Abstract
Hearing loss is a serious burden to physical and mental health worldwide. Aberrant development and damage of hearing organs are recognized as the causes of hearing loss, the molecular mechanisms underlining these pathological processes remain elusive. Investigation of new molecular mechanisms involved in proliferation, differentiation, migration and maintenance of neuromast primordium and hair cells will contribute to better understanding of hearing loss pathology. This knowledge will enable the development of protective agents and mechanism study of drug ototoxicity. In this study, we demonstrate that the zebrafish gene miles-apart, a homolog of sphingosine-1-phosphate receptor 2 (s1pr2) in mammals, has an important role in the development of otic vesicle, neuromasts and survival of hair cells. Whole-mount in situ hybridization of embryos showed that miles-apart expression occurred mainly in the encephalic region and the somites at 24 h.p.f. (hour post fertilization), in the midbrain/hindbrain boundary, the brainstem and the pre-neuromast of lateral line at 48 h.p.f. in a strict spatiotemporal regulation. Both up- and downregulation of miles-apart led to abnormal otoliths and semicircular canals, excess or few hair cells and neuromasts, and their disarranged depositions in the lateral lines. Miles-apart (Mil) dysregulation also caused abnormal expression of hearing-associated genes, including hmx2, fgf3, fgf8a, foxi1, otop1, pax2.1 and tmieb during zebrafish organogenesis. Moreover, in larvae miles-apart gene knockdown significantly upregulated proapoptotic gene zBax2 and downregulated prosurvival gene zMcl1b; in contrast, the level of zBax2 was decreased and of zMcl1b enhanced by miles-apart overexpression. Collectively, Mil activity is linked to organization and number decision of hair cells within a neuromast, also to deposition of neuromasts and formation of otic vesicle during zebrafish organogenesis. At the larva stage, Mil as an upstream regulator of bcl-2 gene family has a role in protection of hair cells against apoptosis by promoting expression of prosurvival gene zMcl1b and suppressing proapoptotic gene zBax2.
Keywords: miles-apart, hair cells, neuromast, otoliths, hearing-associated genes, apoptosis
Hearing loss not only impairs personal physical and mental health but also increases a social and economic burden. Hearing damage can be caused by a wide range of factors, which include defects of hair cells and inner ear structures, but the defect molecular targets remain elusive. Thus, identification of new molecular mechanisms associated with developmental processes of hearing organs such as migration of the neuromast primordium, formation of inner ear, differentiation and maintenance of hair cells, as well as hair cell apoptosis, will contribute to a better understanding of hearing loss pathology. This knowledge developing will facilitate the development of new medicines to prevent ototoxicity and protect hearing.
Sphingosine-1-phosphate (S1P) is a lipid-active mediator and signal molecule, in addition to being a component of the cell membrane. It is involved in regulating multiple physiological functions, including cell movement, migration, growth and survival, vasculogenesis, nervous and immune system function, reproduction, lymphocyte transfer and audition.1, 2, 3 S1P is a ligand for five human G protein-coupled receptors (GPCRs), called S1P1, S1P2, S1P3, S1P4 and S1P5. Dissimilarly, mice possess only three receptors: S1p1, S1p2 and S1p3. Though they are all coupled with G-proteins, the S1P receptors do not demonstrate functional redundancy. Interactions among the receptors are mediated by tyrosine kinase receptors.4 Transduction of S1P signaling occurs mostly by combining with Edg family receptors.5, 6, 7 Although most reports of S1P receptor function relate to the cardiovascular, nervous and immune systems,8, 9, 10, 11 there is some evidence that S1P receptors are related to the function of cochlea and vestibular apparatus. Herr et al.2 found that S1p1, S1p2 and S1p3 are expressed in mouse cochlea, and that S1p2 levels were higher than those of S1p1 and S1p3, and upregulated along with the developmental process. S1p2-knockout mice had normal cochlear and vestibular development initially, but rapid degeneration of these structures led to deep deafness by 22 days of age and regressive lost of vestibular function with their age growth. S1p2/S1p3 double knockout caused increased perinatal lethality and decreased litter size. As in S1p2-knockout mice, significant and progressive degeneration of auditory and vestibular organs also occurred in S1p2/S1p3 double knockout mice with age, culminating in complete hearing loss.1, 2 These results indicate that S1p receptors have important roles in maintaining hair cell functions in mice.
In zebrafish, mutation of the S1P2 ortholog, miles-apart (Mil), resulted in a cardia bifida phenotype. This defect in cardiac development is due to failed migration of heart precursor cells to the midline, revealing an important function of the S1P2 receptor in directing cardio-primordium localization and cardiac tube fusion in zebrafish. Loss of Mil function also caused edematous pericardial sac and epithelium-like blistering in the tail.5, 8, 10 However, there are no reports for zebrafish on the role of Mil in the development of auditory and vestibular organs, such as hair cells and neuromasts. Zebrafish hair cells are similar to their mammalian counterparts in both morphology and function, and reside inside the otic vesicles and in the neuromasts at the lateral line system on the body surface. These characteristics make zebrafish a good model system to study the effects of drug toxicity on hearing and protection and regeneration of hair cells.1, 2 In the zebrafish embryonic stage each otic capsule contains two maculae: the anterior utriculus has a vestibular function, and the posterior sense macula (saccule) has a role in hearing. Each macula is covered by an otolith, which is attached to a kinocilium of hair cells. Semicircular canals begin to form by 48 h.p.f. (hour post fertilization). The number of hair cells in maculae and lateral lines increases rapidly from 3 to 7 days post fertilization (d.p.f.), and by 7 d.p.f. all the key structures have emerged (the lagena, a major auditory macula, does not form until several days later). Neuromasts in the lateral line on the body surface are very similar in structure and function to the sensory maculae of the inner ear.12 These functions are mainly dependent on the physiological activity of the hair cells.
In this study, we explored the functions of zebrafish miles-apart in hair cell and neuromast development and survival and report the first association of miles-apart activity with the development of neuromasts, hair cells and otic vesicles, as well as with the expression of auditory-related genes and bcl-2 gene family. We infer that Mil may be an important regulator for the formation and maintenance of hearing organs in zebrafish. Our findings will contribute to a better understanding of the processes of hearing development and facilitate research of otoprotective agents.
Results
Expression pattern of miles-apart during zebrafish organogenesis
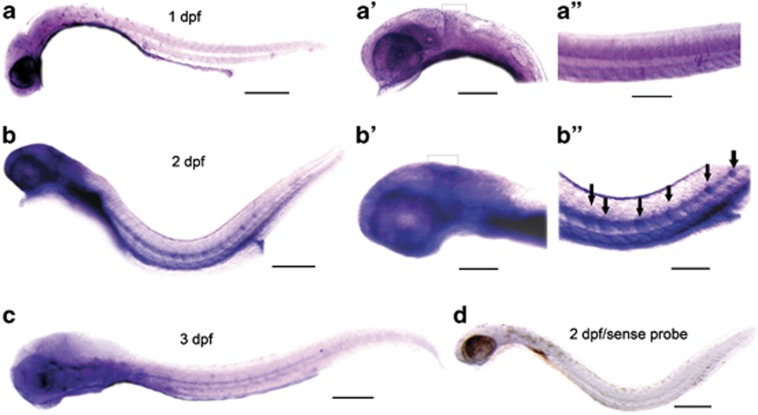
Whole-mount in situ hybridization was used to detect miles-apart spatiotemporal expression pattern during zebrafish embryonic development. The miles-apart expression was mainly aggregated in the encephalic region, particularly in the midbrain/hindbrain boundary and the ventral hindbrain; and weak expression also was observed in somites but not in notochord at 24 h.p.f. (Figures 1a, a′ and a′′). At the pharyngula stage (48 h.p.f.), the miles-apart transcription focused on the midbrain/hindbrain boundary, midbrain, ventral hindbrain area, and in floor plate of the neural tube and vasculature, particularly appeared in a regular and discrete pattern at the positions of the future neuromasts on the lateral lines (Figures 1b, b′ and b′′). However, at the hatching period (72 h.p.f.), the miles-apart mRNA was not detected in the lateral lines and in the posterior floor plate but was still highly expressed in the midbrain/hindbrain boundary, the brainstem and the floor plate (Figure 1c). These results suggest that the miles-apart expression pattern is correlated to development of the nervous system, including the brain, the central nervous tube and the neural sensory system in zebrafish.
Figure 1.
Expression pattern of miles-apart during organogenesis in zebrafish. Hybridization in situ was performed with a miles-apart antisense RNA probe in (a), (b) and (c); miles-apart sense RNA probe was used as a negative control probe in (d). The ventral parts were removed from the embryos for primary presentation of the heads and the trunks. The whole bodies were showed at 1 d.p.f. (a), 2 d.p.f. (b) and 3 d.p.f. (c); the corresponding heads were showed in (a′) and (b′), and their trunks were showed in (a′′) and (b′′). Black arrows indicate the scheduled neuromast positions at the post lateral lines in (b′′). Black half brackets signed the mid/hindbrain boundary in (a′) and (b′). In all the images, the head is toward the left, the tail to the right; the dorsal at the upside, the ventral at the bottom. Scale bars represent 310 μm in (a) and (b), 330 μm in (c), 380 μm in (d), 160 μm in (a′), (b′), 130 μm in (a′′), 180 μm in (b′′)
Dysregulation of miles-apart expression disturbs development of hair cells and otic vesicles
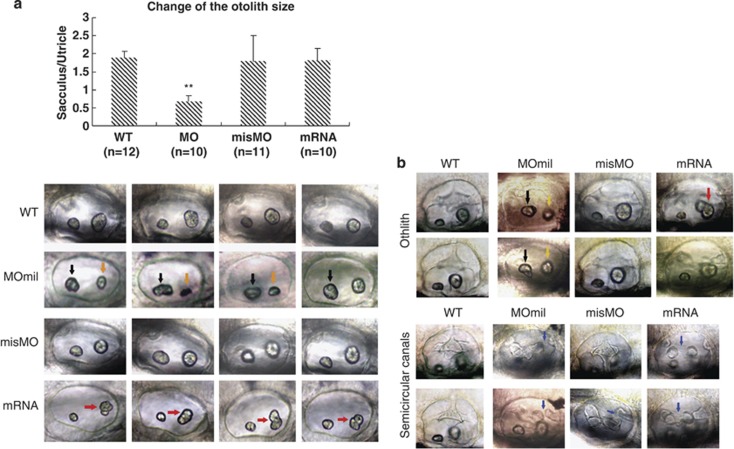
Using the indication that high miles-apart expression occurred in the ventral forebrain and midbrain, midbrain/hindbrain boundary, brainstem and the lateral line neuromasts in 48 h.p.f. WT embryos, we hypothesized that Mil functions may be associated with the formation of neuromasts and hair cells beside its function in development of the central nervous system. We investigated Mil function on development of the otic vesicle in zebrafish embryo using down- and upregulation of miles-apart expression. An antisense morpholino oligo (MOmil) binding to the miles-apart translation initiation site was used for inhibition of miles-apart translation by injection into embryos at the one- to four-cell stage. The morphant embryos displayed aberrant otic vesicle and otoliths that utricles became non-oval, disfigure and bigger than saccule; and in contrast, saccule morphous presented small, deficient and non-spherical structures at 48 h.p.f. (9/10). The size ratio of saccule to utricle changed significantly compared with the WT ratio (Figure 2a). The semicircular canals had defect structure such as a gap or breakage developed between anterior semicircular canal (psc) and posterior semicircular canal (psc), beside the otolith abnormality in 72 h.p.f. embryos (11/15) (Figure 2b). Correspondingly, capped mRNA of Mil was injected into embryos at the same stage for miles-apart overexpression. In these embryos, the utricle appeared shrunk and smaller, and saccular otolith was deformed and divided at 48 h.p.f. (10/10) (Figure 2a). At 72 h.p.f., the middle junction of the semicircular canals was deficient, or even all the semicircular canals totally absent (7/11) (Figure 2b). These data suggest that miles-apart gene dysregulation could considerably result in mis-patterning of the otic vesicle and abnormal structure of otoliths.
Figure 2.
Developmental defects of otoliths and semicircular canals caused by miles-apart dysregulation. Embryos were injected with 40 μM MOmil or 40 ng/ul mil-mRNA at the one-to four-cell stage and were observed at 2 d.p.f. (a) and 3 d.p.f. (b). Compared with WT embryos, abnormal otolith shapes emerged in the morphants at 2 d.p.f., such as enlarged and non-oval utricles (black arrows), and shrink sacculus (orange arrows) by MOmil injection (9/10). Although in misMO control group otoliths present shape (12/13) similar to that in WT (10/10). Embryos treated with miles-apart mRNA injection exhibit deformed, divided or syncytium-like sacculus (red arrows) and smaller utricles (black arrowhead) by miles-apart mRNA injection (11/12). A histogram indicates that variation of otolith sizes caused by miles-apart gene knockdown or overexpression using otolith area scanning, in which area ratio of sacculus to utricle was used as a sign. The means and S.D.'s are derived from the specified otic vesicles. **P-values <0.01. At 3 d.p.f. embryos (b) the top panel shows that the WT embryos developed normal semicircular canals, but deficient semicircular canals were appeared at the posterior part in the morphants (11/15), and deformation and missing occurred at the middle part of semicircular canals with mil-mRNA injection group (7/11). Few of the misMO group had the defect (3/13). Blue arrows show deficient semicircular canals. In addition, the enlarged non-oval utricles (black arrows) and shrink sacculus (orange arrows) by MOmil injection (11/15) and abnormal sacculuses by miles-apart mRNA injection (5/11, red arrows) were seen at 3 d.p.f. The images are left lateral view
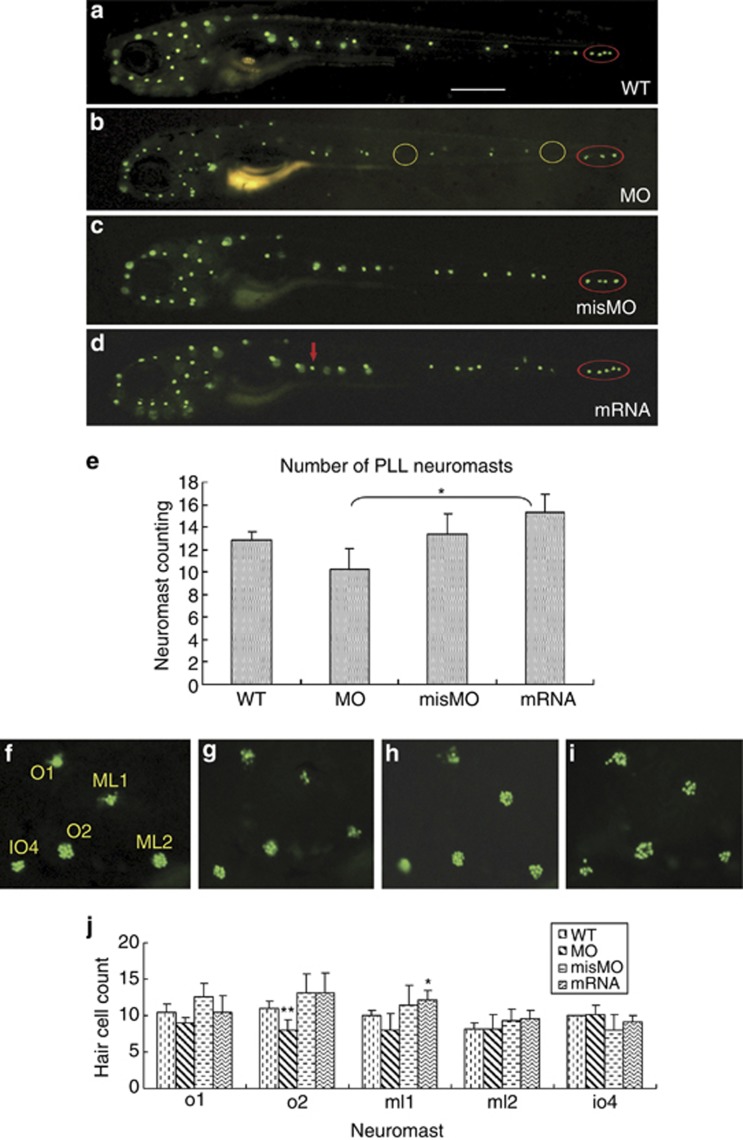
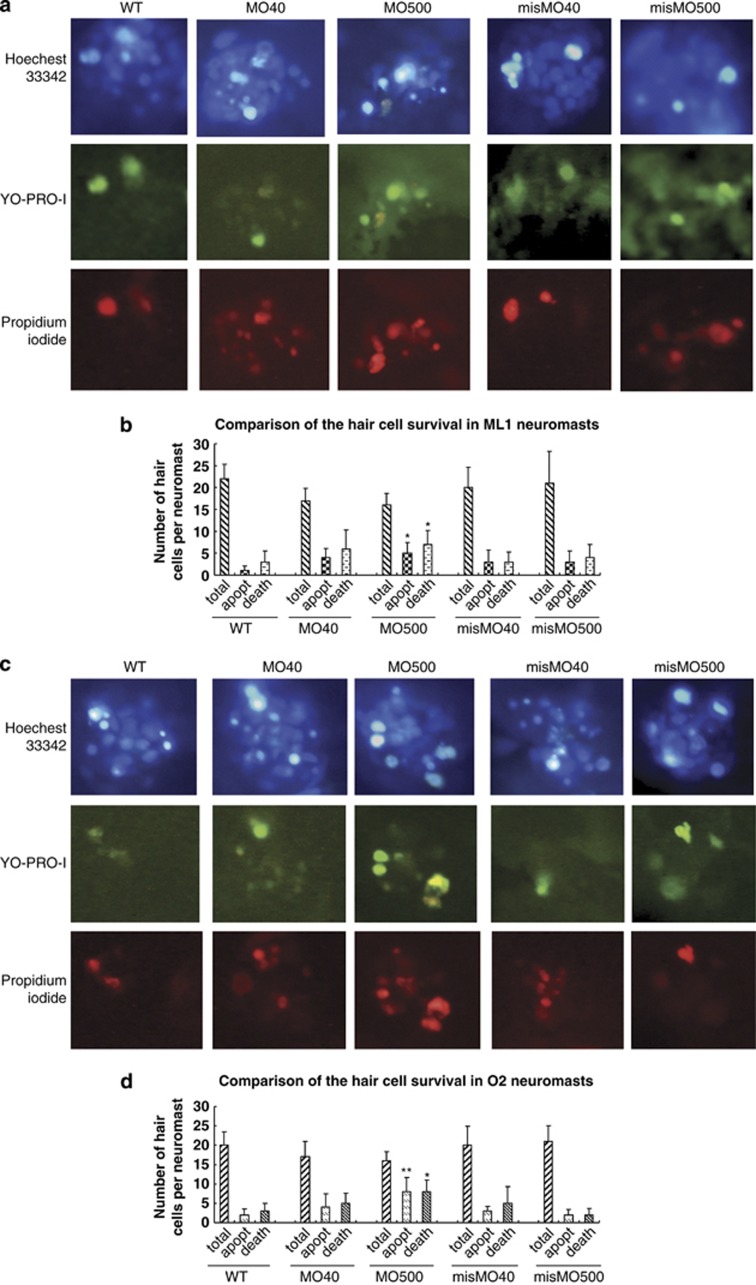
Hair cell staining by YO-PRO-1 was used to examine the impact of miles-apart on the formation and deposition of hair cells and neuromasts in the lateral lines on the surface of zebrafish head and body. The results show that in the 6 d.p.f. morphants at the posterior lateral line several neuromasts were absent or shrunken (Figure 3b); and at the anterior lateral line number of the hair cells in neuromast orbital 1 (o1), middle lateral line 1 (ml1), middle lateral line 1 (ml2), orbital 2 (o2) and infraorbital 4 (io4), which resided at the otic vesicles around, were counted and found that the hair cells were obviously diminished in neuromast o1 and o2 (Figures 3g and j), as opposed to the ordered hair cell aggregation in the WT group and in the mismatched MO (misMO) control group (Figures 3f and h). However, when miles-apart expression was upregulated by mRNA injection, excess neuromasts in the posterior lateral line and ectopic and fluffy hair cells in the five neuromasts around the otic vesicle were observed (Figures 3d, e, I and j). Statistical analysis presents a significant difference of the neuromast numbers between the morphants and mRNA-injected larvae but not between WT larvae and the three injection groups, respectively (Figure 3e). In addition, compared with the WT group the hair cell number of o1 and o2 in the morphants and of ml1 in the mil overexpressed larvae were changed significantly (Figure 3j). These data suggest that one of Mil functions probably is involved in controlling both the number and organization of hair cells in a neuromast, and also in controlling the number and localization of neuromasts in the lateral lines.
Figure 3.
Formation of hair cells and neuromasts disturbed by miles-apart up- or downregulation. All the images were shot at 6 d.p.f. (a) A WT larva shows the normal pattern of neuromasts in the head and in the posterior lateral line. (b) A morphant exhibits small and deficient neuromasts and neuromast-missing positions (yellow rings) on the lateral line. (c) A control larva injected misMO shows the neuromast distribution similar to WT. (d) miles-apart mRNA injection caused extra neuromasts developed in the posterior lateral line (a red arrow and a red cycle). (e) A histogram shows number change of PLL neuromasts. The means and s.d.'s are derived from five larvae. (f) Morphology of five normal neuromasts with well-organized hair cell clusters at an otic vesicle of a WT larva. (g) Fewer, disarranged and deficient hair cells of the five neuromasts around an otic vesicle of a morphant. (h) A larva injected misMO shows the hair cell clusters similar to those of WT. (i) Scattered and excess hair cells in the neuromasts occurred in mRNA-injected larvae. Names of the five neuromasts at the otic vesicle are labeled in f as o1 for orbital 1, ml1 for middle lateral line 1, ml2 for middle lateral line 2, o2 for orbital 2 and io4 for infraorbital 4. Scale bars represent 310 μm in (a–d). Hair cells were stained with dye YO-PRO-1 in (a–d), and (f–i). (j) A histogram indicates variation of hair cell counts by miles-apart gene knockdown or overexpression. The means and S.D's are derived from five larvae. *P-values <0.05, **P-values <0.01
Dysregulation of miles-apart affects expression of hearing-associated genes in zebrafish embryo
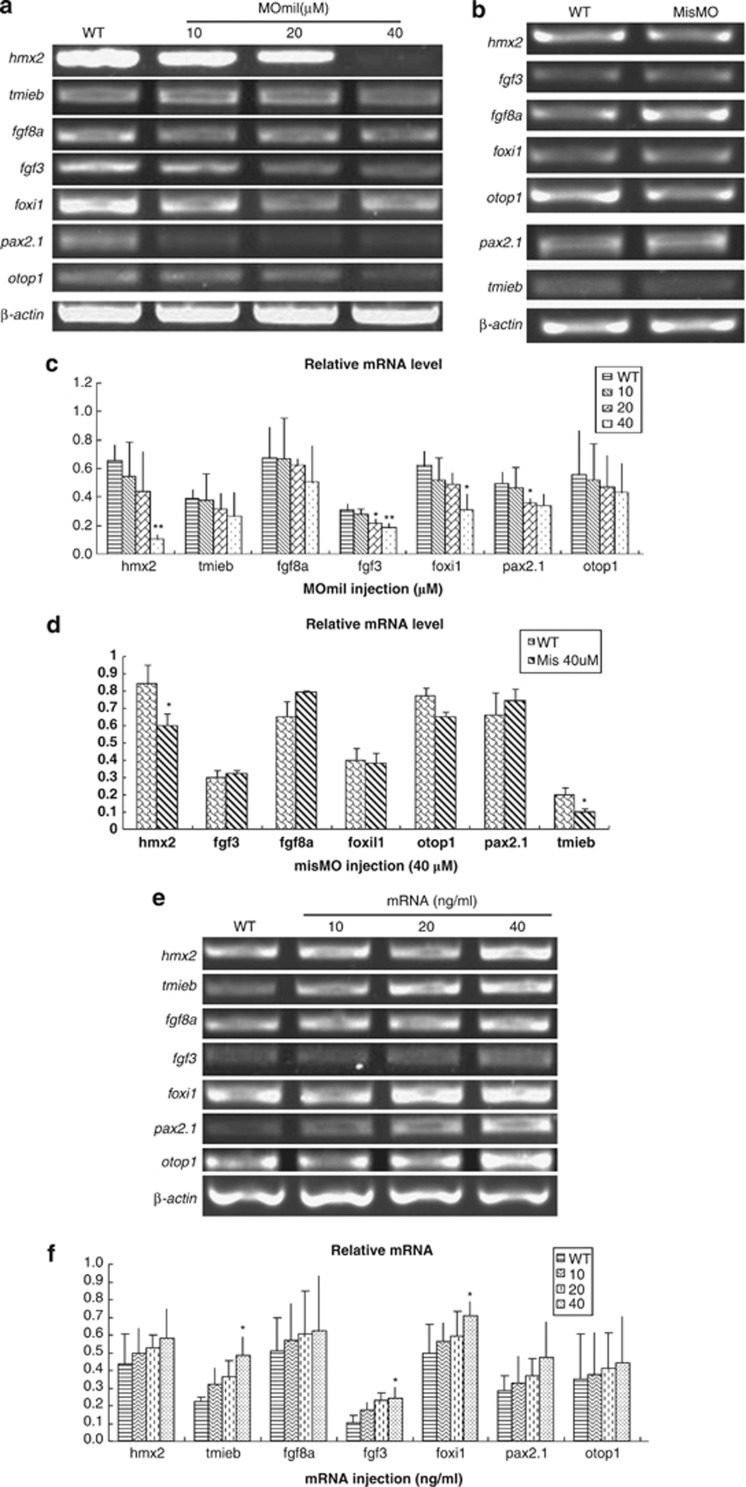
In order to explore whether miles-apart expression is correlated with genes that involved in the developing of hair cells and neuromasts, we examined expression levels of hearing-related genes such as hmx2, fgf3, fgf8a, foxi1, otop1, pax2.1 and tmieb, in 48 h.p.f. embryos. This time point corresponds to the stage while the otic vesicle is developing and the pro-neuromast primordium begins to migrate. The results show that transcription of these genes was reduced in a concentration-dependent manner in MOmil-injected embryos, and four of them hmx2, fgf3, foxi1 and pax2.1 reduced in statistical significance (Figures 4a and c). As injection mock, in the misMO group these gene levels slightly went up or down, and hmx2 and tmieb showed descent significantly (Figures 4b and d). Conversely, these gene levels were elevated in the mRNA-injected embryos and the elevation of fgf3, foxi1 and tmieb are in statistical significance (Figures 4e and f). So, we suppose that these genes may be effectors of Mil activity for the development and functions of hearing organs.
Figure 4.
Expression of hearing-associated genes was down- or upregulated by miles-apart in zebrafish larvae. Transcription of the hearing-associated genes hmx2, fgf3, fgf8a, foxi1, otop1, pax2.1 and tmieb was examined by RT-PCR in 48 h.p.f. embryos, and was found to be reduced in a concentration-dependent manner in morphants (a and c) and elevated in miles-apart mRNA-injected embryos (e and f). The upper panels show representative RT-PCR electrophoresis results for MOmil (a) and miles-apart mRNA-injected groups (e), respectively, and the corresponding histograms from density-scanning of triplicate results of the RT-PCR in (c and f). The mock control group was injected misMO and showed in (b) and (d). *P-values <0.05, **P-values <0.01
Survival of hair cells may be associated with miles-apart function by suppressing zBax2 and promoting zMcl1b
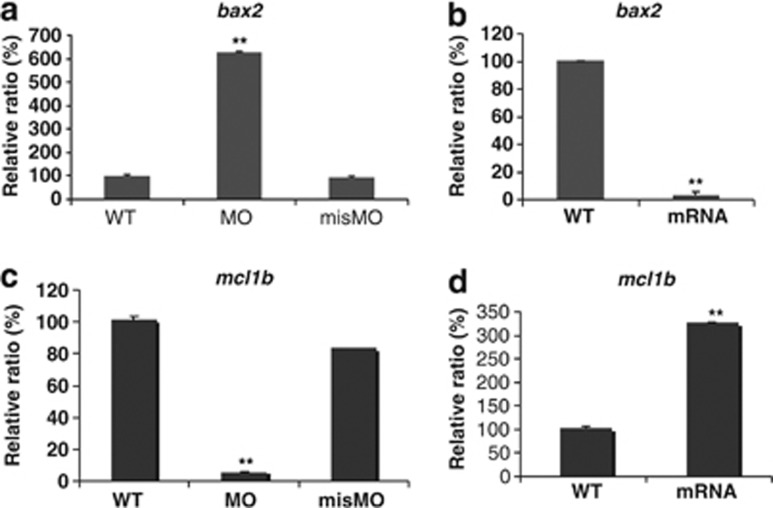
Respecting deficiency of the hair cells and otic vesicles resulted from MOmil injecton, we asked whether miles-apart knockdown prompted hair cell death and which mechanism was involved in? Survival of the larva hair cells was detected using an apoptosis staining kit (Invitrogen, Carlsbad, CA, USA) with the three dyes. Hair cells in neuromast ml1 and o2 were counted and analyzed by statistics. The results show that compared with WT hair cells, number of live hair cells in 6 d.p.f. morphants decreased with dimly stained by Hoechst33324, the apoptotic hair cells with condensed chromatin increased by stained brightly by Hoechst33324 and green fluorescence by YO-PRO-1 dye, and the dead hair cells also grew up with primarily red fluorescence and some green fluorescence in a concentration-dependent manner (Figure 5). Meanwhile, several apoptosis/prosurvival genes including bcl-2 family and caspase family were detected using qPCR. Significantly, zMcl1b, a prosurvival gene was downregulated and a proapoptotic gene, zBax2, was upregulated in the morphants. In contrast, significantly too, zMcl1b was increased and zBax2 decreased in the larva with mil-mRNA injection (Figure 6). So, we suppose that miles-apart knockdown may have sensitized the hair cell to apoptosis by enhancing zBax2 expression and suppressing zMcl1b expression. One of Mil functions is probably involved in hair cell survival.
Figure 5.
Apoptosis of the hair cells was prompted by mil knockdown. Zebrafish larvae at 6 d.p.f. were stained in the three dye mixed solution for 30 min at 28 °C, following the kit instruction. (a) Images of neuromast ml1 in the larvae injected by MOmil at 40 or 500 μM, and by misMO 40 or 500 μM, separately, compared with WT ml1 neuromast. (b) A corresponding histogram for Figure 5a. (c) Images of neuromast O2 treated as the same as the neuromast ml1 by MOmil and misMO, separately. (d) A corresponding histogram for Figure 5c. *P-values <0.05, **P-values <0.01
Figure 6.
Mil may be involved in the regulation of proapoptotic/prosurvival gene expression. The larvae at 6 d.p.f. (for MO and misMO groups) and at 4 d.p.f. (for mRNA group) were collected to examine transcription level of zBax2 and zMcl1b by qPCR. The proapoptotic gene Bax2 mRNA was notably elevated by MOmil injection (a) and reduced by mil-mRNA injection (b) compared with WT and misMO injection. Oppositely, the prosurvival gene Mcl1b was significantly downregulated in the morphants (c) and upregulated in the larvae with mil-mRNA injection (d) **P-values <0.01
Discussion
The inner ear and lateral line systems are important organs for hearing and sensing fluid flow or pressure waves associated with the approach of other animals in vertebrates. The development of inner ear and lateral line system is involve in spatiotemporal patterning of hearing-associated gene expression, expansion of the otic vesicle signaling cascade, cell migration and interaction with neighboring tissues.13, 14 Previous studies of knockout mice showed that the loss of S1p2 disturbed mouse hearing, thus concluding that the S1p2 has an important physiological role for survival and functional maintenance of hair cells in mice.1, 2 However, the role of the zebrafish S1p2 ortholog, Mil, in hair cell function remains to be investigated. In this study, we observed miles-apart expression in the encephalic region including the hindbrain where the otic capsule resides, and at the typical neuromast preset positions in the lateral lines of 48 h.p.f. WT embryos. This is likelihood to predict the future neuromast deposition in the lateral lines. Between 48 and 72 h.p.f., miles-apart expression disappeared from the embryonic trunk, suggesting that miles-apart is subjected to rigorous spatiotemporal regulation for the lateral line system. Using MOmil-mediated gene knockdown and miles-apart capped-mRNA overexpression, a role for Mil in organization and maintenance of hair cells, neuromasts and otic vesicle organ was identified. Further, we explored potential miles-apart targeting genes that are known to be associated with the development and function of the otic vesicle and hair cells. Seven hearing-related genes were found to be coincidentally regulated by miles-apart level variation. They were upregulated, whereas Mil was overexpressed and reduced when miles-apart was knocked down. Thus, we infer that Mil can be an important upstream regulator to the seven genes. In view of the literatures, fgf3 and fgf8 are required for inner ear and hindbrain patterning, induction of otic placode and migration of PLL primordia.14, 15, 16, 17 foxi1 is the earliest marker for otic precursor cells and otic placode formation,18 whereas pax2.1 were essential for the induction of functional hearing cells in both the inner ear and the lateral lines.19, 20 Notably, pax2.1 is specifically expressed in hair cells during differentiation, and is also maintained at high levels in mature hair cells, but is not expressed in support cells.19 hmx2 is involved in the formation of mechanosensory neuromasts,21 and otop1 was identified as being required for otolith initiation and development of otolith size and shape.16, 22 And tmieb was identified as being involved in the functioning of both auditory and vestibular mechanoelectrical transduction.23 These data support the inference in this study that miles-apart gene activity is likely linked to hearing organ development and is probably mediated by the above hearing-associated genes.
Previous research showed that Mil was responsible for directing migration and fusion of cardiac precursor cells at the heart field and for subsequent differentiation into a functional organ in cardiovascular development.5, 8, 24 In the present study, we find that Mil function may be partly involved in migration and development of hair cell precursors and pro-neuromast primodia in ALL and PLL (Figure 3). The functional concordance of Mil in divergent organs suggests a basic common role of Mil in directing precursor cell migrating and positioning in zebrafish organogenesis.
Our results also show that knockdown of miles-apart gene increased the number of apoptotic hair cells and dead hair cells, decreased hair cell survival and caused otic vesicle structure damaged. These phenomena are similar to that of cochlear and vestibular degeneration in S1p2-knockout mice.2 Moreover, the significant results that proapoptotic gene zBax2 and prosurvival gene zMcl1b can be tightly regulated by Mil indicate that Mil may be an upstream coregulator for zBax2 and zMcl1b gene expression. As we know, zMcl and zBax2 interactions regulate the survival/apoptosis pathway in zebrafish. Kratz et al.25 reported that zMcl-1a and zMcl1b function as gatekeepers against apoptosis induction by stimulation of the extrinsic pathway. Knockdown of zMcl-1a and zMcl-1b decreases viability of zebrafish embryos and sensitizes zebrafish embryos to apoptosis induction. zBax2 was homologous to mammalian Bax in sequence and synteny, but functional conservation with human Bak. Coincidentally, in human healthy cells, Bak keeps a silent proapoptotic role by associating with Mcl-1 and Bcl-x(L); releasing of Bak can activate apoptosis process.26 On the basis of these literatures and our current results, we propose a model of Mil mechanism underlining the development and survival of hair cells and neuromast of the lateral lines in zebrafish. During the embryonic organogenesis stage, Mil as a position signal guides the migration and deposition of pro-neuromast primodia at correct positions in ALL and PLL; Mil knockdown led to deficient deposition signals and reduced neuromasts at the lateral lines, which just as a Mil mutant failed to direct cardiomyocytes to migrate to the midline.5 Meanwhile, Mil also participates in activating the expression of hearing genes that subsequently induce development of the otic vesicles and differentiation of pro-neuromast primodia into neuromast and hair cells. Since 2 days post hatching, zebrafish larvae begin to live independently with some behavioral capacity including swim, hiding and looking for food, which means their functional balance and hearing emerged. Execution of these functions is guaranteed by enough normal hair cells and the normal lateral lines. Mil, probably as an upstream regulator of bcl-2 gene family, protects hair cells against apoptosis by upregulating expression of prosurvival gene zMcl1b and downregulating proapoptotic gene zBax2. Knockdown of miles-apart gene can produce excess zBAX2 and deficient zMCL1B, which leads to plenty of free zBAX2 that sensitizes hair cells to apoptosis.
This study first provides evidence that miles-apart gene function is involved in development of the otic vesicles and the lateral line of hair cells and neuromasts in zebrafish, and that change of the Mil level can regulate expression of bcl-2 family gene zBax2 and Mcl1B.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Regulation for the Management of Laboratory Animals of the Ministry of Science and Technology of China. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences (IMBF20060302).
Zebrafish maintenance and embryo collection
Zebrafish (Danio rerio) WT Tuebingen strain was originally obtained from the College of Life Sciences, Beijing University. The fish were raised at 28.5±1 °C in a 14/10 h light–dark cycle.27 Embryos were obtained by natural mating; synchronous embryos at the appropriate stage were collected and recorded by h.p.f. and d.p.f.28 Embryos were fixed with 4% paraformaldehyde in phosphate-buffered saline for in situ hybridization or immersed in Trizol reagent (Invitrogen) for mRNA isolation. Pigmentation in embryos past 24 h.p.f. was prevented by incubating in phenylthiourea (PTU) (Sigma-Aldrich, Saint Louis, MO, USA) before being fixed.
Synthesis of MOs and miles-apart capped-mRNA
Antisense morpholino oligonucleotides of MOmil and misMO were designed and bought from Genetools (Philomath, OR, USA; http://www.gene-tools.com). MOmil was used for the inhibition of miles-apart translation by binding to miles-apart initiation sites, with the sequence 5′-CCGCAAACAGACGGCAAGTAGTCAT-3′. The control misMO' was designed for negative injection control in which five bases were missense and labeled with underline in sequence 5′-CCCCAAACACACCGCAACTACTCAT-3′. Both MOs were redissolved at a stock solution of 1.0 μmol/ml in ddH2O.
The full-length miles-apart cDNA sequence was cloned from 24 h.p.f. WT embryos using Reverse Transcriptase polymerase chain reaction (RT-PCR) and primers designed according to the zebrafish miles-apart sequence logged in GenBank (NM_001159970), and was identified by sequencing (Sangon Biotech, Shanghai, China). This cDNA was used as a template for miles-apart capped mRNA synthesis using an Ambion mMESSAGE mMACHINE T3 kit (Applied Biosystems, Foster City, CA, USA), and the product was tested for quality and yield by electrophoresis.
Microinjection
For hair cell staining, 1 nl of 20 μM MOmil or 20 ng/μl miles-apart capped mRNA was separately injected into WT embryos at one- to four-cell stage; at least five larvae at 6 d.p.f. were used for counting of hair cells of the neuromasts o1, o2, ml1, ml2 and io4 and for counting of neuromasts at the post lateral lines. For otic capsule observation of living larvae and counting of hair cells, 1 nl of 20 μM MOmil or 20 ng/μl miles-apart capped mRNA were injected into embryos at the same stage. For apoptosis staining of hair cells, 1 nl of MOmil at 40 μM and 500 μM, and misMO at the same concentrations, were respectively injected into one- to four-cell embryos; then the larvae were collected at 6 d.p.f. For RT-PCR analysis of hearing-related genes, 1 nl of 10, 20 or 40 μM MOmil, and 10, 20 or 40 ng/μl miles-apart capped mRNA were injected at the same embryonic stage. The embryos were incubated and collected at 48 h.p.f. For qPCR analysis of zBax2 and zMcl1b, 1 nl of 40 μM MOmil and 50 ng/μl miles-apart capped mRNA were separately injected at the same embryonic stage. The embryos were incubated at the same condition above.
Observation of otic vesicles
The morphous and structure of otic vesicles in WT, MOmil- and miles-apart mRNA-injected zebrafish embryos at 48 and 72 h.p.f. were observed under a differential interference contrast IX71 microscope (Olympus, Tokyo, Japan) and recorded by a 500D digital camera (Canon, Tokyo, Japan). Each group has more than 10 larvae for otolith counting and histogram.
Whole-mount in situ hybridization
For in vitro synthesis of the RNA probe, miles-apart gene sequence was inserted into pBluescript KS (Agilent, Santa Clara, CA, USA) then used as a template for miles-apart sense and antisense RNA probe synthesis using a DIG RNA labeling kit (Roche Applied Sciences, Basel, Switzerland). For determination of WT miles-apart expression patterns, embryos were collected at 24, 48 and 72 h.p.f. Embryos treated with MOmil or mRNA injections, as well as WT controls, were collected at 48 h.p.f. Embryos allowed to develop past 24 h.p.f. were pretreated with PTU to suppress pigmentation before fixation in 4% paraformaldehyde overnight at 4 °C. Embryos were treated by DNase before hybridization to remove pseudo-positive interference caused by genomic DNA. Subsequent steps of whole-mount in situ hybridization procedure followed a standard protocol.29
Hair cell staining for counting and apoptosis/death detection
For hair cell counting, zebrafish larvae at 6 d.p.f. were anesthetized in 0.016% Tricaine (Sigma-Aldrich) and incubated in the fluorescent dye YO-PRO-1 (Invitrogen) at final concentration 1 μmol/l was used for staining of hair cell nuclei30 of the five neuromasts, o1, ml1, ml2, o2 and io431 which resided at the surface of the otic vesicle around. The larvae were then visualized using an IX51 inverted phase contrast fluorescence microscope (Olympus). Each group has five larvae for staining and hair-cell counting. Photographs were taken with a Canon 500D Camera.
For apoptosis/death detection of the hair cells, an apoptosis staining kit (Chromatin Condensation/Membrane Permeability/Dead Cell Apoptosis Kit with Hoechst 33342/YO-PRO-1 and PI for Flow Cytometry. Catalog no. V23201; Invitrogen) was used to inspect whether miles-apart gene knockdown can cause hair cell apoptosis. The dye mix solution with the three dyes was prepared in the fish water by following the kit instruction. The living larvae at 6 d.p.f. were soaked in the mixed dye solution and wrapped into tin foil to avoid of light at 28 °C for 30 min, then anesthetized and observed under fluorescence inverted microscope. The five neuromasts, o1, ml1, ml2, o2 and io4 were checked and hair cells in ml1 and o2 were counted and shot. Then, the dyed hair cells were classified based on the kit instruction: ‘Blue-fluorescent Hoechst33324 brightly stains the condensed chromatin of apoptotic cells and more dimly stains the normal chromatin of live cells; Green-fluorescent YO-PRO-1 dye can enter apoptotic cells with intact plasma membrane, whereas red-fluorescent propidium iodide (PI) cannot. Thus, after staining with YO-PRO-1 dye and PI, apoptotic cells show green fluorescence and dead cells show primarily red fluorescence and some green fluorescence' (Invitrogen, Catalog no. V23201).32
RT-PCR and qPCR
Embryos or larvae at selected stages were collected into Trizol Reagent. Total RNA was extracted following the Trizol Reagent RNA extraction kit manual. First-strand cDNAs were synthesized by reverse transcription using the M-MLV RTase cDNA Synthesis Kit (Takara Bio, Shiga, Japan). For the RT-PCR amplification, 0.5 lL of cDNA was used for each reaction with different primer pairs (primer sequences are listed in Table 1). β-Actin was amplified as a template loading control. PCR parameters were 94 °C 60 s, 55 °C 50 s, 72 °C 60 s, for 30 cycles for hearing-related genes, 26 cycles for miles-apart and 25 cycles for β-actin. A final incubation at 72 °C for 10 min was included to ensure complete synthesis. RT-PCR products were subjected to agarose gel electrophoresis. For statistical analysis of density, all results were normalized to β-actin and then to WT. For detection of apoptosis gene zBax2 and prosurvival gene zMcl1b, the larvae at 6 d.p.f. (for MO and misMO injection groups) and at 4 d.p.f. (for mRNA injection group) were collected into Trizol reagent; the different collecting time chosen was based on our preliminary experiments. Transcription levels of zBax2 and zMcl1b were examined by qPCR (Agilent Technologies Stratagene Mx3000P, Santa Clara, CA, USA) and parameters were 95 °C 5 min, 95 °C 30 s, 55 °C 30 s, 72 °C 60 s, for 40 cycles. Then the ct values were analyzed by statistics calculation.
Table 1. Primer sequences for PCR and PCR sizes.
| Gene name | Primer sequence | PCR size (bp) |
|---|---|---|
| mil | F: 5′-GCTTTGTCCGCCTCCGTTTTC-3′ R: 5′-GCTGGTGCGCACAATGAGATAGAT-3′ | 314 |
| hmx2 | F: 5'-GAGGACAGCGGAAGCAAG-3' R: 5'-GGGCATCCCAACCAAAGT-3' | 663 |
| fgf3 | F: 5'-TCCGAGTTTGGAGGAATC-3' R: 5'-TTAAATGTCAGCCCTTCTGT-3' | 730 |
| fgf8a | F: 5'-ACGGTTGAGTTATCTATTCC-3' R: 5'-GTGCGTTTAGTCCGTCTG-3' | 594 |
| foxi1 | F: 5'-CGGCTGGCAGAACTCTAT-3' R: 5'-TGTTGACGCTGAAATGGT-3' | 551 |
| otop1 | F: 5'-CGAGATGGGATACGCAAAC-3' R: 5'-CAGCAGCGAGTAGGTCAGG-3' | 592 |
| pax2.1 | F: 5'-GTCAGGCAAAGAATCGTG-3′ R: 5'-CAGGTGCTTCCGTAAACT-3′ | 621 |
| tmieb | F: 5′-GTAACGGCGGTGTTGATC-3′ R: 5′-GCTGAGGTTTCCACGAGA-3′ | 350 |
| Bax2 | F: 5'-ATAAGCAACAGCCAGGAC-3' R: 5'-CACCAGTGAAGGCAAACA-3' | 442 |
| Mcl1b | F: 5'-CCAGAACTGAAAGCGCATAA-3' R: 5'-ACTCCACAAAGCCATCCC-3' | 514 |
| β-Actin | F: 5'-AATCCCAAAGCCAACAGA-3' R: 5'-GATACCGCAAGATTCCATAC-3' | 492 |
Statistical analysis
Statistical analyses for all the data (means±S.D.) were performed using One way ANOVA tests and P-values, 0.05 were assigned as significant. Data with bars in histograms represent means and S.D.'s that are derived from at least triplicates.
Acknowledgments
We thank Mr Weixian Wang for his supporting in microinjection, and thank professor Bo Zhang (Beijing University) provided zebrafish TU stain seedling. This study was supported by The National Natural Science Foundation of China (No. 30772681 and 81173043), the Chinese National Key Technology R&D Program (2012BAK25B01) and the Presidential Foundation for the governmental public welfare scientific research institutions (No. IMBF 201101).
Glossary
- S1P
Sphingosine-1-phosphate
- WT
wild-type
- d.p.f.
day post fertilization
- h.p.f.
hour post fertilization
- MOmil
morpholino oligo
- Mil
miles-apart
- o1
orbital 1
- ml1
middle lateral line 1
- ml2
middle lateral line 2
- o2
orbital 2
- io4
infraorbital 4
The authors declare no conflict of interest.
Footnotes
Edited by A Stephanou
References
- MacLennan AJ, Benner SJ, Andringa A, Chaves AH, Rosing JL, Vesey R, et al. The S1P2 sphingosine 1-phosphate receptor is essential for auditory and vestibular function. Hear Res. 2006;220:38–48. doi: 10.1016/j.heares.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Herr DR, Grillet N, Schwander M, Rivera R, Müller U, Chun J. Sphingosine 1-phosphate (S1P) signaling is required for maintenance of hair cells mainly via activation of S1P2. J Neurosci. 2007;27:1474–1478. doi: 10.1523/JNEUROSCI.4245-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GW, Burns S, Huang GH, Boyd K, Proia RL, Flavell RA, et al. S1P1 receptor overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol. 2009;10:769–777. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alewijnse AE, Peters SL, Michel MC. Cardiovascular effects of sphingosine-1-phosphate and other sphingomyelin metabolites. Br J Pharmacol. 2004;143:666–684. doi: 10.1038/sj.bjp.0705934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupperman E, An S, Osborne N, Waldron S, Stainier DY. A sphingosine-1-phosphate receptor regulates cell migration during vertebrate heart development. Nature. 2000;406:192–195. doi: 10.1038/35018092. [DOI] [PubMed] [Google Scholar]
- Yelon D. Cardiac patterning and morphogenesis in zebrafish. Dev Dyn. 2001;222:552–563. doi: 10.1002/dvdy.1243. [DOI] [PubMed] [Google Scholar]
- Oskouian B, Saba JD. Death and taxis: what non-mammalian models tell us about sphingosine-1-phosphate. Semin Cell Dev Biol. 2004;15:529–540. doi: 10.1016/j.semcdb.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Matsui T, Raya A, Callol-Massot C, Kawakami Y, Oishi I, Rodriguez-Esteban C, et al. miles-apart-mediated regulation of cell–fibronectin interaction and myocardial migration in zebrafish. Nat Clin Pract Cardiovasc Med. 2007;4:S77–S82. doi: 10.1038/ncpcardio0764. [DOI] [PubMed] [Google Scholar]
- Kimura A, Ohmori T, Kashiwakura Y, Ohkawa R, Madoiwa S, Mimuro J, et al. Antagonism of sphingosine 1-phosphate receptor-2 enhances migration of neural progenitor cells toward an area of brain. Stroke. 2008;39:3411–3417. doi: 10.1161/STROKEAHA.108.514612. [DOI] [PubMed] [Google Scholar]
- Trifilieff A, Baur F, Fozard JR. Role of sphingosine-1-phosphate (S1P) and the S1P(2) receptor in allergen-induced, mast cell-dependent contraction of rat lung parenchymal strips. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:303–309. doi: 10.1007/s00210-009-0438-4. [DOI] [PubMed] [Google Scholar]
- Sensken SC, Gräler MH. Sphingolipids and their medical impact. Dtsch Med Wochenschr. 2009;134:259–263. doi: 10.1055/s-0028-1123990. [DOI] [PubMed] [Google Scholar]
- Shen YC, Jeyabalan AK, Wu KL, Hunker KL, Kohraman DC, Thompson DL. The transmembrane inner ear (tmie) gene contributes to vestibular and lateral line development and function in the zebrafish (Danio rerio) Dev Dyn. 2008;237:941–952. doi: 10.1002/dvdy.21486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkwitz S, Bober E, Baker R. Development of the vertebrate inner ear. Ann N Y Acad Sci. 2001;942:1–14. doi: 10.1111/j.1749-6632.2001.tb03730.x. [DOI] [PubMed] [Google Scholar]
- Whitfield TT, Riley BB, Chiang MY, Phillips B. Development of the zebrafish inner ear. Dev Dyn. 2002;223:427–458. doi: 10.1002/dvdy.10073. [DOI] [PubMed] [Google Scholar]
- Léger S, Brand M. Fgf8 and Fgf3 are required for zebrafish ear placode induction, maintenance and inner ear patterning. Mech Dev. 2002;119:91–108. doi: 10.1016/s0925-4773(02)00343-x. [DOI] [PubMed] [Google Scholar]
- Hughes I, Blasiole B, Huss D, Warchol ME, Rath NP, Hurle B, et al. Otopetrin 1 is required for otolith formation in the zebrafish Danio Rerio. Dev Biol. 2004;276:391–402. doi: 10.1016/j.ydbio.2004.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo VE, Liang J, Behra M, Elkahloun A, Villablanca EJ, Russo V, et al. Molecular dissection of the migrating posterior lateral line primordium during early development in zebrafish. BMC Dev Biol. 2010;10:120. doi: 10.1186/1471-213X-10-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon KS, Kudoh T, Dawid IB, Fritz A. Zebrafish foxi1 mediates otic placode formation and jaw development. Development. 2003;130:929–940. doi: 10.1242/dev.00308. [DOI] [PubMed] [Google Scholar]
- Riley BB, Chiang M, Farmer L, Heck R. The deltaA gene of zebrafish mediates lateral inhibition of hair cells in the inner ear and is regulated by pax2.1. Development. 1999;126:5669–5678. doi: 10.1242/dev.126.24.5669. [DOI] [PubMed] [Google Scholar]
- McDermott BM, Jr, Asai Y, Baucom JM, Jani SD, Castellanos Y, Gomez G, et al. Transgenic Labeling of Hair Cells in the Zebrafish Acousticolateralis System. Gene Expr Patterns. 2010;10:113–118. doi: 10.1016/j.gep.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Xu Q. Pivotal role of hmx2 and hmx3 in zebrafish inner ear and lateral line development. Dev Biol. 2010;339:507–518. doi: 10.1016/j.ydbio.2009.12.028. [DOI] [PubMed] [Google Scholar]
- Hughes I, Thalmann I, Thalmann R, Ornitz DM. Mixing model systems: Using zebrafish and mouse inner ear mutants and other organ systems to unravel the mystery of otoconial development. Brain Res. 2006;1091:58–74. doi: 10.1016/j.brainres.2006.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason MR, Nagiel A, Jamet S, Vologodskaia M, López-Schier H, Hudspeth AJ. The Transmembrane inner ear (Tmie) protein is essential for normal hearing and balance in the zebrafish. PNAS. 2009;106:21347–21352. doi: 10.1073/pnas.0911632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoura A, Hla T. Regulation of vascular physiology and pathology by the S1P2 receptor subtype. Cardiovasc Res. 2009;82:221–228. doi: 10.1093/cvr/cvp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz E, Eimon PM, Mukhyala K, Stern H, Zha J, Strasser A, et al. Functional characterization of the Bcl-2 gene family in the zebrafish. Cell Death Differ. 2006;13:1631–1640. doi: 10.1038/sj.cdd.4402016. [DOI] [PubMed] [Google Scholar]
- Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M.THE ZEBRAFISH BOOK: A guide for the laboratory use of zebrafish (Danio rerio) University of Oregon Press: Eugene, OR, USA; 2000. 4th ednon line http://zfin.org/zf_info/zfbook/zfbk.html . [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the Zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- Coffin AB, Ou H, Owens KN, Santos F, Simon JA, Rubel EW, et al. Chemical screening for hair cell loss and protection in the zebrafish lateral line. Zebrafish. 2010;7:3–11. doi: 10.1089/zeb.2009.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AL, Yu-chi S, Babb-Clendenon SG, Rostedt J, Liu B, Barald KF, et al. Cadherin-4 plays a role in the development of Zebrafish q. Dev Dyn. 2007;236:893–902. doi: 10.1002/dvdy.21085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiøtz BL, Baekkevold ES, Poulsen LC, Mjaaland S, Gjøen T. Analysis of host- and strain-dependent cell death responses during infectious salmon anemia virus infection in vitro. Virol J. 2009;6:91. doi: 10.1186/1743-422X-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]