Abstract
Proliferation and epithelial–mesenchymal transition (EMT) of lens epithelium cells (LECs) may contribute to anterior subcapsular cataract (ASC) and posterior capsule opacification (PCO), which are important causes of visual impairment. Histone deacetylases (HDACs)-mediated epigenetic mechanism has a central role in controlling cell cycle regulation, cell proliferation and differentiation in a variety of cells and the pathogenesis of some diseases. However, whether HDACs are involved in the regulation of proliferation and EMT in LECs remain unknown. In this study, we evaluated the expression profile of HDAC family (18 genes) and found that class I and II HDACs were upregulated in transforming growth factor β2 (TGFβ2)-induced EMT in human LEC lines SRA01/04 and HLEB3. Tricostatin A (TSA), a class I and II HDAC inhibitor, suppressed the proliferation of LECs by G1 phase cell cycle arrest not only through inhibition of cyclin/CDK complexes and induction of p21 and p27, but also inactivation of the phosphatidylinositol-3-kinase/Akt, p38MAPK and ERK1/2 pathways. Meanwhile, TSA strongly prevented TGFβ2-induced upregulation of fibronectin, collagen type I, collagen type IV, N-cadherin, Snail and Slug. We also demonstrated that the underlying mechanism of TSA affects EMT in LECs through inhibiting the canonical TGFβ/Smad2 and the Jagged/Notch signaling pathways. Finally, we found that TSA completely prevented TGFβ2-induced ASC in the whole lens culture semi-in vivo model. Therefore, this study may provide a new insight into the pathogenesis of ASC and PCO, and suggests that epigenetic treatment with HDAC inhibitors may be a novel therapeutic approach for the prevention and treatment of ASC, PCO and other fibrotic diseases.
Keywords: histone deacetylase inhibitor, lens epithelium cells (LECs), proliferation, epithelial–mesenchymal transition (EMT), anterior subcapsular cataract (ASC), posterior capsule opacification (PCO)
Cataract is the most common cause of visual impairment in the elderly all over the world.1 Anterior subcapsular cataract (ASC) and posterior capsule opacification (PCO) are two types of cataract but share many cellular and molecular features.2, 3 PCO, also known as a secondary cataract, is a major long-term complication of cataract surgery, occurring in 20–40% of patients after cataract surgery, particularly, in children and infants.4, 5 In the last few decades, although advances in surgical techniques, intraocular lens materials and designs have reduced the PCO rate, it is still a significant problem. Currently, there is no generally accepted pharmacologic agent that can prevent or slow down the onset or the progression of these diseases. Cataract surgery and Nd:YAG laser capsulotomy are the only effective treatments for ASC and PCO, nevertheless, they carry vision-related complications and risks, and put a significant financial burden on the health-care system.
The growing body of evidence shows that proliferation and epithelial–mesenchymal transition (EMT) of lens epithelial cells (LECs) are the major pathologic changes in development of ASC6, 7 and PCO.8, 9 Transforming growth factor β (TGFβ), especially TGFβ2, the major isoform in the aqueous humor of the eye, is the most important factor driving the trans-differentiation and pathologic fibrosis of LECs.10 PCO is caused by a wound-healing response of residual LECs at the equator and under the anterior lens capsule. After surgery, the residual LECs proliferate and undergo EMT, which leads to the formation of fibroblasts and the expression of numerous extracellular matrix proteins, such as collagen type I (Col I), Col IV and fibronectin (FN).11 Moreover, LECs undergo cytoskeletal rearrange and loss of epithelial phenotype, then migrate away from the original location onto the posterior capsule, and finally contribute to the development of PCO.7 ASC is a primary cataract, which is caused by the proliferation and EMT of LECs in situ. These fibroblasts form subcapsular plaques beneath the lens capsule, and similar to the transdifferentiated cells in PCO.2 Based on the above observations, inhibition of LECs proliferation, migration and TGFβ-induced EMT may be a promising strategy to prevent ASC and PCO.
Histone deacetylases (HDACs)-mediated epigenetic mechanism has a central role in controlling cell cycle regulation, cell proliferation and differentiation in a variety of cells and the pathogenesis of some diseases.12, 13 Multiple studies suggest that HDACs are vital targets in various diseases, including cancers, inflammatory diseases and metabolic disorders.13 For this reason, HDAC inhibitors now are potential antitumor drugs.14, 15 To date, 18 human HDAC isoforms have been characterized and divided into four major classes: class I HDACs (HDAC1, 2, 3 and 8), class II HDACs (HDAC4, 5, 6, 7, 9 and 10), class III HDACs (SIRT1, 2, 3, 4, 5, 6 and 7) and class IV HDAC (HDAC11).16 Class II HDACs are further subdivided into class IIa (HDAC4, 5, 7, 9) and class IIb (HDAC6 and 10) forms.16 Recently, HDAC inhibitors are also shown to be promising new compounds for the therapy of fibrotic diseases.17, 18 A class I and II HDAC inhibitor, tricostatin A (TSA), inhibited trans-differentiation of hepatic stellate cells into myofibroblasts,19 and also abrogated TGFβ2-induced EMT in human renal epithelial cells and skin fibroblasts.17, 20, 21 Spiruchostatin A, a class I HDAC inhibitor, suppresses proliferation and differentiation of fibroblasts in human pulmonary fibrosis.22 Despite its definite effects on proliferation and EMT inhibition, the underlying mechanisms remain to be clarified.17 Moreover, the function of HDAC inhibitor in LECs proliferation and EMT is still unknown.
In current study, we reported, for the first time, that some of class I and class II HDACs were upregulated in TGFβ2-induced EMT in human LEC lines SRA01/04 and HLEB3. Inhibition of HDAC activity with TSA strongly suppressed proliferation and TGFβ2-induced EMT of LECs. Moreover, we also demonstrated that TSA completely prevented TGFβ2-induced ASC in the whole lens culture semi-in vivo model. These results suggest that inhibition of HDAC activity may be of benefit in the treatment of ASC and PCO.
Results
Expression of HDACs in TGFβ2-induced EMT of LECs
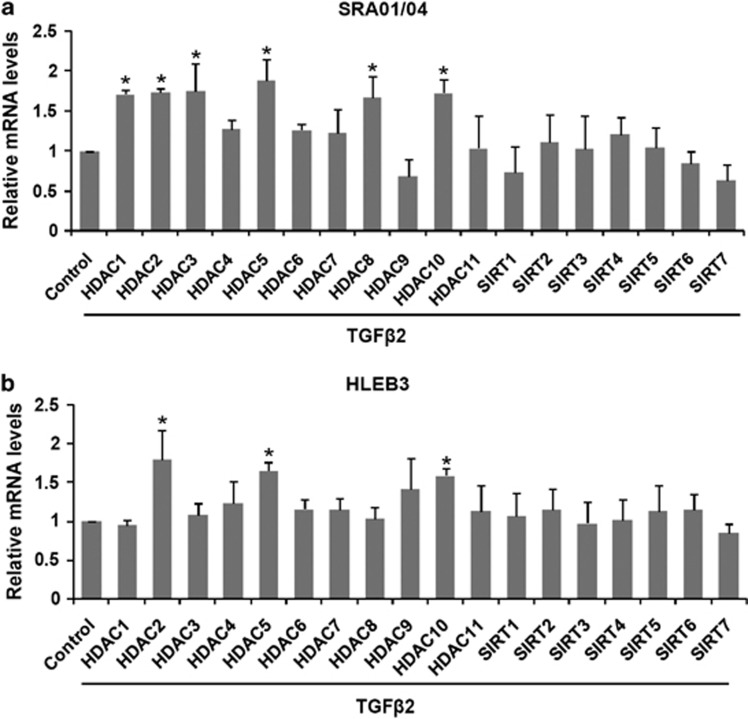
Aberrant expression of different HDACs has been observed in various tumors and linked to tumor progression and poor outcome.23 So we first evaluated the expression pattern of the HDAC family (18 genes) in TGFβ2-induced EMT in human LEC lines SRA01/04 and HLEB3 by real-time PCR. The result showed that HDAC1, HDAC2, HDAC3, HDAC5, HDAC8 and HDAC10 were upregulated in TGFβ2-induced SRA01/04 cells, meanwhile, HDAC2, HDAC5 and HDAC10 were upregulated in HLEB3 cells, whereas the expression of other HDAC family members were not significantly changed after 24 h TGFβ2 treatment (Figures 1a and b:*P<0.05 versus the control group). These results suggest that several family members of class I and II HDACs are upregulated in TGFβ2-induced EMT in LECs.
Figure 1.
Expression of HDACs in TGFβ2-induced EMT in LECs. Cells were cultured in the presence or absence of TGFβ2 (10 ng/ml) for 24 h, and the mRNA expression levels of HDAC family (18 isoforms) were detected by real-time quantitative PCR. (a) Expression of HDACs in TGFβ2-induced EMT in SRA01/04 cells. (b) Expression of HDACs in TGFβ2-induced EMT in HLEB3 cells. All experiments were repeated three times with similar results. The expression of each HDAC was normalized separately to its untreated counterpart. *P<0.05 versus the control group
The HDAC inhibitor TSA suppressed proliferation of LECs by cell cycle arrest
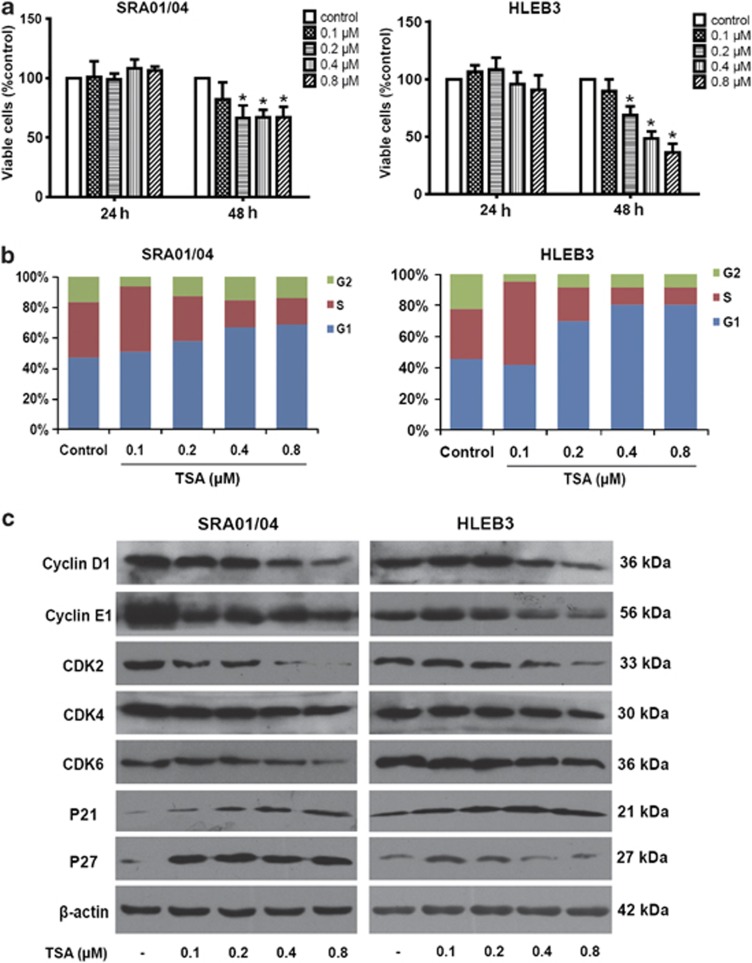
Proliferation of LECs is the fundamental step during ASC and PCO, so we first examined the effect of the HDAC inhibitor TSA on proliferation of LEC lines SRA01/04 and HLEB3. As shown in Figure 2a, TSA (0.2, 0.4 and 0.8 μM) exerted strongly inhibitory effects on the proliferation of both SRA01/04 and HLEB3 cells after treatment for 48 h (P<0.05), but has no effect after 24 h (P>0.05). To determine if the cell growth inhibition observed was due to apoptosis induced by TSA, the apoptosis of cells were analyzed using flow cytometry. The results showed that TSA at high doses 0.4 and 0.8 μM caused an increase in the number of cells death in both SRA01/04 and HLEB3 cells after treatment for 48 h (Supplementary Figure 1). These results indicate that the inhibitory effect of low dose of TSA (0.2 μM) on growth inhibition is mainly due to the suppression of proliferation, whereas the effect of high doses of TSA is partly due to induction of apoptosis. We further examined cell cycle progression to determine whether the anti-proliferative effect of TSA might result from cell cycle arrest. As shown by the results of flow cytometry, SRA01/04 and HLEB3 cells cultured with various concentrations of TSA for 24 h showed an accumulation of cells in G1 phase of the cell cycle, with concomitant decreases in the proportion of those in G2 and S phases (Figure 2b). To investigate the underlying mechanism of cell cycle arrest, the effect of TSA on the expression of cell cycle-regulated proteins were examined by western blot analysis. The results revealed that TSA significantly decreased the protein levels of cyclin D1, cyclin E1, cyclin-dependent kinase (CDK) 2, CDK4 and CDK6, whereas increased the expression of P21 and P27 in a concentration-dependent manner in SRA01/04 and HLEB3 cells (Figure 2c). These results suggest that TSA inhibits the proliferation of LEC lines SRA01/04 and HLEB3 by G1 phase cell cycle arrest through inhibition of cyclin D1/CDK4/6 and cyclin E1/CDK2 complexes, and induction of P21 and P27.
Figure 2.
The HDAC inhibitor TSA suppressed proliferation of LECs by cell cycle arrest. (a) SRA01/04 and HLEB3 cell lines were treated with TSA at various concentrations (0.1, 0.2, 0.4, and 0.8 μM) for 24 and 48 h, then the percentage of viable cells was determined by CCK-8 kit. *P<0.05 versus the control group. (b) Cell cycle analysis of SRA01/04 and HLEB3 cell lines were quantified by PI staining followed by flow cytometry analyses after treatment for 24 h. Bar graphs represent the mean ±S.E.M. of three independent experiments. (c) The protein expression levels of cyclin D1, cyclin E1, CKD2, CDK4 CDK6, P21 and P27 were detected by western blot analysis after treatment for 24 h in SRA01/04 and HLEB3 cell lines. All experiments were repeated three times with similar results
TSA inhibited LECs proliferation through suppressing the PI3K/Akt, p38MAPK and ERK1/2 signaling pathways
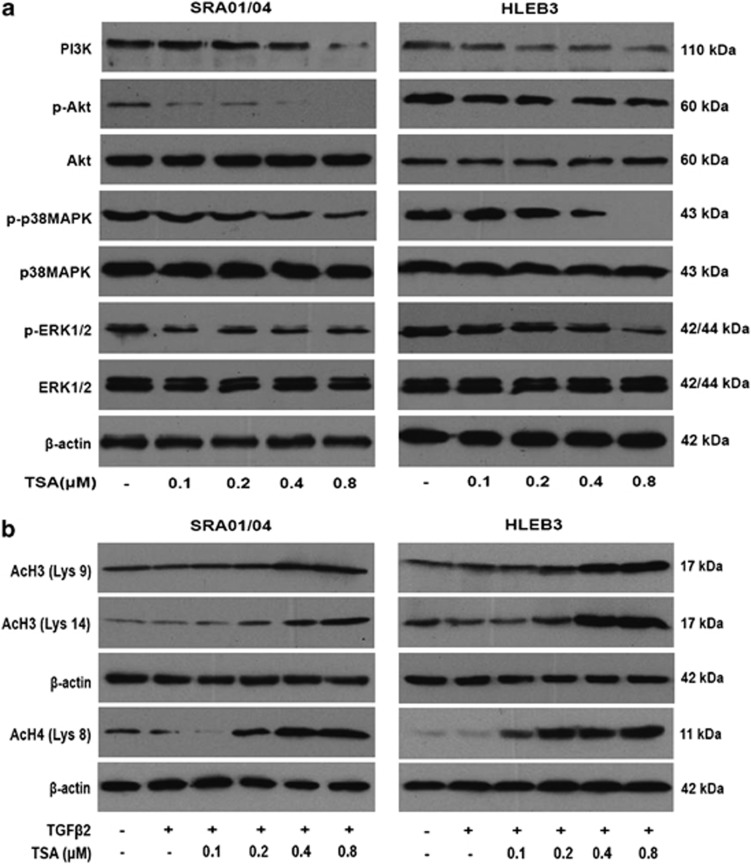
Many reports have demonstrated that the phosphatidylinositol-3-kinase (PI3K)/Akt, mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinase (ERK) and p38 pathways have been demonstrated to have key roles in promoting cell proliferation, adhesion, survival and motility.24, 25 To elucidate the mechanisms responsible for the inhibitory effect of TSA on proliferation of LECs, we examined the phosphorylation of Akt, p38MAPK and ERK1/2 in SRA01/04 and HLEB3 cells. As shown in Figure 3a, TSA decreased the expression of PI3K, as well as the phosphorylation of Akt, p38MAPK and ERK1/2 in a dose-dependent manner without affecting their total protein levels at 60 min after TSA treatment in both cell lines. These results suggest that TSA potently inhibits the proliferation of LECs by inactivating PI3K/Akt, p38MAPK and ERK1/2 signaling pathways.
Figure 3.
(a) TSA inhibited LECs proliferation through suppressing the PI3K/Akt, p38MAPK and ERK1/2 signaling pathways. SRA01/04 and HLEB3 cell lines were treated with TSA at various concentrations (0.1, 0.2, 0.4 and 0.8 μM) for 60 min, then the protein expression levels of PI3K, p-Akt, Akt, p-p38MAPK, p38MAPK, p-ERK1/2 and ERK1/2 were detected by western blot analysis. (b) TSA increased the levels of acetylated histones H3 and H4. The expression of acetylated histones H3 (Lys 9 and 14) and H4 (Lys8) were detected after 12 h treatment. β-Actin, loading control. All experiments were repeated three times with similar results
TSA prevented TGFβ2-induced EMT and migration in LECs
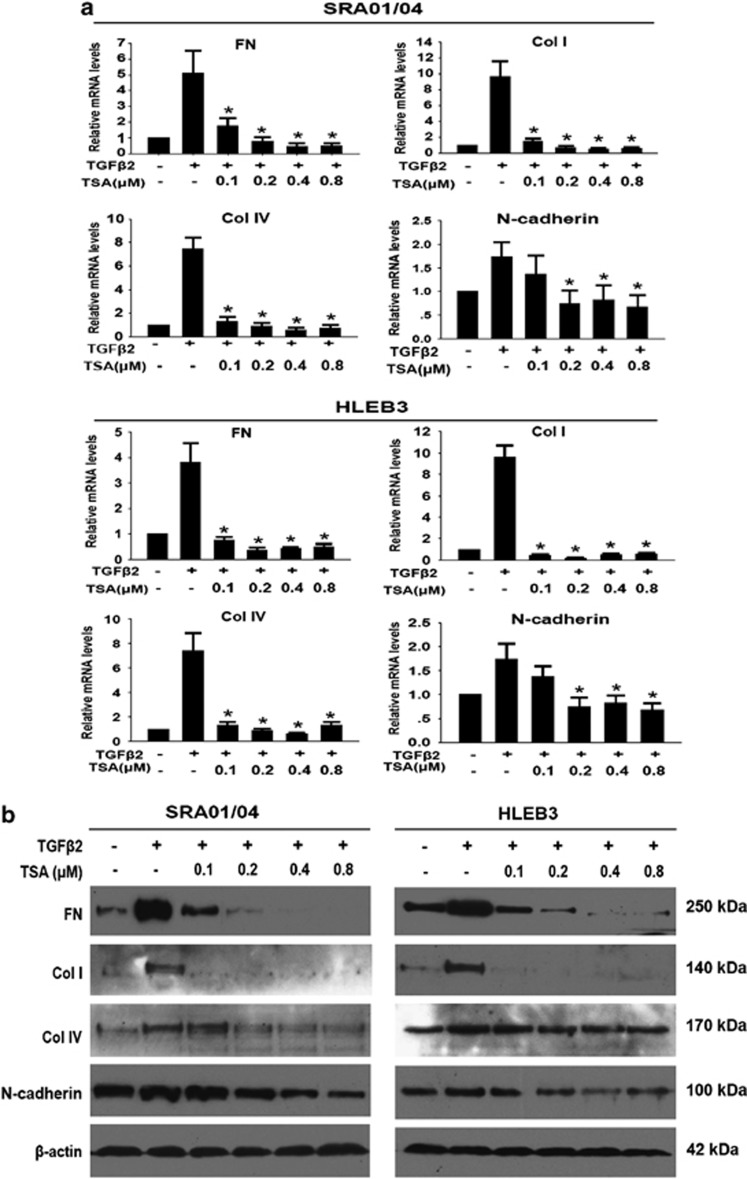
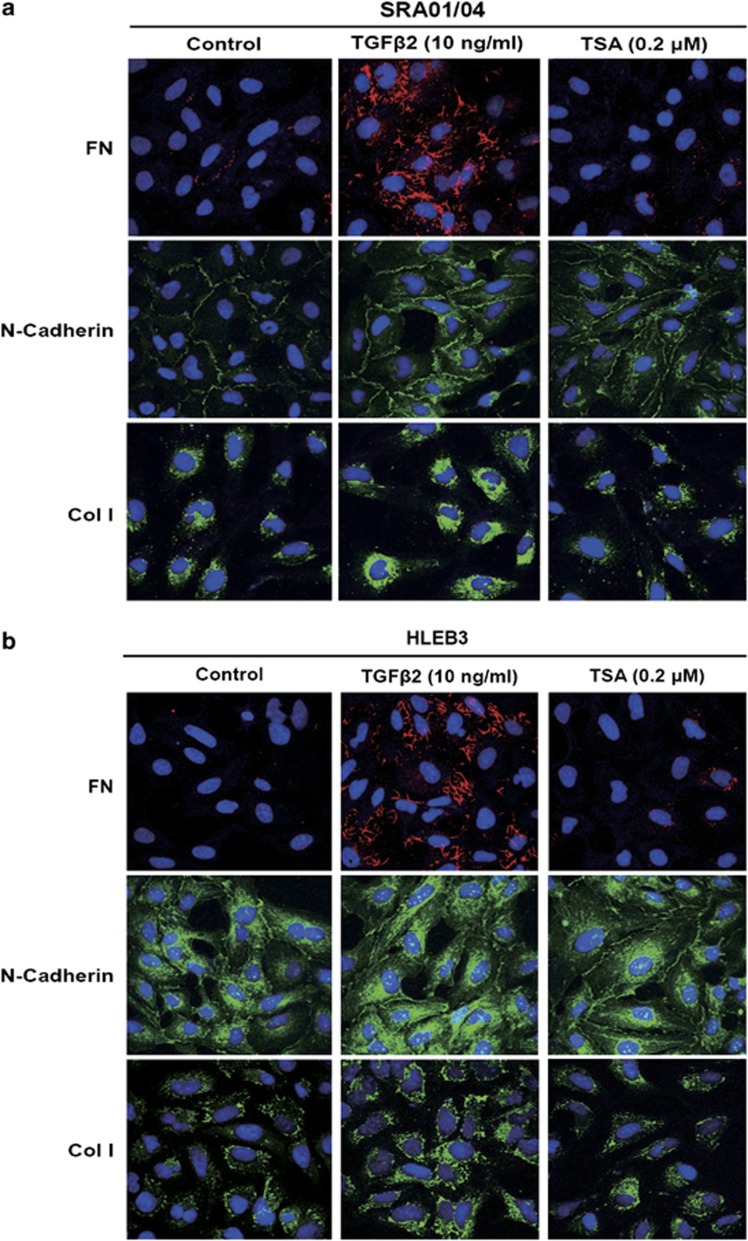
To explore whether HDAC inhibition could prevent TGFβ2-induced EMT in LECs, EMT markers, such as FN, Col I, Col IV, N-cadherin, were investigated at mRNA and protein levels by real-time PCR and western blot analysis, respectively. As shown in Figure 4, TGFβ2 significantly increased the expression of FN, Col I, Col IV, N-cadherin at both mRNA (Figure 4a) and protein levels in SRA01/04 and HLEB3 cells (Figure 4b). In agreement with these, immunofluorescence staining of FN, Col I and N-cadherin were also enhanced obviously (Figures 5a and b). On the contrary, TSA completely abrogated the upregulation of FN, Col I, Col IV and N-cadherin, as well as weakened the staining of FN, Col I and N-cadherin in SRA01/04 and HLEB3 cells (Figures 4a and b: *P<0.05 versus TGFβ2 treated with DMSO group; Figures 5a and b). Furthermore, the effect of TSA on migration of LECs stimulated by TGFβ2 was assessed using scratch wound assay. HLEB3 cells treated with TGFβ2 exhibited an enhanced ability to migrate and the area of wound decreased after injury for 24 h, whereas the wound area remained wide in TGFβ2 co-treatment with TSA group (Supplementary Figures b and d, *P<0.05 versus TGFβ2 treated with DMSO group). Meanwhile, TGFβ2 did not induce SRA01/04 cells to migrate, just as the cells cultured without TGFβ2, however, the wound of the cells treated with TSA was wider than the control and TGFβ2 treatment groups (Supplementary Figures 2a and c, *P<0.05 versus the control and TGFβ2 treated with DMSO groups). Taken together, these data indicate that HDAC inhibitor TSA can powerfully attenuate TGFβ2-induced EMT and migration in LECs.
Figure 4.
TSA prevents TGFβ2-induced EMT in LECs. Cells were cultured in the absence or presence of TGFβ2 with TSA or DMSO for 24 h. (a) The mRNA expression levels of FN, Col I, Col IV and N-cadherin in SRA01/04 and HLEB3 cell lines were determined by real-time quantitative PCR. Gene expression levels were normalized to the glyceraldehyde 3-phosphate dehydrogenase control. *P<0.05 versus TGFβ2 treated with DMSO group. (b) The protein expression levels of FN, Col I, Col IV and N-cadherin in SRA01/04 and HLEB3 cell lines were detected by western blot analysis. β-Actin, loading control. All experiments were repeated three times with similar results
Figure 5.
TSA prevents TGFβ2-induced EMT in LECs. Cells were cultured in the absence or presence of TGFβ2 with TSA (0.2 μM) or DMSO for 24 h. Immunofluorescence analysis of FN (red), N-cadherin (green) and Col I (green) in SRA01/04 (a) and HLEB3 (b) cell lines were observed using confocal microscopy. Representative images are shown (magnification, × 400). All experiments were repeated three times with similar results
TSA abrogated TGFβ2-induced upregulation of Snail and Slug
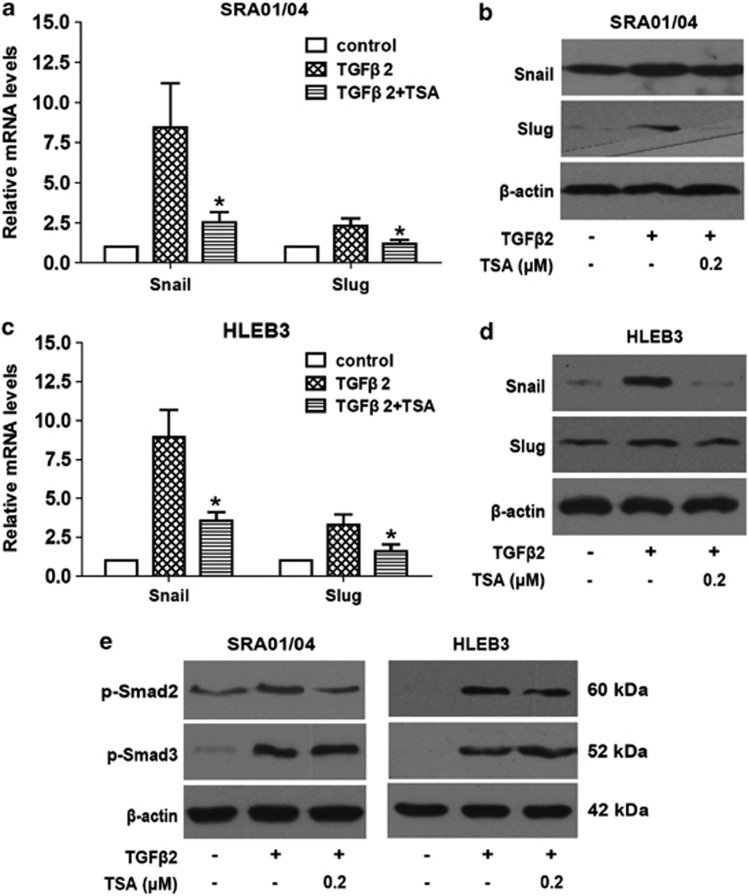
Snail and Slug are the most widely recognized as important regulators in EMT. In fact, most known EMT events during cancer development and fibrosis appear to be associated with Snail and Slug activation.26 Therefore, we next investigated whether the effects of TSA on LECs EMT are mediated through regulating the expression of Snail and Slug. As documented in Figure 6, TGFβ2 treatment distinctly increased Snail and Slug expression at mRNA and protein levels, nevertheless, TSA dramatically abrogated the upregulation of Snail and Slug induced by TGFβ2 in SRA01/04 and HLEB3 cells. These results strongly suggest that inhibition of HDACs contributes to downregulation of Snail and Slug, resulting in the prevention of EMT phenotype in LECs.
Figure 6.
TSA abrogates TGFβ2-induced upregulation of Snail and Slug, and phosphorylation of Smad2. Cells were cultured in the absence or presence of TGFβ2 with TSA (0.2 μM) or DMSO for 24 h, the mRNA and protein expression of Snail and Slug in SRA01/04 were detected by real-time PCR (a) and western blot analysis (b), respectively. (c) The mRNA expression of Snail and Slug in HLEB3 were detected by real-time PCR. (d) The protein expression of Snail and Slug in HLEB3 were detected by western blot analysis. Gene expression levels were normalized to the glyceraldehyde 3-phosphate dehydrogenase control. *P<0.05 versus TGFβ2 treated with DMSO group. (e) The phosphorylation levels of Smad2 and Smad3 were detected by western blot analysis after 60 min treatment. β-Actin, loading control. All experiments were repeated three times with similar results
TSA inhibited TGFβ2 signaling pathway by suppressing phosphorylation of Smad2
To clarify whether the inhibitory effect of TSA on LECs EMT is partly via blocking the TGFβ2 signaling pathway, the impact of TSA on activation of receptor-regulated Smad proteins Smad2 and Smad3 were investigated. As shown in Figure 6e, TGFβ2 alone activated Smad2 and Smad3 via the stimulated phosphorylation after treatment for 60 min, whereas co-treatment with TSA inhibited phosphorylation of Smad2, but did not alter phosphorylation of Smad3 in both SRA01/04 and HLEB3 cell lines. These results suggest that TSA inhibits TGFβ2 signaling pathway transduction via suppressing phosphorylation of Smad2.
TSA increased acetylated histones H3 and H4
To investigate whether the observed effects of TSA are due to direct modification of histone acetylation, the levels of acetylated histones H3 and H4 were analyzed by western blot analysis. As shown in Figure 3b, the amount of acetylated histones H3 (Lys9 and Lys14) and H4 (Lys8) were sharply increased by treatment with TSA in a concentration-dependent manner in SRA01/04 and HLEB3 cells. These results indicate that the effects of TSA on LECs proliferation and EMT are primarily owing to direct modification of histone acetylation.
TSA abrogated TGFβ2-induced EMT via downregulating the Notch pathway
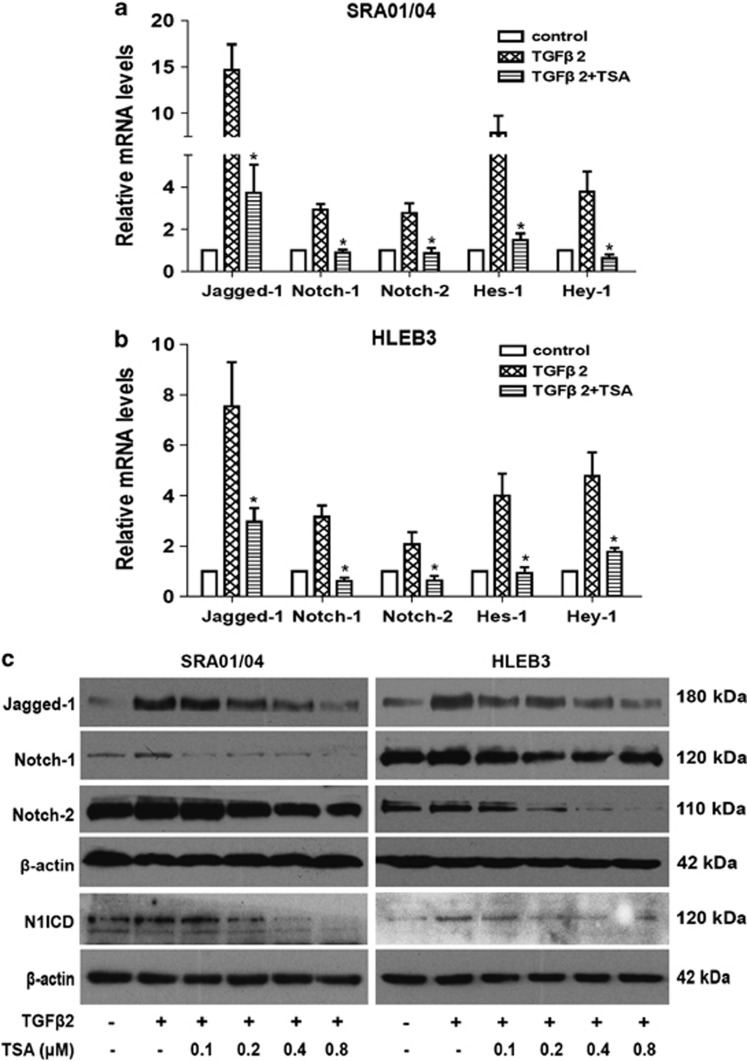
Emerging evidence suggest that the Notch signaling pathway is a vital regulator in the induction of EMT during embryonic development, fibrotic diseases and cancer metastasis.27 Our previous studies also demonstrated that Jagged/Notch pathway is required in TGFβ2-induced EMT in human LECs and retinal pigment epithelial cell in association with the induction of Notch ligands and receptors, whereas blockade of Notch pathway reverses TGFβ2-induced EMT (data unpublished). To investigate whether the inhibitory effect of TSA on LECs EMT is partly mediated via regulating the Notch signaling, the impact of TSA on Notch pathway was further accessed. In accordance with our previous research, TGFβ2 treatment significantly increased the expression of Jagged-1, Notch-1, Notch-2, Notch-1 intracellular domain (N1ICD) as well as their downstream genes Hes-1 and Hey-1 in LECs. In contrast, TSA could completely attenuate the TGFβ2-induced upregulation of Jagged-1, Notch-1, Notch-2, N1ICD, together with target genes Hes-1 and Hey-1 in both SRA01/04 and HLEB3 cell lines (Figures 7a and b: *P<0.05 versus TGFβ2 treated with DMSO group; Figure 7c). Altogether, these results suggest that TSA abrogates TGFβ2-induced EMT through downregulating the Jagged/Notch pathway.
Figure 7.
TSA abrogates TGFβ2-induced EMT via downregulating the Notch pathway. (a) The mRNA expression levels of Jagged-1, Notch-1, Notch-2, Hes-1 and Hey-1 were detected by real-time PCR in SRA01/04 cell line treated with TGFβ2 in the presence of TSA (0.2 μM) or DMSO for 24 h. (b) The mRNA expression levels of Jagged-1, Notch-1, Notch-2, Hes-1 and Hey-1 in HLEB3 cell line were also detected by real-time PCR. Gene levels were normalized to control glyceraldehyde 3-phosphate dehydrogenase. *P<0.05 versus TGFβ2 treated with DMSO group. (c) Representative Jagged-1, Notch-1, Notch-2 and N1ICD immunoblot from SRA01/04 and HLEB3 cell lines treated with TGFβ2 in the presence of TSA (0.1, 0.2 0.4 and 0.8 μM) or DMSO for 24 h. All experiments were repeated three times with similar results
TSA abrogated TGFβ2-induced ASC in the whole lens culture semi-in vivo model
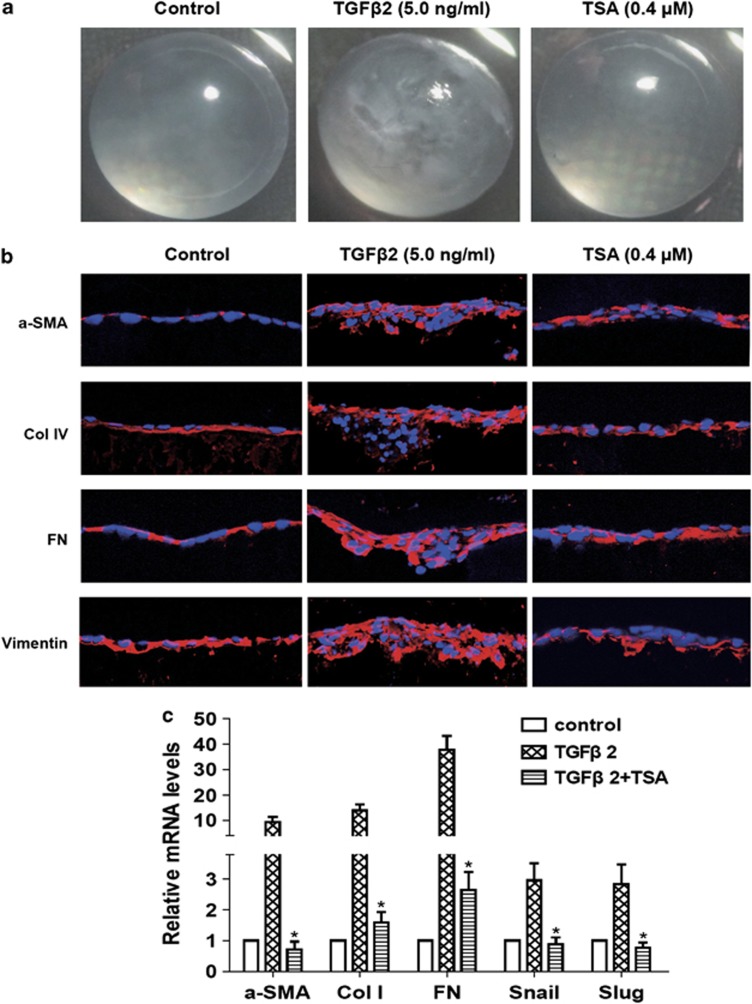
Previous studies have identified that TGFβ can induce the whole lens cultured in vitro to form opacities that contain morphologic and biochemical markers for ASC.28 To further investigate whether TSA can reverse TGFβ2-induced EMT in eye lens in a more complicated system, we used the whole lens culture semi-in vivo model. When the lenses from 21 to 22 days rats were cultured with 5 ng/ml of TGFβ2 for 7 days, lenses developed obvious clumpy anterior opacities just beneath the lens capsule in each lens, whereas TSA abrogated TGFβ2-induced ASC and the lenses remained transparent as cultured without TGFβ2 (Figure 8a). As shown in Figure 8b, the morphology of the frozen sections showed that the aberrant cells were colocalized with the subcapsular clumpy opacities. The clumps contained accumulations of FN and Col IV as shown by staining of the sections. The expression of α-SMA and vimentin were increased in the clumps. In contrast, the lenses cultured with TSA remained transparent, retained normal lens morphology, and did not have accumulation of FN, Col IV, α-SMA and vimentin, but instead, just as lenses cultured without TGFβ2 (Figures 8a and b). In addition, we also demonstrated that TSA dramatically reduced the upregulation of mRNA expression of α-SMA, Col I, FN, Snail and Slug in rat lenses induced by TGFβ2 for 7 days (Figure 8c: *P<0.05 versus TGFβ2 treated with DMSO group). Taken together, these data indicate that inhibition of HDACs activity can completely abrogated TGFβ2-induced ASC in rat lens.
Figure 8.
TSA abrogated TGFβ2-induced ASC in the whole lens culture semi-in vivo model. Lenses were cultured in the absence or presence of TGFβ2 with TSA (0.4 μM) or DMSO for 7 days. (a) The morphology of the lenses was photographed using a dissecting microscope. n=12. (b) The staining of frozen sections for α-SMA (red), Col IV (red), FN (red) and vimentin (red). Images were captured using a fluorescence confocal microscope. n=6. Bar, 20 μm. (c) The mRNA expression levels of α-SMA, Col I, FN, Snail and Slug in lenses were detected by real-time PCR. n=6. Gene levels were normalized to control glyceraldehyde 3-phosphate dehydrogenase. *P<0.05 versus TGFβ2 treated with DMSO group
Discussion
In this study, we evaluated the expression profile of HDAC family in TGFβ2-induced EMT for the first time, and reported that several family members of class I and II HDACs were upregulated in TGFβ2-induced EMT in human LECs. Inhibition of HDAC activity with TSA, a class I and II HDAC inhibitor, dramatically suppressed the proliferation of LECs by cell cycle arrest and inhibition of the PI3K/Akt, p38MAPK and ERK1/2 pathways. Moreover, TSA strongly prevented TGFβ2-induced EMT in LECs through blocking the canonical TGFβ/Smad2 signal transduction and downregulating the Jagged/Notch signaling pathway. We also demonstrated that TSA completely prevented TGFβ2-induced ASC in the whole lens culture semi-in vivo model. Therefore, our study provides convincing evidence that epigenetic regulators, HDAC inhibitors, may be a novel strategy for the prevention and treatment of ASC and PCO.
The mammalian cell cycle is controlled by complexes containing cyclins and CDKs and their related pathways.29 Cyclins bind and activate their selected CDKs in specific phases of cell cycle, following which they phosphorylate their target proteins to promote cell cycle progression. For example, cyclin D1 and cyclin E1 bind and activate CDK4/6 and CDK2, respectively, and promote the G1 to S phase transition.30 Nevertheless, CDK inhibitors, such as P21 and P27, negatively regulate cell cycle progression by inhibiting the activity of the cyclin D1/CDK4/6 and cyclin E/CDK2 complexes.30 Therefore, targeting cyclin/CDK complexes is considered a promising strategy for a number of cancers treatment. In this study, TSA treatment has a significant, dose-dependent inhibitory effect on cyclin D1/CDK4/6 and cyclinE1/CDK2 complexes, and a stimulative effect on P21 and P27 expression in both SRA01/04 and HLEB3 cell lines. These results provide convincing evidence that TSA exerts its inhibitory effects on cell cycle progression primarily via inhibiting cyclin D1/CDK4/6 and cyclin E1/CDK2 complexes, and inducing P21 and P27. Furthermore, G1 cell cycle arrest is a potential mechanism of growth inhibition effect of TSA in LECs.
As is well known, the PI3K/Akt and MAPK signaling pathways are important signal transducers for cell proliferation, differentiation and survival, and also serve as key factors in the regulation of cancer cell invasion and metastasis.24, 25 Therefore, recent studies have focused on inhibiting the PI3K/Akt and MAPKs pathways as potential targets for treating cancers. In addition, the PI3K/Akt and MAPKs pathways are also involved in the proliferation and migration of LECs.31, 32 In our study, TSA inhibited the expression of PI3K, as well as reduced the phosphorylation of Akt, p38MAPK and ERK1/2 in a dose-dependent manner in both SRA01/04 and HLEB3 cell lines. These results indicate that TSA potently inhibits the activation of the PI3K/Akt, p38MAPK and ERK1/2 pathways, leading to a significant decrease in cell growth. This may be another potential mechanism of growth inhibition effect of TSA in LECs.
Emerging evidence is increasingly showing that aberrant expression of HDACs may contribute to the development and progression of various cancers and many other diseases.33, 34 Recent clinical trials about HDAC inhibitors have shown encouraging results in different cancers.16 In present study, we identified that HDAC1, HDAC2, HDAC3, HDAC5, HDAC8 and HDAC10 were upregulated in TGFβ2-induced EMT in SRA01/04 cells, meanwhile, HDAC2, HDAC5 and HDAC10 were upregulated in TGFβ2-induced HLEB3 cells. Earlier studies have reported that HDAC1 is required for TGFβ-induced EMT and cell migration in hepatocytes, and HDAC inhibitors completely suppress TGFβ-induced EMT in murine hepatocytes.20 HDAC2 is also upregulated in TGFβ-induced EMT in rat kidney tubular epithelial cells and streptozotocin-induced diabetic nephropathy.20, 35 Wu et al. also revealed that HDAC3 is essential for hypoxia-induced EMT in liver cells.36 Taken together, our results coincide with these previous findings, however, there is no existing report concerning the role of HDAC5, HDAC8 and HDAC10 in EMT. So it will be interesting and valuable to determine their possible roles in EMT in future studies.
To testify our hypothesis that inhibition of HDACs can abrogate TGFβ-induced EMT in LECs, we used TSA, a class I and II HDAC inhibitor. As expected, the results showed that inhibition of HDACs is capable of reducing the expression of mesenchymal markers (i.e., FN, Col I, Col IV and N-cadherin) and transcription factors (Snail and Slug) completely in both SRA01/04 and HLEB3 cell lines. Next, we wished to determine the underlying molecular mechanism of EMT prevention effect of TSA in LECs.
TGFβ signaling has been established as a central mediator in numerous fibrotic diseases. Specific targeting of the TGFβ signaling pathway is a critical and effective therapeutic strategy for fibrosis. The canonical TGFβ signaling transmits its signals through binding to two transmembrane type I and type II receptors, which subsequently recruits and activates receptor-regulated Smad proteins-Smad2 and/or Smad3.37 The phosphorylation of Smad2 and Smad3, leads them to be dissociated from TGFβ receptor I and form complexes with Smad4, then translocate to the nuclear and regulate target genes expression.38 Therefore, the inhibition of phosphorylation of Smad2 and Smad3 is able to arrest TGFβ signaling pathway transduction. In our study, we demonstrated that TSA inhibited TGFβ2 induced-phosphorylation of Smad2, but has no effect on Smad3 in both SRA01/04 and HLEB3 cell lines. Hence, this indicates that TSA abrogates TGFβ2-induced EMT via partly affecting the canonical TGFβ/Smad2 signaling.
Recently, an increasing number of studies show that the Notch signaling pathway is a key regulator in inducing EMT during embryonic development, fibrotic diseases and cancer metastasis.27 Elevated Jagged/Notch signaling has been verified in a large range of fibrotic diseases in the kidney, liver and lung.39 Moreover, our previous study also demonstrated that the Notch signaling is required in TGFβ2-induced EMT in LECs and retinal pigment epithelium cells (data unpublished). In TGFβ2-induced EMT in LECs, the elements of the Notch signaling pathway, including Jagged-1, Notch-1, Notch-2, together with their target genes Hes-1 and Hey-1, were upregulated, whereas blockade of Notch pathway inhibited TGFβ2-induced EMT (data unpublished). In this study, we found that TSA can markedly attenuate TGFβ2-induced upregulation of Jagged-1, Notch-1, Notch-2, N1ICD, Hes-1 and Hey-1. Collectively, these results provide convincing evidence that the inactivation of Jagged/Notch signaling by TSA is another potential mechanism of EMT abrogation in LECs.
Finally, to further investigate whether TSA can prevent TGFβ2-induced EMT in eye lens in a more complicated system, we used the whole lens culture semi-in vivo model. The ocular lens is an excellent model to study cell behavior.3 The lens is made up of an anterior monolayer of LECs that overlie a mass of aligned fiber cells, all surrounded by the lens capsule. Numerous studies have shown that abnormal TGFβ signaling in the eye results in EMT of LECs that bear a morphologic and molecular resemblance to some forms of human cataract, including ASC and PCO.6, 28, 40 In present study, using the whole lens culture model, we found that inhibition of HDAC activity with TSA in cultured lenses effectively blocked TGFβ2-induced EMT and cataract formation in this semi-in vivo model.
In summary, our results provided, for the first time, evidence that several family members of class I and II HDACs were upregulated in TGFβ2-induced EMT in human LECs. Inhibition of HDAC activity with TSA strongly inhibits the proliferation and TGFβ2-induced EMT in human LECs. The mechanism underlying proliferation suppression by TSA is the cell cycle arrest and inactivation of the PI3K/Akt, p38MAPK and ERK1/2 pathways. Meanwhile, the underlying mechanism of EMT inhibition by TSA is blockade of the canonical TGFβ/Smad2 and Jagged/Notch signaling pathways. Therefore, this study provides a new insight into the pathogenesis of ASC and PCO in an epigenetic aspect. Epigenetic regulators, represented by HDAC inhibitors, may be novel therapeutic agents for the prevention and treatment of ocular fibrotic diseases, such as ASC and PCO.
Materials and Methods
Reagents and antibodies
Recombinant human TGFβ2 was purchased from Cell Signaling (Danvers, MA, USA). TSA (a class I and II HDAC inhibitor) was purchased from Sigma-Aldrich (Louis, MO, USA). Antibodies against cyclin D1, cyclin E1, CDK2, CDK4, CDK6, P21, P27, acetyl-histone H3 (Lys9), acetyl-histone H3 (Lys14), acetyl-histone H4 (Lys8), Jagged-1, Notch-1, Notch-2, p-Smad2/3, Snail, Slug, PI3K, p-Akt, Akt, p38MAPK, p-p38MAPK, ERK1/2, p-ERK1/2, horse anti-mouse and goat anti-rabbit horseradish peroxidase conjugates secondary antibodies, Alexa Fluor 488-conjugated goat anti-rabbit and Alexa Fluor 555-conjugated donkey anti-mouse secondary antibodies were purchased from Cell Signaling. Antibodies against β-actin, FN, Col I, Col IV, N-cadherin, α-SMA, vimentin and N1ICD were purchased from Abcam (Cambridge, UK). CCK-8 kit was purchased from Dojindo (Shanghai, China).
Cells culture and treatment
The human lens epithelial cell line SRA01/04 and HLEB3 were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. The cells were grown at 37 °C in a humidified atmosphere containing 5% CO2 and dissociated with 0.25% trypsin-0.02% EDTA solution.
For TGFβ2 and TSA treatments, the cells were grown in six-well plates and treated with 10 ng/ml recombinant human TGFβ2 and various concentrations of TSA for different time points.
Cell proliferation assay
To assess proliferation, LEC lines SRA01/04 and HLEB3 were seeded into 96-well plates at the density of 5 × 103 cells/well and grown overnight. Then the cells were treated with increasing doses of TSA for 24 and 48 h, respectively. At the end of the treatment period, 10 μl of CCK-8 solution was added to each well and incubated for 2 h. The absorbance (A) at 450 nm was measured using a microplate reader.
Cell apoptosis assay
Cells were seeded on six-well plates and treated with increasing doses of TSA for 48 h, and then cells were harvested, washed with PBS and stained with Annexin V-FITC/PI (Becton Dickinson, Franklin Lakes, NJ, USA) at room temperature for 15 min. Samples were analyzed by flow cytometry.
Cell-cycle analysis
Cells were treated with increasing doses of TSA for 24 h, and then cells were harvested and fixed in cold 70% ethanol overnight at 4 °C. The cells were centrifuged down and resuspended in 0.2 ml propidium iodide staining solution for 45 min incubation at room temperature. Finally, the DNA content of cells was measured using a flow cytometry (BD Biosciences, San Jose, CA, USA).
Scratch wound assay
The effect of TSA on TGFβ2-induced migration of LECs was evaluated by scratch wound assay. In brief, LECs was wounded using a 100-μl yellow micropipette tip and incubated with TGFβ2 in the presence or absence of TSA for 24 h. Wound closure was monitored under an inverted phase-contrast microscope and photographed in digital format ( × 50) after incubation for 24 h. For quantitative assessment, the area of the wound was determined by Image-Pro Plus software 5.1 (Media Cybernetics, Inc. Silver Spring, MD, USA).
Real-time PCR analysis for gene expression
Total RNA was isolated from LECs and rat lenses using Trizol reagent (Invitrogen, Carlsbad, CA, USA). The concentration of total RNA was quantified by spectrophotometry and cDNA was synthesized with a reverse transcription kit (Takara; Siga, Japan). For quantitative analysis of mRNA expression, SYBR PrimeScript RT-PCR kit (Takara) was used to amplify the target genes and reactions were performed with the ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA, USA) according to the manufacture's protocol. Glyceraldehyde 3-phosphate dehydrogenase was used as an internal control.
Immunofluorescence
LECs (1 × 105) were grown on cover slips in six-well plates and treated as described previously. After fixed with acetone for 10 min and permeabilized with 0.1% Triton X-100 for 5 min, cells were blocked with 1% bovine serum albumin (BSA) in PBS for 1 h. Then they were incubated with different primary antibodies (diluted in blocking solution) at 4 °C overnight. On the next day, cells were incubated with Alexa Fluor 555-conjugated donkey anti-mouse or Alexa Fluor 488-conjugated goat anti-rabbit secondary antibody (1 : 200) for 1 h at room temperature. After washing with PBS containing 0.1% Tween-20, cells were incubated with 50 ng/ml DAPI for 5 min to stain nuclei. The slides were mounted with anti-fade fluorescent mounting medium and images were acquired by a fluorescence confocal microscope (LSM510; Carl Zeiss, Oberkochen, Germany).
Western blot analysis for protein expression
Cells were lysed in RIPA buffer and protein was collected after centrifugation and mixed with 5 × SDS sample buffer. The samples were separated by 10% SDS-PAGE, and then transferred to PVDF membranes. The membranes were blocked in 5% nonfat milk for 1 h and subsequently incubated with different primary antibodies at 4 °C overnight. After 1 h incubation with horseradish peroxidase-conjugated secondary antibodies, the protein bands were detected with chemiluminescence detection reagents. β-Actin was used as loading control.
Lens culture and treatment
All experimental procedures conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Lenses of 21–22 days Wistar rats were culture as described previously.28, 41 Briefly, whole lenses were carefully removed using forceps and a pair of fine scissors, and maintained in 4 ml serum-free M199 medium (two lenses per dish) containing 0.1% BSA, 0.1 mg/ml L-glutamine, 50 IU/ml penicillin and 50 mg/ml streptomycin. TGFβ2 and TSA were added to the culture medium at the final concentrations of 5 ng/ml and 0.4 μM, respectively. Culture medium was renewed every second day throughout the culture period. Lenses were cultured for up to 7 days and photographed using a dissecting microscope.
Immunofluorescent staining of frozen sections
At the end of the culture period, the lenses were embedded in optimal cutting temperature compound at −20 °C. Sections (6 μm) were cut using a cryostat and fixed in cold acetone for10 min, then permeabilized with 0.1% Triton X-100 for 5 min. Sections were then blocked with 1% BSA in PBS for 1 h followed by overnight incubation with different primary antibodies (dilution in above buffer) at 4 °C in a humidity chamber. Next day, the sections were incubated with Alexa Fluor 555-conjugated donkey anti-mouse secondary antibody (1 : 200) for 1 h at room temperature. After staining the nuclei, sections were mounted and images were captured using a fluorescence confocal microscope.
Statistical analysis
Experiments presented in the figures were representative of three or more different repetitions. All data were expressed as mean±S.E.M. and analyzed with SPSS 15.0 software (SPSS Inc., Chicago, IL, USA). One-way analysis of variance was used to compare differences among groups. A value of P<0.05 was considered statistically significant.
Acknowledgments
The research was funded by the grant from the National Natural Science Foundation of China (no. 81270981 and 81000371).
Glossary
- ASC
anterior subcapsular cataract
- PCO
posterior capsule opacification
- EMT
epithelial–mesenchymal transition
- LECs
lens epithelial cells
- TGFβ
transforming growth factor β
- Col I
collagen type I
- Col IV
collagen type IV
- HDAC
histone deacetylases
- CDK
cyclin-dependent kinase
- PI3K
phosphatidylinositol-3-kinase
- MAPKs
mitogen-activated protein kinases
- ERK
extracellular signal-regulated kinase
- N1ICD
Notch-1 intracellular domain
- FN
fibronectin
- TSA
tricostatin A
- BSA
bovine serum albumin
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by A Finazzi-Agró
Supplementary Material
References
- McCarty CA, Taylor HR. Recent developments in vision research: light damage in cataract. Invest Ophthalmol Vis Sci. 1996;37:1720–1723. [PubMed] [Google Scholar]
- Nathu Z, Dwivedi DJ, Reddan JR, Sheardown H, Margetts PJ, West-Mays JA. Temporal changes in MMP mRNA expression in the lens epithelium during anterior subcapsular cataract formation. Exp Eye Res. 2009;88:323–330. doi: 10.1016/j.exer.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin EH, Basson MA, Robinson ML, McAvoy JW, Lovicu FJ. Sprouty is a negative regulator of transforming growth factor beta-induced epithelial-to-mesenchymal transition and cataract. Mol Med. 2012;18:861–873. doi: 10.2119/molmed.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apple DJ, Solomon KD, Tetz MR, Assia EI, Holland EY, Legler UF, et al. Posterior capsule opacification. Surv Ophthalmol. 1992;37:73–116. doi: 10.1016/0039-6257(92)90073-3. [DOI] [PubMed] [Google Scholar]
- Hodge WG. Posterior capsule opacification after cataract surgery. Ophthalmology. 1998;105:943–944. doi: 10.1016/S0161-6420(98)96040-7. [DOI] [PubMed] [Google Scholar]
- Srinivasan Y, Lovicu FJ, Overbeek PA. Lens-specific expression of transforming growth factor beta1 in transgenic mice causes anterior subcapsular cataracts. J Clin Invest. 1998;101:625–634. doi: 10.1172/JCI1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Iongh RU, Wederell E, Lovicu FJ, McAvoy JW. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs. 2005;179:43–55. doi: 10.1159/000084508. [DOI] [PubMed] [Google Scholar]
- Wallentin N, Wickstrom K, Lundberg C. Effect of cataract surgery on aqueous TGF-beta and lens epithelial cell proliferation. Invest Ophthalmol Vis Sci. 1998;39:1410–1418. [PubMed] [Google Scholar]
- Meacock WR, Spalton DJ, Stanford MR. Role of cytokines in the pathogenesis of posterior capsule opacification. Br J Ophthalmol. 2000;84:332–336. doi: 10.1136/bjo.84.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JB, Davidson MG, Nasisse MP, Fleisher LN, McGahan MC. The lens influences aqueous humor levels of transforming growth factor-beta 2. Graefes Arch Clin Exp Ophthalmol. 1998;236:305–311. doi: 10.1007/s004170050083. [DOI] [PubMed] [Google Scholar]
- Awasthi N, Guo S, Wagner BJ. Posterior capsular opacification: a problem reduced but not yet eradicated. Arch Ophthalmol. 2009;127:555–562. doi: 10.1001/archophthalmol.2009.3. [DOI] [PubMed] [Google Scholar]
- Reichert N, Choukrallah MA, Matthias P. Multiple roles of class I HDACs in proliferation, differentiation, and development. Cell Mol Life Sci. 2012;69:2173–2187. doi: 10.1007/s00018-012-0921-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Yan H, Zhuang S. Histone deacetylases as targets for treatment of multiple diseases. Clin Sci (Lond) 2013;124:651–662. doi: 10.1042/CS20120504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Heckman CA, Boxer LM. Histone deacetylase inhibitors down-regulate bcl-2 expression and induce apoptosis in t(14;18) lymphomas. Mol Cell Biol. 2005;25:1608–1619. doi: 10.1128/MCB.25.5.1608-1619.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson C, Mizzau M, Paroni G, Maestro R, Schneider C, Brancolini C. Role of caspases, Bid, and p53 in the apoptotic response triggered by histone deacetylase inhibitors trichostatin-A (TSA) and suberoylanilide hydroxamic acid (SAHA) J Biol Chem. 2003;278:12579–12589. doi: 10.1074/jbc.M213093200. [DOI] [PubMed] [Google Scholar]
- Barneda-Zahonero B, Parra M. Histone deacetylases and cancer. Mol Oncol. 2012;6:579–589. doi: 10.1016/j.molonc.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts K, Niki T, Greenwel P, Vandermonde A, Wielant A, Hellemans K, et al. Trichostatin A, a histone deacetylase inhibitor, suppresses collagen synthesis and prevents TGF-beta(1)-induced fibrogenesis in skin fibroblasts. Exp Cell Res. 2002;278:184–197. doi: 10.1006/excr.2002.5577. [DOI] [PubMed] [Google Scholar]
- Van Beneden K, Geers C, Pauwels M, Mannaerts I, Wissing KM, Van den Branden C, et al. Comparison of trichostatin A and valproic acid treatment regimens in a mouse model of kidney fibrosis. Toxicol Appl Pharmacol. 2013;271:276–284. doi: 10.1016/j.taap.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Niki T, Rombouts K, De Bleser P, De Smet K, Rogiers V, Schuppan D, et al. A histone deacetylase inhibitor, trichostatin A, suppresses myofibroblastic differentiation of rat hepatic stellate cells in primary culture. Hepatology. 1999;29:858–867. doi: 10.1002/hep.510290328. [DOI] [PubMed] [Google Scholar]
- Lei W, Zhang K, Pan X, Hu Y, Wang D, Yuan X, et al. Histone deacetylase 1 is required for transforming growth factor-beta1-induced epithelial-mesenchymal transition. Int J Biochem Cell Biol. 2010;42:1489–1497. doi: 10.1016/j.biocel.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M, Hishikawa K, Marumo T, Fujita T. Inhibition of histone deacetylase activity suppresses epithelial-to-mesenchymal transition induced by TGF-beta1 in human renal epithelial cells. J Am Soc Nephrol. 2007;18:58–65. doi: 10.1681/ASN.2005111187. [DOI] [PubMed] [Google Scholar]
- Rishikof DC, Ricupero DA, Liu H, Goldstein RH. Phenylbutyrate decreases type I collagen production in human lung fibroblasts. J Cell Biochem. 2004;91:740–748. doi: 10.1002/jcb.10742. [DOI] [PubMed] [Google Scholar]
- Kerl K, Ries D, Unland R, Borchert C, Moreno N, Hasselblatt M, et al. The histone deacetylase inhibitor SAHA acts in synergism with fenretinide and doxorubicin to control growth of rhabdoid tumor cells. BMC Cancer. 2013;13:286. doi: 10.1186/1471-2407-13-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems L, Tamburini J, Chapuis N, Lacombe C, Mayeux P, Bouscary D. PI3K and mTOR signaling pathways in cancer: new data on targeted therapies. Curr Oncol Rep. 2012;14:129–138. doi: 10.1007/s11912-012-0227-y. [DOI] [PubMed] [Google Scholar]
- Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Li Y, Kong D, Sarkar FH. The role of Notch signaling pathway in epithelial-mesenchymal transition (EMT) during development and tumor aggressiveness. Curr Drug Targets. 2010;11:745–751. doi: 10.2174/138945010791170860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales AM, Chamberlain CG, McAvoy JW. Cataract induction in lenses cultured with transforming growth factor-beta. Invest Ophthalmol Visual Sci. 1995;36:1709–1713. [PubMed] [Google Scholar]
- Hallstrom TC, Nevins JR. Balancing the decision of cell proliferation and cell fate. Cell Cycle. 2009;8:532–535. doi: 10.4161/cc.8.4.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulappa T, Reddy RS, Suman S, Nyakeriga AM, Damodaran C. Molecular interplay between cdk4 and p21 dictates G0/G1 cell cycle arrest in prostate cancer cells. Cancer Lett. 2013;337:177–183. doi: 10.1016/j.canlet.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Park SY, Joo CK. Hepatocyte growth factor induces proliferation of lens epithelial cells through activation of ERK1/2 and JNK/SAPK. Invest Ophthalmol Visual Sci. 2004;45:2696–2704. doi: 10.1167/iovs.03-1371. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Zhou C, Bi Z, Wan Y. EGF-induced cell migration is mediated by ERK and PI3K/AKT pathways in cultured human lens epithelial cells. J Ocul Pharmacol Ther. 2006;22:93–102. doi: 10.1089/jop.2006.22.93. [DOI] [PubMed] [Google Scholar]
- Gloghini A, Buglio D, Khaskhely NM, Georgakis G, Orlowski RZ, Neelapu SS, et al. Expression of histone deacetylases in lymphoma: implication for the development of selective inhibitors. Br J Haematol. 2009;147:515–525. doi: 10.1111/j.1365-2141.2009.07887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Janadi A, Chandana SR, Conley BA. Histone deacetylation: an attractive target for cancer therapy. Drugs R&D. 2008;9:369–383. doi: 10.2165/0126839-200809060-00003. [DOI] [PubMed] [Google Scholar]
- Noh H, Oh EY, Seo JY, Yu MR, Kim YO, Ha H, et al. Histone deacetylase-2 is a key regulator of diabetes- and transforming growth factor-beta1-induced renal injury. Am J Physiol Renal Physiol. 2009;297:F729–F739. doi: 10.1152/ajprenal.00086.2009. [DOI] [PubMed] [Google Scholar]
- Wu MZ, Tsai YP, Yang MH, Huang CH, Chang SY, Chang CC, et al. Interplay between HDAC3 and WDR5 is essential for hypoxia-induced epithelial-mesenchymal transition. Mol Cell. 2011;43:811–822. doi: 10.1016/j.molcel.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Akhurst RJ, Hata A. Targeting the TGFbeta signalling pathway in disease. Nat Rev Drug Discov. 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi ME, Ding Y, Kim SI. TGF-beta signaling via TAK1 pathway: role in kidney fibrosis. Sem Nephrol. 2012;32:244–252. doi: 10.1016/j.semnephrol.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A. Targeting the jagged/notch pathway: a new treatment for fibrosis. J Cell Commun Signaling. 2010;4:197–198. doi: 10.1007/s12079-010-0101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor TB, Jr, Roberts AB, Sporn MB, Danielpour D, Dart LL, Michels RG, et al. Correlation of fibrosis and transforming growth factor-beta type 2 levels in the eye. J Clin Invest. 1989;83:1661–1666. doi: 10.1172/JCI114065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales AM, Chamberlain CG, McAvoy JW. Susceptibility to TGFbeta2-induced cataract increases with aging in the rat. Invest Ophthalmol Vis Sci. 2000;41:3544–3551. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.