [PubMed] [Google Scholar]
- Hattori T., Muller C., Gebhard S., Bauer E., Pausch F., Schlund B., Bosl M.R., Hess A., Surmann-Schmitt C., von der Mark H., de Crombrugghe B., and von der Mark K. (2010). SOX9 is a major negative regulator of cartilage vascularization, bone marrow formation and endochondral ossification. Development 137, 901–911 [DOI] [PubMed] [Google Scholar]
- Hiramatsu K., Sasagawa S., Outani H., Nakagawa K., Yoshikawa H., and Tsumaki N. (2011). Generation of hyaline cartilaginous tissue from mouse adult dermal fibroblast culture by defined factors. J. Clin. Invest. 121, 640–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Chung U.I., Kronenberg H.M., and de Crombrugghe B. (2001). The chondrogenic transcription factor Sox9 is a target of signaling by the parathyroid hormone-related peptide in the growth plate of endochondral bones. Proc. Natl. Acad. Sci. USA 98, 160–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieda M., Fu J.D., Delgado-Olguin P., Vedantham V., Hayashi Y., Bruneau B.G., and Srivastava D. (2010). Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142, 375–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii R., Kami D., Toyoda M., Makino H., Gojo S., Ishii T., and Umezawa A. (2012). Placenta to cartilage: Direct conversion of human placenta to chondrocytes with transformation by defined factors. Mol. Biol. Cell 23, 3511–3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S., and Eghbali-Fatourechi G.Z. (2006). Circulating cells with osteogenic potential. Ann. NY Acad. Sci. 1068, 489–497 [DOI] [PubMed] [Google Scholar]
- Kim S.Y., and Im G.I. (2011). The expressions of the SOX trio, PTHrP (parathyroid hormone-related peptide)/IHH (Indian hedgehog protein) in surgically induced osteoarthritis of the rat. Cell Biol. Int. 35, 529–535 [DOI] [PubMed] [Google Scholar]
- Kondo S., Shukunami C., Morioka Y., Matsumoto N., Takahashi R., Oh J., Atsumi T., Umezawa A., Kudo A., Kitayama H., Hiraki Y., and Noda M. (2007). Dual effects of the membrane-anchored MMP regulator RECK on chondrogenic differentiation of ATDC5 cells. J. Cell Sci. 120, 849–857 [DOI] [PubMed] [Google Scholar]
- Lenas P., Moos M., and Luyten F.P. (2009a). Developmental engineering: a new paradigm for the design and manufacturing of cell-based products. Part I: From three-dimensional cell growth to biomimetics of in vivo development. Tissue Eng. Part B Rev. 15, 381–394 [DOI] [PubMed] [Google Scholar]
- Lenas P., Moos M., and Luyten F.P. (2009b). Developmental engineering: A new paradigm for the design and manufacturing of cell-based products. Part II: From genes to networks: tissue engineering from the viewpoint of systems biology and network science. Tissue Eng. Part B Rev. 15, 395–422 [DOI] [PubMed] [Google Scholar]
- Livak K.J., and Schmittgen T.D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- Maes C., Kobayashi T., Selig M.K., Torrekens S., Roth S.I., Mackem S., Carmeliet G., and Kronenberg H.M. (2010). Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 19, 329–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedlich S.U., Zalutskaya A., Zhu E.D., and Demay M.B. (2010). Phosphate-induced apoptosis of hypertrophic chondrocytes is associated with a decrease in mitochondrial membrane potential and is dependent upon Erk1/2 phosphorylation. J. Biol. Chem. 285, 18270–18275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifune Y., Matsumoto T., Kawamoto A., Kuroda R., Shoji T., Iwasaki H., Kwon S.M., Miwa M., Kurosaka M., and Asahara T. (2008). Local delivery of granulocyte colony stimulating factor-mobilized CD34-positive progenitor cells using bioscaffold for modality of unhealing bone fracture. Stem Cells 26, 1395–1405 [DOI] [PubMed] [Google Scholar]
- Peacock J.D., Huk D.J., Ediriweera H.N., and Lincoln J. (2011). Sox9 transcriptionally represses Spp1 to prevent matrix mineralization in maturing heart valves and chondrocytes. PLoS One 6, e26769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provot S., Kempf H., Murtaugh L.C., Chung U.I., Kim D.W., Chyung J., Kronenberg H.M., and Lassar A.B. (2006). Nkx3.2/Bapx1 acts as a negative regulator of chondrocyte maturation. Development 133, 651–662 [DOI] [PubMed] [Google Scholar]
- Roach H.I. (1992). Trans-differentiation of hypertrophic chondrocytes into cells capable of producing a mineralized bone matrix. Bone Miner. 19, 1–20 [DOI] [PubMed] [Google Scholar]
- Roberts S.J., Geris L., Kerckhofs G., Desmet E., Schrooten J., and Luyten F.P. (2011). The combined bone forming capacity of human periosteal derived cells and calcium phosphates. Biomaterials 32, 4393–4405 [DOI] [PubMed] [Google Scholar]
- Scotti C., Piccinini E., Takizawa H., Todorov A., Bourgine P., Papadimitropoulos A., Barbero A., Manz M.G., and Martin I. (2013). Engineering of a functional bone organ through endochondral ossification. Proc. Natl. Acad. Sci. USA 110, 3997–4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro F. (2008). Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur. Cells Mater. 15, 53–76 [DOI] [PubMed] [Google Scholar]
- Sommer C.A., Christodoulou C., Gianotti-Sommer A., Shen S.S., Sailaja B.S., Hezroni H., Spira A., Meshorer E., Kotton D.N., and Mostoslavsky G. (2012). Residual expression of reprogramming factors affects the transcriptional program and epigenetic signatures of induced pluripotent stem cells. PLoS One 7, e51711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., and Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., and Yamanaka S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 [DOI] [PubMed] [Google Scholar]
- Vierbuchen T., Ostermeier A., Pang Z.P., Kokubu Y., Sudhof T.C., and Wernig M. (2010). Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss H.E., Roberts S.J., Schrooten J., and Luyten F.P. (2012). A semi-autonomous model of endochondral ossification for developmental tissue engineering. Tissue Eng. Part A 18, 1334–1343 [DOI] [PubMed] [Google Scholar]
- Yamashita S., Andoh M., Ueno-Kudoh H., Sato T., Miyaki S., and Asahara H. (2009). Sox9 directly promotes Bapx1 gene expression to repress Runx2 in chondrocytes. Exp. Cell Res. 315, 2231–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G., Zheng Q., Engin F., Munivez E., Chen Y., Sebald E., Krakow D., and Lee B. (2006). Dominance of SOX9 function over RUNX2 during skeletogenesis. Proc. Natl. Acad. Sci. USA 103, 19004–19009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.