Dear Editor,
Anti-apoptotic Bcl-2 family proteins Bcl-2, Bcl-xL, and Mcl-1 have become prime targets for anti-cancer agents in an effort to overcome blocks in apoptosis exhibited by cancer cells.1 Marinopyrrole A, a marine natural product originally developed for its activity against Methicillin-resistant Staphylococcus aureus,2, 3 was recently reported by Doi et al.4 to be a selective Mcl-1 antagonist that binds to Mcl-1 and targets it for degradation. This publication was received with great interest because the discovery of a specific Mcl-1 inhibitor had thus far eluded researchers. Indeed, the only agents reported to target Mcl-1 to date are general transcription inhibitors, which show limited selectivity for Mcl-1 due to its short half-life.5, 6 However, previous studies in human colon carcinoma cells reported actin as the primary target of marinopyrrrole A.2 This raised the possibility that Mcl-1 may not be the primary target for marinopyrrole A in cancer cells. We therefore sought to determine whether marinopyrrole A was indeed a bona fide-specific Mcl-1 inhibitor.
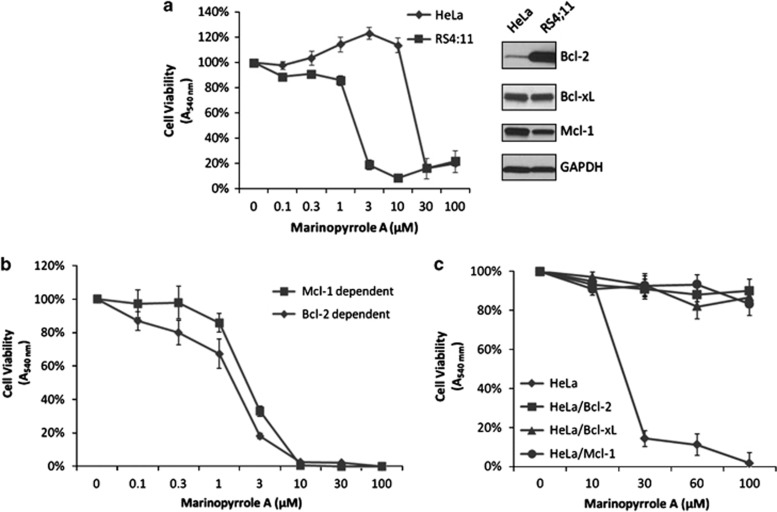
Our recent studies testing the viability of HeLa cells after systematic knockdown of individual anti-apoptotic Bcl-2 proteins demonstrated strict dependence on Mcl-1 (Eichhorn et al., manuscript submitted). We first compared the sensitivity to marinopyrrole of Mcl-1-dependent HeLa cells versus RS4;11 human leukemia cells which have previously been shown to be dependent on Bcl-2 for survival.7 HeLa and RS4;11 cells were treated with increasing concentrations of natural marinopyrrole A (0.1–100 μM) and cell viability assessed after 72 h (Figure 1a). Relative expression levels of Bcl-2, Bcl-xL, and Mcl-1 (Figure 1a, right) highlight Bcl-2 overexpression in the RS4;11 cell line. Surprisingly, marinopyrrole A was much more effective against Bcl-2-dependent RS4;11 cells (IC50: 2 μM) when compared to Mcl-1-dependent HeLa cells (IC50: 20 μM) (Figure 1a). Immunoblotting indicated that marinopyrrole A treatment did not affect Mcl-1 expression levels (data not shown).
Figure 1.
Marinopyrrole A is not selectively cytotoxic for Mcl-1-dependent cell lines. Cell viability was assessed using an MTT ((3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) cell viability assay. Cells were untreated (100%) or treated with marinopyrrole A (0.1–100 μM) for 72 h. Results given are mean±s.d. (n=6). (a) Left: cell viability curves for HeLa and RS4:11 (Bcl-2 dependent) cells. Right: immunoblot analysis of relative levels of anti-apoptotic Bcl-2, Bcl-xL, and Mcl-1. GAPDH was used as a loading control. (b) Cell viability curves for Mcl-1-dependent and Bcl-2-dependent leukemia cells. (c) Cell viability curves for control HeLa cells and HeLa cells generated to stably overexpress untagged full-length Bcl-2, Bcl-xL, or Mcl-1. Cell extraction, immunoblotting, and cell viability assays using MTT dye were performed as described previously.9 Antibodies against Bcl-2 (sc-509) and Mcl-1 (sc-819) were from Santa Cruz (Santa Cruz, CA) and antibodies against Bcl-xL (2762) and GAPDH (14C10) were from Cell Signaling (Beverly, MA)
We next tested whether natural marinopyrrole A would show selective cytotoxicity against Mcl-1- versus Bcl-2-dependent leukemia cell lines as reported by Doi et al.4 For this purpose we chose two murine leukemia cell lines generated from transgenic mice and established via multiple criteria to be either dependent on Mcl-1 or Bcl-2.8 Marinopyrrole A was equally effective against Mcl-1-dependent leukemia cells (IC50: 2.5 μM) as Bcl-2-dependent cells (IC50: 2 μM; Figure 1b).
It is important to note that data presented by Doi et al.4 utilized a racemic mixture of synthetic marinopyrrole A, whereas our data and previous work done by Hughes et al.2 were performed using the natural M-(-)-enantiomer. Therefore, cell viability assays were conducted in the Mcl-1- and Bcl-2-dependent leukemia cell lines using the non-natural P-(+)-enantiomer, and no statistical difference between the two enantiomers was observed (results not shown).
Finally, we tested whether overexpression of Bcl-2, Bcl-xL, or Mcl-1 would protect HeLa cells from marinopyrrole A. HeLa cells stably overexpressing Bcl-2, Bcl-xL, or Mcl-1 were treated with marinopyrrole A for 72 h and cell viability was assessed. Strikingly, overexpression of each anti-apoptotic Bcl-2 family member conferred complete resistance to marinopyrrole A (Figure 1c). Thus, marinopyrrole A failed to show selective cytotoxicity towards cells overexpressing Mcl-1 in contrast to previously reported data.4 The data in Figure 1c indicate that the death signals generated from marinopyrrole A activate the intrinsic apoptotic pathway.
The results reported here are in stark contrast to previously reported data showing that marinopyrrole A induces cell death selectively in Mcl-1-dependent but not Bcl-2-dependent cells.4 While further studies are needed to establish the basis of this discrepancy, taken together, our results seriously question the validity of marinopyrrole A as a specific Mcl-1 inhibitor.
Acknowledgments
Thank you to Dr. Anthony Letai for generously providing the Mcl-1- and Bcl-2-dependent cell lines.
The authors declare no conflict of interest.
References
- Letai AG. Nat Rev Cancer. 2008. pp. 121–132. [DOI] [PubMed]
- Hughes CC, et al. J Am Chem Soc. 2009. pp. 12094–12096. [DOI] [PMC free article] [PubMed]
- Haste NM, et al. Antimicrobial Agents Chemother. 2011. pp. 3305–3312. [DOI] [PMC free article] [PubMed]
- Doi K, et al. J Biol Chem. 2012. pp. 10224–10235. [DOI] [PMC free article] [PubMed]
- Wei G, et al. Cancer Cell. 2012. pp. 547–562. [DOI] [PMC free article] [PubMed]
- Zhang S, et al. Cell Signalling. 2012.
- Del Gaizo Moore V, et al. Blood. 2008. pp. 2300–2309. [DOI] [PMC free article] [PubMed]
- Brunelle JK, et al. J Cell Biol. 2009. pp. 429–442. [DOI] [PMC free article] [PubMed]
- Sakurikar, et al. J Biol Chem. 2012. p. 39193. [DOI] [PMC free article] [PubMed]