Abstract
Induced pluripotent stem cells (iPSCs) are usually generated by reprogramming somatic cells through transduction with a transcription factor cocktail. However, the low efficiency of this procedure has kept iPSCs away from the study of the clinical application of stem cell biology. Our research shows that continuous passage increases the efficiency of reprogramming. Compared with conventional method of establishment of iPSCs, more embryonic stem cell (ESC)-like clones are generated by continuous passage during early reprogramming. These inchoate clones, indistinguishable from genuine ESC clones, are closer to fully reprogrammed cells compared with those derived from classical iPSC induction, which increased the expression of pluripotent gene markers and the levels of demethylation of Oct4 and Nanog. These results suggested that full reprogramming is a gradual process that does not merely end at the point of the activation of endogenous pluripotency-associated genes. Continuous passage could increase the pluripotency of induced cells and accelerate the process of reprogramming by epigenetic modification. In brief, we have provided an advanced strategy to accelerate the reprogramming and generate more nearly fully reprogrammed iPSCs efficiently and rapidly.
Introduction
Somatic cells can be reprogrammed to a pluripotent state through the ectopic expression of four transcription factors, Oct4, Klf4, Sox2, and c-Myc (Gao et al., 2013; Lorenzo et al., 2012; Takahashi and Yamanaka, 2006). The transformation of differentiated somatic cells to induced pluripotent cells (iPSCs) has opened a new horizon of regenerative medicine in cell transplantation therapies; however, there are several limitations in using iPSCs as a valuable tool for studying disease modeling. For example, not all clones that appear in the induced process are fully reprogramminged (Hanna et al., 2009; Vierbuchen and Wernig, 2012). In addition, the difficulty of picking clones and the generation of Oct4 promoter–labeled iPSCs are processes that are too expensive for use by researchers. The current standard strategy for iPSCs generation relies on a nearly 2-week ectopic expression of four Yamanaka factors and takes 2–3 additional weeks for expansion (Kou et al., 2010; Slack, 2009). Although there are several alternatives to this strategy, including the use of some other transcriptional factors, signaling factors, and pharmacological molecules (Liu et al., 2010; Su et al., 2013), previous data has shown that early-passage iPSCs cannot abrogate the epigenetic memory of their original cells, and this affects their potential ability to differentiate into other cell types (Bilic and Izpisua Belmonte, 2012; Hewitt et al., 2011). Recent research has shown than the continuous passage of iPSCs can attenuate transcriptional, epigenetic, and functional differences (Hanna et al., 2010; Sullivan et al., 2010). Another study has identified that donor cell–specific gene expression patterns of human iPSCs in early passages are different from those in late-passage cells (Ghosh et al., 2010), suggesting an influence of continuous passage on the molecular properties of the resultant iPSCs. However, both studies only focused on the expression of differentiated genes and did not examine the effect on the expression of pluripotent genes in iPSCs by continuous passage. Therefore, we doubted whether continuous passage of iPSCs could change the expression pattern of endogenous pluripotent genes, and whether the efficiency of cellular reprogramming could be increased by continuous passage.
Many studies have shown that the inhibition of the p53/p16 pathway enhances the generation of iPSCs, and a high cell division rate accelerates the process of direct reprogramming slightly (Banito et al., 2009; Gao et al., 2013; Lin and Ying, 2012; Smith et al., 2010). Indeed, our research found that continuous passage of mouse iPSCs not only upregulated pluripotent genes, but also resulted in a highly increased demethylated change in the promoters of both Oct4 and Nanog. Thus, continuous passage during the early stage could significantly increase the pluripotency of iPSCs with epigenetic modification.
Materials and Methods
Cell culture
Mouse embryonic fibroblasts (MEFs) were isolated from E13.5 B6D2F1 mouse embryos and washed in phosphate-buffered saline (PBS). The head and visceral tissues were removed from isolated embryos. The remaining bodies were washed in fresh PBS, minced to 1- to 3-mm pieces using a pair of scissors, transferred into a tube with 0.1 mM trypsin/1 mM EDTA solution, and incubated for 3 min. After incubation, Dulbecco's modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS) was added to stop trypsinization, and the supernatant was transferred into a new tube. Cells were resuspended in fresh medium and cultured on 100-mm dishes at 37°C with 5% CO2.
R1 cells were cultured in DMEM medium with 15% embryonic cell–qualified FBS (ES-FBS; Invitrogen), 0.1 mM nonessential amino acids (NEAA; Invitrogen), 0.1 mM β-mercaptoethanol, and 1000 U/mL leukemia inhibitory factor (LIF; Gibco) and passaged every other day. Plat-E packaging cells (Cyagen), which were used to produce retroviruses, were maintained in DMEM medium containing 10% FBS, 50 mg/mL penicillin/streptomycin, 1 mg/mL puromycin (Sigma), and 100 mg/mL of blasticidin S (Merck).
Establishment and in vitro differentiation of iPSCs
Retroviral production was performed as described by Yamanaka with minor modifications. Plat-E cells were seeded at 8×106 cells per 100-mm dish. The following day, pMXs-based retroviral vectors of Oct4, Sox2, Klf4, and c-Myc were introduced into Plat-E cultures using Lipofectamine® LTX & PLUS transfection reagent (Invitrogen). Virus-containing supernatants were collected 48 h later and filtered through a 0.45- μm cellulose acetate filter (Millipore) and supplemented with 4 mg/mL Polybrene (Sigma). Equal amounts of supernatants containing each of the four retroviruses were transferred to the fibroblasts dish and incubated overnight. On the next day, MEFs were infected again. After 24 h (labeled day 0), the medium was replaced with MEF medium. After the infected MEFs were seeded on mitomycin C–treated MEFs, the medium was changed to knockout DMEM medium with 15% Knockout Serum Replacement (KOSR; Invitrogen), 0.1 mM NEAA, 0.1 mM β-mercaptoethanol, and 1000 U/mL LIF. The ESC-like clones were picked and amplified.
After the generation of iPSCs, the cells were harvested by trypsinization and transferred to low-attachment Petri dishes in the ESC medium without LIF. After 4 days, aggregated cells were plated onto gelatin-coated tissue culture dishes and incubated for another 4 days. The cells were stained with germ layer antibody [Tuj1, smooth muscle actin [SMA], α-fetoprotein (AFP)].
Teratoma formation assay
iPSCs were suspended at 1×107cells/mL in PBS. A 100- μL amount of the cell suspension was used for subcutaneous injection into the dorsal flank of nonobese diabetic–severe combined immunodeficient (NOD-SCID) mice. Four weeks after the injection, tumors were surgically dissected from the mice, fixed with 4% paraformaldehyde, and embedded in paraffin. Sections were stained with Hematoxylin & Eosin.
Alkaline phosphatase and immunofluorescent staining
For alkaline phosphatase (AKP) staining, cells were first washed with PBS, fixed in 4% paraformaldehyde (PFA), and washed again with PBS. The cells were stained with AKP solution according to the manufacturer's guidelines (Beyotime Institute of Biotechnology).
For immunocytochemistry, cells were fixed with 4% PFA for 30 min, permeabilized with 1% Triton X-100 for 15 min, and blocked with 2% goat serum in PBS for 1 h. Primary antibodies used were anti-Oct3/4 (Abcam), anti-stage-specific embryonic antigen-1 (SSEA1) (Santa Cruz), anti-AFP (Boster), anti-Tuj1 (Santa Cruz), and anti-SMA (Boster). After incubation overnight with primary antibodies, cells were washed with PBS twice and incubated with secondary antibodies (Santa Cruz). After washing with PBS, the samples were counterstained with Hoechst 33258 (Invitrogen). Fluorescent images were taken under a Nikon microscope using appropriate filters.
RT-PCR and real-time PCR analysis
Total RNA was isolated with TRIzol and treated with DNase (Promega). RNA was reverse transcribed using a High Capacity cDNA Reverse Transcription Kit (ABI, cat. no. 4368814). PCR cycling parameters were 5 min at 94°C followed by thirty cycles of 30 sec at 94°C, 30 sec at 60°C, 30 sec at 72°C, and a final 10-min extension at 72°C. PCR products were separated on a 2% agarose gel, stained with ethidium bromide, visualized, and photographed on a UV transilluminator. Real-time PCR reactions were performed based on a 1- μL cDNA sample, 10 μL of TransStart™ Top Green qPCR SuperMix (TransGen, AQ131), and gene-specific primers in a 20- μL reaction system on CFX96 Real-Time System (Bio-Rad, USA). Each sample was analyzed in triplicate with glyceraldehyde 3-phophate dehydrogenase (Gapdh), H2a, and β-actin as the internal controls. Primer sequences used in this study are shown in Table S1 (Supplementary Data are available at www.liebertpub.com/cell/.) The Student's t-test was used to assess differences between groups. p<0.05 was considered statistically significant.
Bisulfite genomic sequencing
Bisulfite treatment was performed using an EZ DNA Methylation Kit (Zymo Research) according to the manufacturer's recommendations. Amplified products were cloned into pCR2.1-TOPO (Invitrogen). Ten randomly selected clones were sequenced with the M13 forward and M13 reverse primers for each gene. We obtained PCR primer sequences for SSLP from the Mouse Genome Informatics website. Allele sizes were approximated on the basis of the known allele sizes in various inbred strains.
Results
Passaging culture increased the generation of ESC-like clones
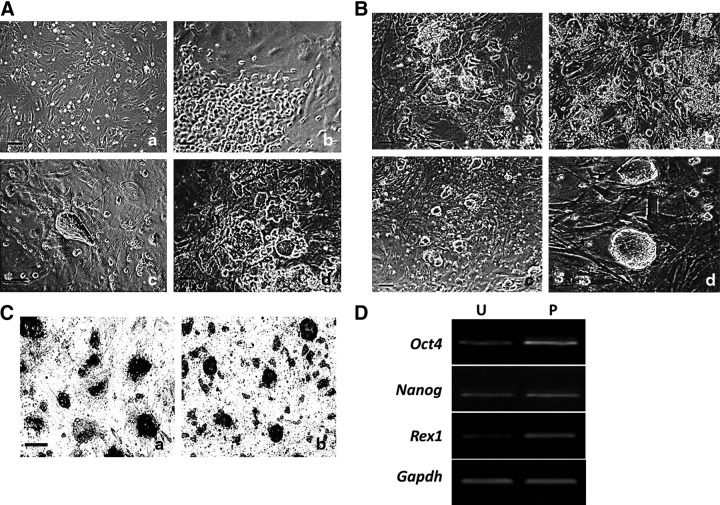
We infected B6D2F1 MEFs by retrovirus with cDNAs carrying four Yamanaka factors as described in the Material and Methods section. Infected MEFs were transferred onto feeder layers at day 3 after infection and cultured in ESC medium (Fig. 1Aa). Cell groups with an epithelium-like morphology first emerged at day 6 (Fig. 1Ab). ESC-like colonies were large and round with clear boundaries at day 8 (Fig. 1Ac). At day 10, cells with different morphology filled the plate and included ESC-like populations, a separate population of small, round, rapidly dividing cells (Fig. 1Ad). Instead of picking the clones, we passaged the cells as an entire plate, which gave rise to a heterogeneous culture.
FIG. 1.
Generation of miPSCs cells by heterogeneous culture. (A) Morphology of induced MEFs at different stages. a, Day 3, MEFs morphology; b, day 6, epithlium-like morphology; c, day 8, ESC-like clone morphology; d, day 10, different morphology of induced MEFs. (B) Morphology of heterogeneous culture cells at different passages. a, Unpassaged cells; b, passage 2; c, passage 4; d, passage 4. (C) AKP staining of reprogrammable MEFs. a, Unpassaged cells; b, heterogeneous cultured cells. (D) RT-PCR analysis examining Oct4, nanog, and res1 expression, with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as loading controls (n=3, p<0.05). U, unpassaged; P, continuously passaged. Scale bar, 100 μm (A, Ba–c, C) and 50 μm (Bd).
After several passages, it turned out that the more cells were passaged, the more numbers of ESC-like colonies were produced in heterogeneous culture cells (Fig. 1B). The heterogeneous culture clones exhibited more multicellular, clearly marginal, and regular morphology (Fig. 1Bd). AKP staining showed that more AKP-positive cells were found in heterogeneous cultured cells compared with unpassaged cells (Fig. 1C). RT-PCR results showed the expression of pluripotent gene markers, such as Oct4, Nanog, and Rex1, were higher in heterogeneous cultured cells than that in unpassaged induced cells (Fig. 1D).
Afterward, we randomly picked 10 ESC-like colonies from unpassaged cells and heterogeneous cultured cells by morphological criteria, and then generated 6 or 10 iPSCs, respectively. Some clones derived from unpassaged cells did not form iPSCs by morphological criteria. The efficiency of induction by heterogeneous culture was significantly higher than in unpassaged cultures. These results suggested cell passage might play an important role in early MEF reprogramming.
Identification of unpassaged and passaged cultured iPSCs
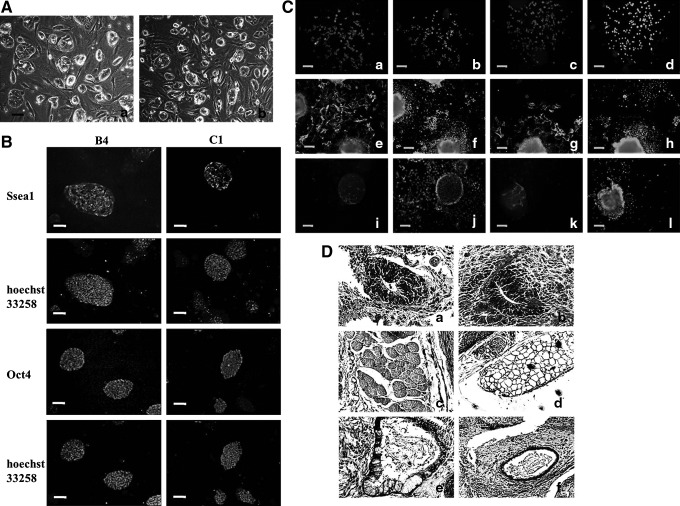
B4 iPSCs cells derived from unpassaged cultured cells and C1 iPSCs from passaged (also called heterogeneous) cultured cells were randomly chosen for further identification. Both B4 and C1 iPSCs had been stably expanded for over 30 passages and maintained a normal karyotype. The two iPSCs lines were morphologically indistinguishable from classic mouse ESCs, displayed typical ESC-like morphology, including a high nucleus-to-cytoplasm ratio, and had clear boundaries (Fig. 2A). They both expressed the typical ESC marker genes Oct4 and Ssea1 (Fig. 2B). After culture in differentiated medium for 8 days, the two iPSC lines expressed the three germ layer markers, Tuj1 (ectoderm), SMA (mesoderm), and AFP (endoderm), as seen by by immunofluorescence (Fig. 2C). Furthermore, the two iPSC lines were injected subcutaneously into nude mice; after 4 weeks, this resulted in teratomas comprising tissues derived from the three germ layers (Fig. 2D). These results showed that the pluripotency of iPSCs was indistinguishable after longer passage.
FIG. 2.
miPSCs are generated from unpassaged and passaged cultured cells. (A) The miPSC colonies were observed after stable expansion for 30 passages. a, B4 iPSCs; b, C1 iPSCs. (B) The expression of SSEA-1 (green) and Oct4 (green) by miPSCs was detected by immunocytochemistry. (C) Immunohistochemistry for germ layer markers. a, AFP (red, B4 iPSCs); c, AFP (red, C1 iPSCs); e, SMA (green, B4 iPSCs); g, SMA (green, C1 iPSCs); i, Tuj1 (red, B4 iPSCs); k, Tuj1(red, C1 iPSCs); b, d, f, h, j, l, nuclei staining (blue). (D) Teratomas containing multiple tissues are analyzed histologically with Hematoxylin & Eosin staining. a, Neural (derived from B4 iPSCs); b, nerviduct (derived from C1 iPSCs); c, skeletal muscle (derived from B4 iPSCs); d, adipose tissue (derived from C1 iPSCs); e, glandular structures (derived from B4 iPSCs); f, glandular structures (derived from C1 iPSCs). Scale bar, 100 μm.
Continuous passage affects the pluripotency of induced cells
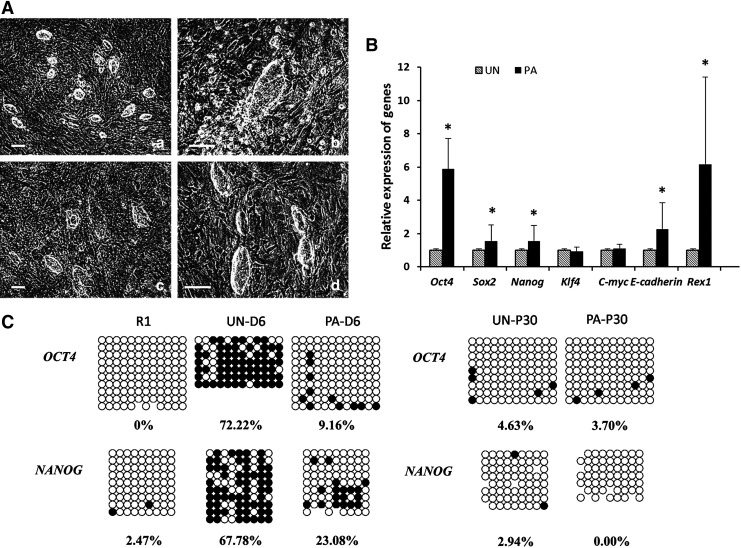
All analysis above suggested that passage could accelerate the process of reprogramming when early iPSCs cells were being established. To verify the effect of passage on the reprogramming, we divided the induced cells into two groups after infection. One group (UN, n=5) was passaged every 3 days after picking ESC-like clones, and the medium was changed every day; the other group (PA, n=5) was passaged after picking ESC-like clones every day. Although all colonies were cultured in the same medium, the morphology of clones in the PA group was different from the UN group after 3 days. All clones of PA group exhibited a round surface with a clear margin whereas some clones of the UN group remained flat or separated into a population of small round cells (Fig. 3A). After 6 days of culture, we examined the expression levels of several pluripotent genes in two groups by real-time PCR. Endogenous Oct4, Sox2, Nanog, Rex1, and E-cadherin were upregulated in the PA group compared with the UN group (Fig. 3B). Afterward, we investigated DNA methylation status at the promoter regions of Oct4 and Nanog in two groups by bisulfite genomic sequence analysis. The data demonstrated that the levels of methylation in the promoter regions of Oct4 and Nanog were decreased during the reprogramming. The extent of demethylation of Oct4 in the PA group was more significant than that of Nanog, which was becoming similar to that in R1 cells (Fig. 3C). However, after culturing for passage 30, both Oct4 and Nanog were nearly absolutely demethylated (Fig. 3C). In this case, continuous passage of miPSCs could promote the expression of pluripotent genes by epigenetic modification.
FIG. 3.
Comparison of unpassaged and continuously passaged cells during early reprogramming. (A) Morphology of unpassaged and continuously passaged cells at day 3 after picking clones. a and b, Unpassaged cells; c and d, passaged cells. (B) Expression levels of Oct4, Sox2, Nanog, Klf4, C-myc, Rex1, and E-cadherin genes in unpassaged (UN group) and continuous passaged cells (PA group) relative to GAPDH (a loading control) as assessed by real-time PCR. The values from unpassaged cells were set to 1. The error bar indicates standard deviation (SD). (C) Methylation analysis of OCT4 and NANOG promoter regions in iPSCs. Genomic DNA from R1ES cells, unpassaged cells, and continuously passaged cells after culturing for 6 days, and unpassaged and continuously passaged cells after culturing for passage 30 days. Open and filled circles indicate unmethylated and methylated CpG dinucleotides, respectively. Scale bar, 100 μm.
Discussion
Previous studies on iPSCs had demonstrated their application not only in the study of stem cell biology, but also in clinical utility (Gandre-Babbe et al., 2013; Hunt, 2011; Wichterle and Przedborski, 2010). However, low efficiency was a significant barrier for generation of iPSCs. Notably, some other reports identified that cellular origin influences the in vitro differentiation potentials of early-passage iPSCs into the other cell types. Correspondingly, it takes a long time for the complete reprogramming converting MEFs to iPSCs. Our study showed rapid upregulation of pluripotent genes by continuous passage of miPSCs during early reprogramming, which suggested that full reprogramming was a gradual process that did not merely end at the point of the activation of endogenous pluripotency-associated genes. The upregulated genes included Oct4, Sox2, and Nanog during continuous-passage culture, especially the high expression of Oct4. There was no significant difference in the expression of c-Myc between the PA group and the UN group. This is in accordance with the previous report that c-Myc is at a low level at the end of reprogramming process, and that it plays a vital role as transcriptional activator (Ohi et al., 2011).
During the process of induction, we found that it was difficult to generate 100% iPSCs by only morphological selection because some ESC-like clones may not be genuine fully reprogrammed cells, which would be time-consuming and a waste of money. However, if the ESC-like clones were picked after culturing for several passages, almost all would generate fully reprogrammed iPSCs; this increased the efficiency of establishing these cells. This suggested that the early passage could accelerate the generation of pluripotency. To verify this hypothesis, we detected the effect of passage on the pluripotency. The results showed that compared with the long-time passage (passage each 3 days), the morphology of induced cells was closer to that of ESCs with clear borders. Molecular analysis showed continuous passage increased the expression of pluripotent gene markers, such as Oct4 and Nanog, which were key genes involved in the regulated network of ESCs. However, pluripotent expression of these genes became similar after long passage (>10 passages). This was in accordance with what was reported previously, that the transcriptional pattern in early-passage iPSCs cells is different from the transcriptional pattern in late-passage iPSCs (Barrero and Izpisua Belmonte, 2011; Lister et al., 2011). This suggested that the effect of passage on pluripotency occurred during the early reprogramming. Furthermore, we investigated the regulatory mechanism of continuous passage. We found that continuous passage increased the level of demethylation of Oct and Nanog, demonstrating that the passage regulates the expression of pluripotent gene markers by epigenetic modification.
Although the induction of iPSCs is a long and unstable process, it can be modified by other methods, such as changing the vectors carrying transcription factors or adding small molecules in induced cells (Mochiduki and Okita, 2012). The research reported here revealed that continuous passage could accelerate reprogramming by increasing the pluripotency in early induced cells, thus providing an advanced strategy for rapid iPSCs generation. Interestingly, we wondered whether partially reprogrammed iPSCs could turn into fully-reprogrammed iPSCs by continuous passage. Future research will focus on this aspect.
In conclusion, our study showed that continuous passage could affect the pluripotency of induced cells and accelerate the process of reprogramming by promoting transcriptional reactivation and modifying epigenetic inheritance. This is a meaningful tool for studying the mechanism of reprogramming of somatic cells.
Supplementary Material
Acknowledgments
The authors are grateful to the laboratory of Professor Liu at North East Agricultural University for their valuable technical assistance. This article is funded by the National Natural Science Foundation of China (grant no. 31000645), the National Natural Science Foundation of China (grant no. 30900413), Ph.D. Programs Foundation of Ministry of Education of China (grant no. 20092307120016 ), and The National Natural Science Heilongjiang Outstanding Youth Fund (grant no. JC200905).
Author Disclosure Statement
No competing financial interests exist.
References
- Banito A., Rashid S.T., Acosta J.C., Li S., Pereira C.F., Geti I., Pinho S., Silva J.C., Azuara V., Walsh M., Vallier L., and Gil J. (2009). Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev 23, 2134–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrero M.J., and Izpisua Belmonte J.C. (2011). iPS cells forgive but do not forget. Nat. Cell Biol. 13, 523–525 [DOI] [PubMed] [Google Scholar]
- Bilic J., and Izpisua Belmonte J.C. (2012). Concise review: Induced pluripotent stem cells versus embryonic stem cells: Close enough or yet too far apart? Stem Cells 30, 33–41 [DOI] [PubMed] [Google Scholar]
- Gandre-Babbe S., Paluru P., Aribeana C., Chou S.T., Bresolin S., Lu L., Sullivan S.K., Tasian S.K., Weng J., Favre H., Choi J.K., French D.L., Loh M.L., and Weiss M.J. (2013). Patient-derived induced pluripotent stem cells recapitulate hematopoietic abnormalities of juvenile myelomonocytic leukemia. Blood 121, 4925–4929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Yang L., Chen L., Wang X., Wu H., Ai Z., Du J., Liu Y., Shi X., Wu Y., Guo Z., and Zhang Y. (2013). Vitamin C facilitates pluripotent stem cell maintenance by promoting pluripotency gene transcription. Biochimie 95, 2107–2113 [DOI] [PubMed] [Google Scholar]
- Ghosh Z., Wilson K.D., Wu Y., Hu S., Quertermous T., and Wu J.C. (2010). Persistent donor cell gene expression among human induced pluripotent stem cells contributes to differences with human embryonic stem cells. PLoS One 5, e8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J., Saha K., Pando B., van Zon J., Lengner C.J., Creyghton M.P., van Oudenaarden A., and Jaenisch R. (2009). Direct cell reprogramming is a stochastic process amenable to acceleration. Nature 462, 595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J., Cheng A.W., Saha K., Kim J., Lengner C.J., Soldner F., Cassady J.P., Muffat J., Carey B.W., and Jaenisch R. (2010). Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc. Natl. Acad. Sci. USA 107, 9222–9227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt K.J., Shamis Y., Hayman R.B., Margvelashvili M., Dong S., Carlson M.W., and Garlick J.A. (2011). Epigenetic and phenotypic profile of fibroblasts derived from induced pluripotent stem cells. PLoS One 6, e17128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C.J. (2011). Cryopreservation of human stem cells for clinical application: A review. Transfus. Med. Hemother. 38, 107–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou Z., Kang L., Yuan Y., Tao Y., Zhang Y., Wu T., He J., Wang J., Liu Z., and Gao S. (2010). Mice cloned from induced pluripotent stem cells (iPSCs). Biol. Reprod. 83, 238–243 [DOI] [PubMed] [Google Scholar]
- Lin S.L., and Ying S.Y. (2012). Mechanism and method for generating tumor-free iPS cells using intronic microRNA miR-302 induction. Methods Mol. Biol. 936, 295–312 [DOI] [PubMed] [Google Scholar]
- Lister R., Pelizzola M., Kida Y.S., Hawkins R.D., Nery J.R., Hon G., Antosiewicz-Bourget J., O'Malley R., Castanon R., Klugman S., Downes M., Yu R., Stewart R., Ren B., Thomson J.A., Evans R.M., and Ecker J.R. (2011). Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature 471, 68–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Luo G.Z., Yang W., Zhao X., Zheng Q., Lv Z., Li W., Wu H.J., Wang L., Wang X.J., and Zhou Q. (2010). Activation of the imprinted Dlk1-Dio3 region correlates with pluripotency levels of mouse stem cells. J. Biol. Chem. 285, 19483–19490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo I.M., Fleischer A., and Bachiller D. (2012). Generation of mouse and human induced pluripotent stem cells (iPSC) from primary somatic cells. Stem Cell Rev. 9, 435–450 [DOI] [PubMed] [Google Scholar]
- Mochiduki Y., and Okita K. (2012). Methods for iPS cell generation for basic research and clinical applications. Biotechnol. J. 7, 789–797 [DOI] [PubMed] [Google Scholar]
- Ohi Y., Qin H., Hong C., Blouin L., Polo J.M., Guo T., Qi Z., Downey S.L., Manos P.D., Rossi D.J., Yu J., Hebrok M., Hochedlinger K., Costello J.F., Song J.S., and Ramalho-Santos M. (2011). Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nat. Cell. Biol. 13, 541–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack J.M. (2009). Metaplasia and somatic cell reprogramming. J. Pathol. 217, 161–168 [DOI] [PubMed] [Google Scholar]
- Smith Z.D., Nachman I., Regev A., and Meissner A. (2010). Dynamic single-cell imaging of direct reprogramming reveals an early specifying event. Nat. Biotechnol. 28, 521–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Wang L., Huang W., Qin D., Cai J., Yao X., Feng C., Li Z., Wang Y., So K.F., Pan G., Wu W., and Pei D. (2013). Immediate expression of Cdh2 is essential for efficient neural differentiation of mouse induced pluripotent stem cells. Stem Cell Res. 10, 338–348 [DOI] [PubMed] [Google Scholar]
- Sullivan G.J., Bai Y., Fletcher J., and Wilmut I. (2010). Induced pluripotent stem cells: epigenetic memories and practical implications. Mol. Hum. Reprod. 16, 880–885 [DOI] [PubMed] [Google Scholar]
- Takahashi K., and Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 [DOI] [PubMed] [Google Scholar]
- Vierbuchen T., and Wernig M. (2012). Molecular roadblocks for cellular reprogramming. Mol. Cell 47, 827–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H., and Przedborski S. (2010). What can pluripotent stem cells teach us about neurodegenerative diseases? Nat. Neurosci. 13, 800–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.