Abstract
Compound C, a well-known inhibitor of AMP-activated protein kinase (AMPK), has been reported to induce apoptosis in some types of cells. However, the underlying mechanisms remain largely unclear. Using a DNA microarray analysis, we found that the expression of many genes was downregulated upon treatment with compound C. Importantly, compound C caused transcriptional repression with the induction of p53, a well-known marker of transcriptional stress response, in several cancer cell lines. Compound C did not induce the phosphorylation of p53 but dramatically increased the protein level of p53 similar to some other transcriptional inhibitors, including 5,6-dichloro-1-β-D-ribobenzimidazole (DRB). Consistent with previous reports, we found that compound C initiated apoptotic death of cancer cells in an AMPK-independent manner. Similar to DRB and actinomycin D (ActD), two classic transcription inhibitors, compound C not only resulted in the loss of Bcl-2 and Bcl-xl protein but also induced the phosphorylation of eukaryotic initiation factor-alpha (eIF2α) on Ser51. Hence, the phosphorylation of eIF2α might be a novel marker of transcriptional inhibition. It is noteworthy that compound C-mediated apoptosis of cancer cells is correlated with decreased expression of Bcl-2 and Bcl-xl and the phosphorylation of eIF2α on Ser51. Remarkably, compound C exhibits potent anticancer activities in vivo. Taken together, our data suggest that compound C may be an attractive candidate for anticancer drug development.
Keywords: compound C, transcriptional inhibition, apoptosis, cancer
Finding therapies that target and kill cancer cells is a major goal of most cancer therapeutic approaches. Several cell death modalities, including programmed cell death, necrosis and mitotic catastrophe can initiate cancer cells death.1, 2, 3, 4, 5 Irradiation and drugs used for cancer chemotherapy always result in DNA damage and transcriptional stress response, which can lead to apoptotic death, in some cancer cells.4
Transcription is an essential process for gene expression in both prokaryotic and eukaryotic cells. Genes coding for proteins in eukaryotes are transcribed by the concerted action of a number of transcription-related factors, chief among them is the RNA polymerase II.6, 7, 8 With the inhibition of transcription over a certain period of time, cells will be destroyed by apoptosis.9, 10, 11 It has been shown that DNA-damaging agents and transcriptional inhibitors can induce cell apoptosis in a time-dependent manner.11, 12 Transcriptional inhibitors are drugs that inhibit global transcription by different mechanisms. Transcriptional inhibitors induce apoptosis partially by inhibiting the expression of labile antiapoptotic proteins because of their ability to downregulate proteins of short half-life.10, 13, 14, 15 The accumulation of p53 is one of the hallmarks of transcriptional stress.11 However, transcriptional repression results in apoptosis by both p53-dependent or -independent mechanisms.11 Importantly, transformed cells appear to be more sensitive to transcriptional stress induced by disruption of RNA synthesis than normal cells.12, 16 General transcriptional inhibitors may be useful in cancer therapies and, in some instances, have been shown to work as antiviral agents.13, 17 Some of these drugs, such as flavopiridol and seliciclib, are potential drugs against different types of cancer.18, 19, 20, 21, 22, 23
AMP-activated protein kinase (AMPK) is a principal intracellular energy sensor, which has an essential role as a master regulator of cellular energy homeostasis. AMPK is activated by an increasing cellular AMP/ATP ratio caused by metabolic stresses that interfere with ATP production or that accelerate ATP consumption.24, 25, 26, 27, 28, 29 AMPK maintains the energy balance through the direct phosphorylation of target proteins or via transcriptional control of target genes.30, 31 Compound C, also known as dorsomorphin, has been described as a pharmacological AMPK inhibitor that efficiently blocks metabolic actions of AMPK.32 It has been reported that compound C attenuates the apoptotic activities of AMPK in various cell types.33, 34, 35, 36, 37, 38, 39 In contrast, it has also been suggested that compound C treatment directly causes apoptosis in some cancer cells, including breast cancer cells and skin cancer cells.40, 41, 42, 43
In this study, we investigated the potential association between compound C and apoptosis in different cancer cells. We demonstrated that compound C is a potent apoptosis inducer in various cancer cells. Interestingly, compound C initiates transcriptional inhibition and p53 induction in cancer cells. Further, we found that compound C-mediated apoptosis is associated with the decreased expression of Bcl-2 and Bcl-xl, and the phosphorylation of eIF2α. Importantly, compound C exhibits potent anticancer activities in vivo.
Results
Compound C initiates apoptosis in cancer cells
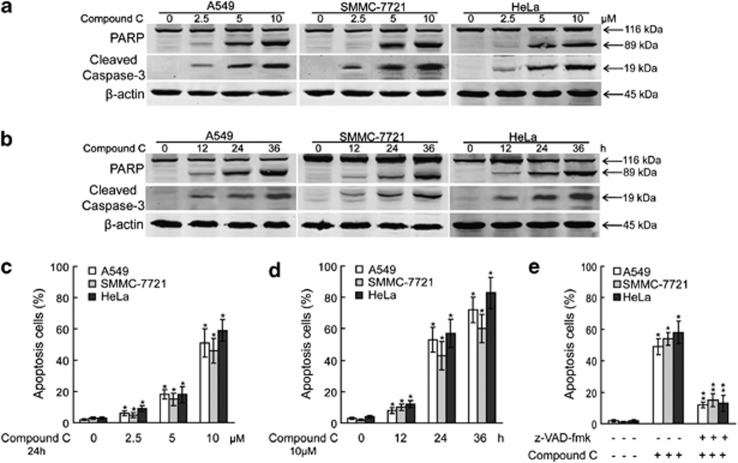
To investigate the potential role of compound C in the induction of apoptosis in cancer cells, we first examined the proapoptotic effects of compound C in A549, SMMC-7721 and HeLa cells. Western blot analysis revealed that compound C treatment induced the cleavage of PARP and caspase-3 in A549, SMMC-7721 and HeLa cells in a concentration-dependent manner (Figure 1a). Moreover, compound C resulted in the activation of caspase-3 and PARP cleavage in A549, SMMC-7721 and HeLa cells in a time-dependent manner (Figure 1b). The effects of compound C on apoptosis induction in cancer cells in a concentration- and time-dependent manner were reconfirmed by annexin V-FITC apoptosis analysis (Figures 1c and d). Importantly, compound C-mediated apoptosis was obviously inhibited by the pretreatment of z-VAD-fmk, a specific inhibitor of caspase (Figure 1e). Thus, compound C induces apoptosis in a caspase-dependent manner.
Figure 1.
Compound C induces apoptosis. (a) A549, SMMC-7721 and HeLa cells were treated with indicated amounts of compound C for 24 h and cell lysates were analyzed for the levels of indicated proteins. (b) A549, SMMC-7721 and HeLa cells were treated with 10 μmol/l compound C for indicated periods and cell lysates were analyzed for the levels of indicated proteins. (c) A549, SMMC-7721 and HeLa cells were treated with indicated amounts of compound C for 24 h and apoptosis were analyzed using flow cytometry after staining with FITC-conjugated Annexin V and propidium iodide. (d) A549, SMMC-7721 and HeLa cells were treated with 10 μmol/l compound C for indicated periods and apoptosis were analyzed using flow cytometry after staining with FITC-conjugated Annexin V and propidium iodide. (e) A549, SMMC-7721 and HeLa cells were treated with 10 μmol/l compound C for 24 h with or without z-VAD-fmk (50 μmol/l) pretreatment for 1 h and apoptosis were analyzed using flow cytometry after staining with FITC-conjugated Annexin V and propidium iodide. Data are presented as mean values±S.D. of three measurements. Columns, mean of three individual experiments; bars, S.D. * Significantly different from control value; ** Significantly different from *value
To make sure whether inhibition of AMPK is involved in compound C-mediated apoptosis, specific small-interfering RNA (siRNA) was used to block the expression of MAPKα. The results showed that AMPKα knockdown had no evident effects on compound C-mediated apoptosis in A549, SMMC-7721 and HeLa cells (Supplementary Figure 1). Taken together, these data suggest that compound C initiates apoptosis of cancer cells in an AMPK-independent manner.
Compound C inhibits transcription in cancer cells
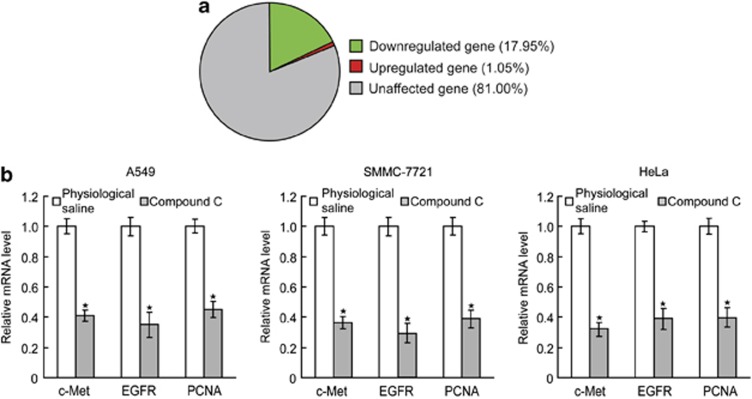
To identify the molecules involved in the proapoptotic activities of compound C, we compared the gene expression profiles of compound C-treated A549 cells with physiological saline-treated A549 cells using microarray analysis. Using a DNA microarray, we analyzed about 32 000 genes for changes in expression upon compound C treatment. Statistical analysis indicated that 6030 genes had a significant change in expression levels, of which 5713 (17.85% of total genes) and 317 (0.99% of total genes) were more than twofold down- or upregulated (Figure 2a). We tested some of the downregulated genes, such as hepatocyte growth factor receptor (c-Met), epidermal growth factor receptor (EGFR) and proliferating cell nuclear antigen (PCNA), using real-time RT-PCR analysis. The results reconfirmed that c-Met, EGFR and PCNA were downregulated in A549, SMMC-7721 and HeLa cells after compound C treatment for 24 h (Figure 2b).
Figure 2.
Compound C represses gene expression. (a) A549 cells were treated with 10 μmol/l compound C for 24 h and gene expressions were analyzed using DNA microarray analysis. (b) A549, SMMC-7721 and HeLa cells were treated with 10 μmol/l compound C for 24 h, and c-Met, EGRR and PCNA mRNA levels were analyzed using real-time RT-PCR. Data are presented as mean values±S.D. of three measurements. Columns, mean of three individual experiments; bars, S.D. * Significantly different from control value
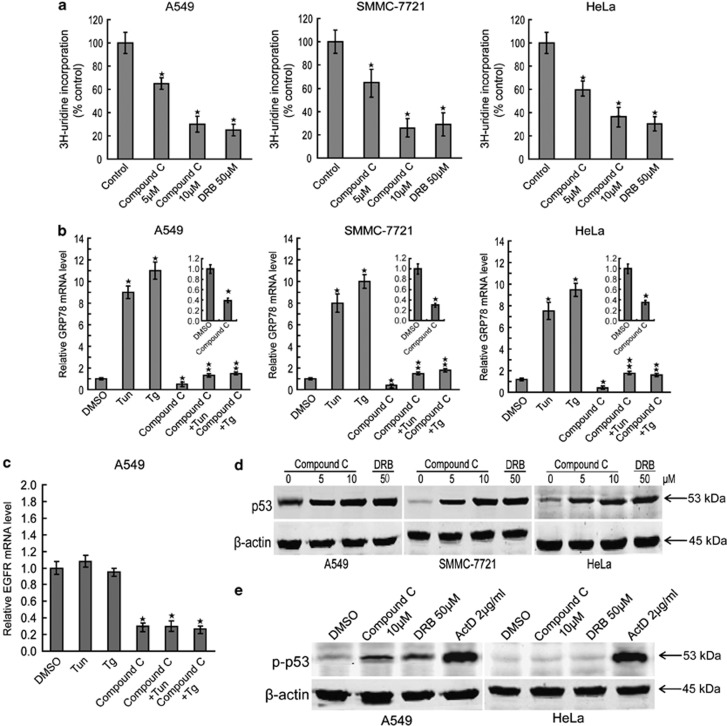
To investigate the impact of compound C on transcription, mRNA synthesis was measured in compound C-treated cancer cells in the presence of [3H]uridine. It is noteworthy that compound C inhibited mRNA synthesis in a concentration-dependent manner in A549, SMMC-7721 and HeLa cells (Figure 3a), indicating that compound C inhibits transcription in cancer cells. As endoplasmic reticulum stress (ER stress) activation can induce the expression of specific mRNA,44, 45, 46 such as 78 kDa glucose-regulated protein (GRP78) mRNA, the impact of compound C on ER stress-induced GRP78 mRNA synthesis was tested. Importantly, compound C treatment not only decreased GRP78 mRNA but also blocked ER stress inducers tunicamycin (Tun)- and thapsigargin (Tg)-induced GRP78 mRNA synthesis in A549, SMMC-7721 and HeLa cells (Figure 3b). These results reconfirm the function of transcriptional inhibition of compound C. Further, compound C induced the downregulation of EGFR mRNA, which is not regulated by ER stress, under ER stress conditions (Figure 3c), suggesting that the effects of compound C on ER stress-induced transcription inhibition is nonspecific.
Figure 3.
Compound C inhibits transcription. (a) A549, SMMC-7721 and HeLa cells were treated with indicated amounts of compound C or physiological saline for 24 h in medium containing 0.5% FBS. The cells were pulsed with [3H]thymidine (1 μCi per well) for 6 h, and then fixed with 5% trichloroacetic acid and solubilized before scintillation counting. DRB was used as the positive control. (b) A549, SMMC-7721 and HeLa cells were treated with 2.5 μg/ml tunicamycin (Tun) or 1 μmol/l thapsigargin (Tg) for 12 h with or without 10 μmol/l compound C pretreatment for 1 h, and GRP78 mRNA levels were analyzed using real-time RT-PCR. (c) A549 cells were treated with 2.5 μg/ml Tun or 1 μmol/l Tg for 12 h with or without 10 μmol/l compound C pretreatment for 1 h, and EGFR mRNA levels were analyzed using real-time RT-PCR. (d) A549, SMMC-7721 and HeLa cells were treated with indicated amounts of compound C for 24 h and cell lysates were analyzed for the levels of indicated proteins. DRB was used as the positive control. (e) A549 and HeLa cells were treated with 10 μmol/l of compound C for 24 h and cell lysates were analyzed for the levels of indicated proteins. DRB was used as the negative control and ActD was used as the positive control. Data are presented as mean values±S.D. of three measurements. Columns, mean of three individual experiments; bars, S.D. * Significantly different from control value; ** Significantly different from *value
As p53 is one of the hallmarks of global transcriptional repression,10, 11, 47, 48, 49 it is reasonable to suggest that compound C might initiate the induction of p53. This notion is supported by the data that compound C resulted in the accumulation of p53 in A549, SMMC-7721 and HeLa cells (Figure 3d). In line with DRB, compound C induced the accumulate p53 proteins that lacks evident phosphorylation on Ser15 site (Figure 3e). However, ActD efficiently initiated the phosphorylation of p53 on Ser15 (Figure 3e). Taken together, these data indicate that compound C is a potent suppressor of transcription.
Bcl-2 and Bcl-xl downregulation promotes compound C-induced apoptosis in cancer cells
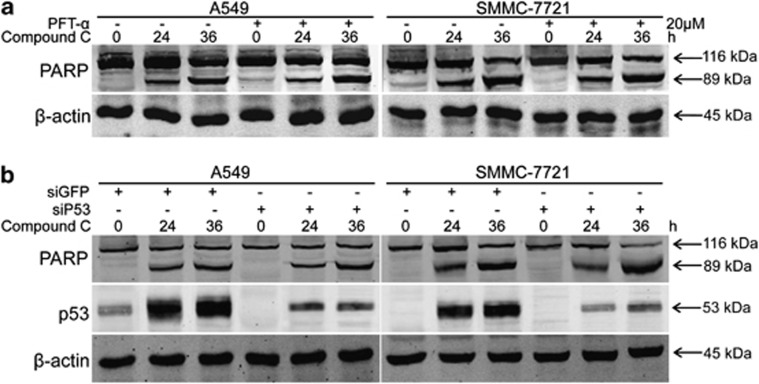
As transcriptional stress can initiate cell apoptosis in a p53-dependent or p53-independent manner,11, 50 we assessed whether p53 is involved in compound C-mediated apoptosis in cancer cells. It is noteworthy that p53 inhibitor PFT-α pretreatment had no demonstrable effects on compound C-mediated apoptosis in A549 and SMMC-7721 cells (Figure 4a). Further, suppression of p53 expression also had no evident effects on compound C-mediated apoptosis (Figure 4b). These data indicate that p53 is not involved in compound C-mediated apoptosis in cancer cells.
Figure 4.
The induction of p53 is not involved in compound C-induced apoptosis. (a) A549 and SMMC-7721 cells were treated with 10 μmol/l compound C for indicated periods with or without PFT-α (20 μmol/l) pretreatment for 1 h and cell lysates were analyzed for the levels of indicated proteins. (b) After GFP or P53 siRNAs transient transfection for 48 h, A549 and SMMC-7721 cells were treated with 10 μmol/l compound C for indicated periods and cell lysates were analyzed for the levels of indicated proteins
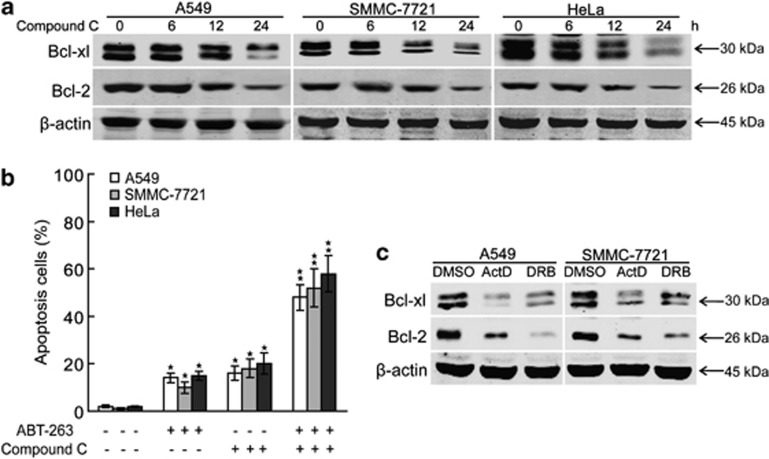
As Bcl-2 and Bcl-xl are essential for inhibiting the activation of caspases,51, 52, 53, 54 we investigated whether Bcl-2 and Bcl-xl are involved in compound C-mediated apoptosis. The expression of Bcl-2 and Bcl-xl in compound C-treated cancer cells was examined. As shown in Figure 5a, compound C decreased the protein levels of Bcl-2 and Bcl-xl in a time-dependent manner. More importantly, Bcl-2 and Bcl-xl inhibitor ABT-263 preincubation promoted compound C-induced apoptosis in A549, SMMC-7721 and HeLa cells (Figure 5b). Further, transcriptional inhibitors ActD and DRB also induced Bcl-2 and Bcl-xl decreasing in A549 and SMMC-7721 cells (Figure 5c), indicating that the loss of Bcl-2 and Bcl-xl promotes transcriptional inhibition-mediated apoptosis. Together, these findings indicate that the compound C-mediated decreasing of Bcl-2 and Bcl-xl is involved partially in the apoptosis of cancer cells under compound C-induced transcriptional stress.
Figure 5.
The downregulation of Bcl-2 and Bcl-xl promotes compound C-induced apoptosis. (a) A549, SMMC-7721 and HeLa cells were treated with 10 μmol/l compound C for indicated periods and cell lysates were analyzed for the levels of indicated proteins. (b) A549, SMMC-7721 and HeLa cells were treated with 5 μmol/l compound C for 24 h with or without ABT-263 (1 μmol/l) pretreatment for 1 h, and apoptosis were analyzed using flow cytometry after staining with FITC-conjugated Annexin V and propidium iodide. (c) A549 and SMMC-7721 cells were treated with 10 μg/ml ActD or 50 μmol/l DRB for 24 h and cell lysates were analyzed for the levels of indicated proteins. Data are presented as mean values±S.D. of three measurements. Columns, mean of three individual experiments; bars, S.D. * Significantly different from control value; ** Significantly different from *value
The phosphorylation of eIF2α promotes compound C-induced apoptosis in cancer cells
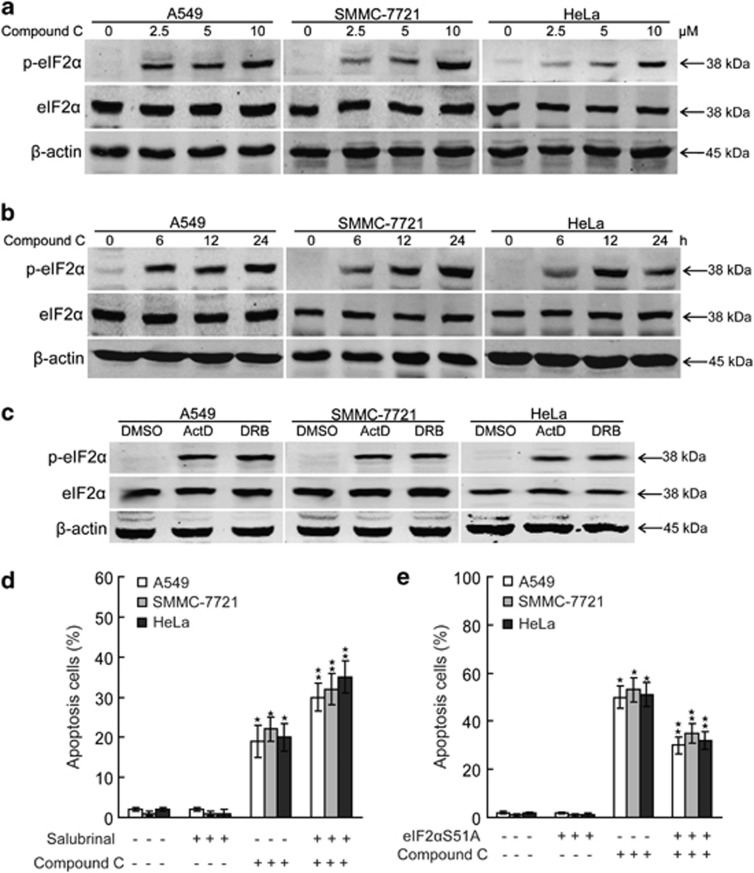
It is known that the eIF2α pathway, a critical pathway that regulates the integrated stress response, can be activated by various stress stimuli.55 We therefore studied whether compound C regulates the eIF2α pathway in cancer cells. We found that compound C induced the phosphorylation of eIF2α on Ser51 in a dose- and time-dependent manner (Figures 6a and b), indicating the eIF2α pathway is implicated in compound C-induced transcriptional stress response. To further test the role of the eIF2α pathway in transcriptional stress response, we investigated the phosphorylation of eIF2α on Ser51 upon ActD or DRB treatment. The data showed that ActD and DRB induced the phosphorylation of eIF2α on Ser51 in A549, SMMC-7721 and HeLa cells (Figure 6c), indicating that transcriptional stress activates the eIF2α pathway.
Figure 6.
The phosphorylation of eIF2α promotes compound C-induced apoptosis. (a) A549, SMMC-7721 and HeLa cells were treated with indicated amounts of compound C for 24 h and cell lysates were analyzed for the levels of indicated proteins. (b) A549, SMMC-7721 and HeLa cells were treated with 10 μmol/l compound C for indicated periods and cell lysates were analyzed for the levels of indicated proteins. (c) A549, SMMC-7721 and HeLa cells were treated with 10 μg/ml ActD or 50 μmol/l DRB for 12 h and cell lysates were analyzed for the levels of indicated proteins. (d) A549, SMMC-7721 and HeLa cells were treated with 10 μmol/l compound C for 20 h with or without salubrinal (25 μmol/l) pretreatment for 1 h, and apoptosis were analyzed using flow cytometry after staining with FITC-conjugated Annexin V and propidium iodide. (e) After eIF2αS51A expression plasmids transient transfection for 24 h, A549, SMMC-7721 and HeLa cells were treated with 10 μmol/l compound C for 24 h, and apoptosis were analyzed using flow cytometry after staining with FITC-conjugated Annexin V and propidium iodide. Data are presented as mean values±S.D. of three measurements. Columns, mean of three individual experiments; bars, S.D. * Significantly different from control value; ** Significantly different from *value
To make sure whether the phosphorylation of eIF2α has a role in compound C-induced apoptosis, salubrinal, a selective inhibitor of eIF2α dephosphorylation, was used in our study. As depicted in Figure 6d, salubrinal pretreatment promoted A549, SMMC-7721 and HeLa cells apoptosis upon compound C treatment. Further, transfection of A549, SMMC-7721 and HeLa cells with the phosphorylation-mutant expression plasmid eIF2αS51A, which inhibits the phosphorylation of eIF2α on Ser51, inhibited compound C-mediated apoptosis (Figure 6e). Taken together, these results suggest the phosphorylation of eIF2α is involved in part in the apoptosis of cancer cells under compound C-mediated transcriptional stress.
Compound C suppresses growth of cancer cells in mice
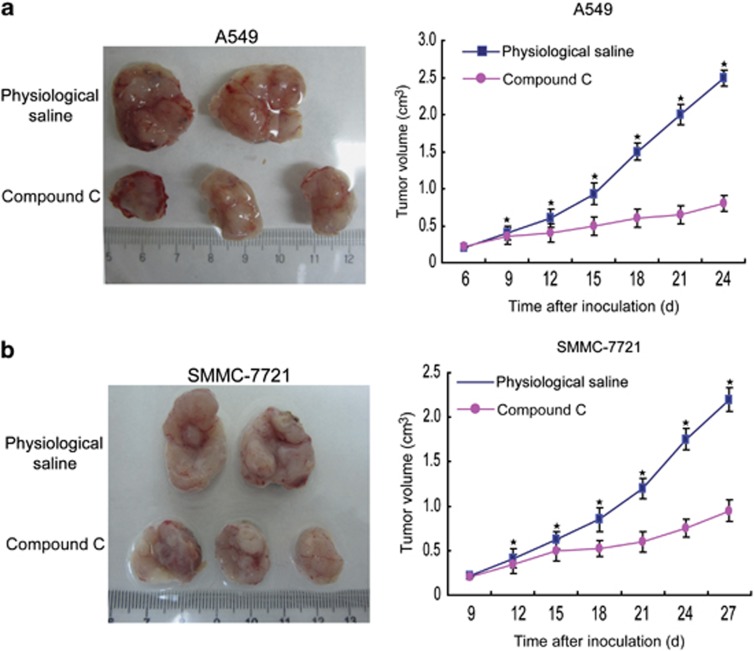
We assessed the potential of compound C in inhibition of tumor formation using an in vivo mouse model. The subcutaneous tumor model was chosen in our experiments for its high implant survival rate and ease in separating tumor from host tissue for accurate measurements of tumor growth. Treatment with compound C significantly reduced tumor burden of A549 and SMMC-7721 cells as compared with the control group (Figures 7a and b). These data demonstrated that compound C suppresses the growth of cancer cells in xenograft mouse models, which imply a potential use of compound C in repressing the progression of cancer cells.
Figure 7.
Compound C suppresses growth of cancer cells in mice. (a and b) After mice were subcutaneously inoculated with 1 × 107 A549 (a) or SMMC-7721 (b) cells for 7 days, intratumoral injection was performed to the experimental group mice with 15 mg/kg compound C twice weekly. The control group mice were treated by direct intratumoral injection of physiological saline twice weekly. Representative subcutaneous tumors are shown (left panel). Tumor size was measured every 3 days from day 6 (a) or 9 (b) through days 24 (a) or 27 (b) after inoculation of A549 (a) or SMMC-7721 (b) cells into mice (right panel). Error bars stand for S.D. of six animals
Discussion
Although compound C is implicated in the induction of apoptosis, it remains largely unclear how compound C exerts its proapoptotic action.40, 41, 42, 43 The present work reveals that compound C represses transcription and initiates apoptosis in cancer cells partially by decreasing the expression of Bcl-2 and Bcl-xl, and by inducing the phosphorylation of eIF2α.
It has been suggested that compound C induces apoptosis in some types of cells.40, 41, 42, 43 In agreement with these reports, we saw a similar effect in several cancer cell lines upon treatment with compound C. On the basis of the data that blocking AMPKα had no effects on compound C-induced apoptosis, we suggest compound C induces apoptosis in an AMPK-independent manner in cancer cells. As compound C induces autophagy through AMPK inhibition-independent mechanisms,56 it is necessary to address whether the induction of autophagy is involved in apoptosis of cancer cells upon compound C treatment. Compound C induced autophagy in A549 and HeLa cells (Supplementary Figure 2a), but the activation of autophagy was not responsible for compound C-mediated apoptosis (Supplementary Figure 2b). Apoptosis in mammalian cells is a multistep process that results in the activation of caspases, followed by execution of cell death. We found that compound C-induced apoptosis is caspase dependent.
A noteworthy property of compound C is its ability to inhibit transcription in various cancer cells. A recent report indicated that compound C inhibits ER stress-induced transcription program depending on the glucose deprivation conditions through a mechanism independent of AMPK.57 However, our data indicate that compound C blocks ER stress-induced transcription through nonspecific transcriptional blockade. A striking feature of compound C-mediated apoptosis is that it is p53 independent. We have shown that A549, SMMC-7721 and HeLa cells along with their p53 inhibition or knockdown undergo apoptosis to the same extent upon treatment with compound C. This is in agreement with the observation that p53 is a marker but not a mediator of transcriptional inhibitors ARC- and flavopiridol-induced apoptosis.10, 48 However, some of other transcriptional inhibitors, such as DRB and α-amanitin, were suggested to induce p53-dependent apoptosis.47, 58 It was shown recently that p53 knockdown cancer cells were much more resistant to compound C-induced apoptosis.42 In our study, however, although the accumulation of p53 was observed in response to compound C treatment, it did not lead to enhanced apoptosis. The reason for this discrepancy is unclear. The differences in the cell lines or experimental approaches used in studies could be the reason behind the difference in outcome.
An important question now before us is how compound C induces apoptosis under transcription stress conditions. On the basis of our findings that compound C resulted in Bcl-2 and Bcl-xl decrease, we propose that Bcl-2 and Bcl-xl might be involved in compound C-mediated apoptosis. This speculation was demonstrated by the findings that blocking the function of Bcl-2 and Bcl-xl promoted compound C-induced apoptosis. In our case, it is reasonable to speculate that the ability of compound C to downregulate antiapoptotic proteins, including Bcl-2 and Bcl-xl, has an important role in transcription repression-induced apoptosis upon treatment with compound C.
As compound C induced the phosphorylation on Ser51 of eIF2α, which is an important regulator of the decision between survival and apoptosis in response to diverse stress,55 we focused on the role of eIF2α signaling in regulating compound C-mediated apoptosis. As salubrinal, the selective inhibitor of eIF2α dephosphorylation, pretreatment promoted cancer cells apoptosis upon compound C treatment, it is reasonable to suggest that the phosphorylation of eIF2α contributed to compound C-induced apoptosis. This is further supported by the observation that blocking the phosphorylation of eIF2α by the phosphorylation-mutant expression plasmid eIF2αS51A inhibited compound C-induced apoptosis. Interestingly, other transcriptional inhibitors, including ActD and DRB, also induced the phosphorylation of eIF2α on Ser51, indicating that the eIF2α pathway is an important regulator of transcriptional stress. The fact that compound C repressed tumor growth in mice models makes a strong case for consideration of compound C as a potential antitumor agent. Hence, compound C could be a promising candidate for in vivo studies.
In brief, the present work reveals that transcriptional repression has an essential role in compound C-induced apoptosis in cancer cells. The ability of compound C to inhibit tumor growth in vivo implies its potent anticancer activities. More detailed studies on the function of compound C upon transcriptional repression will contribute to the development of compound C as anticancer drug.
Materials and Methods
Chemicals and antibodies
AMPK inhibitor compound C, eIF2α phosphatase enzymes inhibitor salubrinal and autophagy inhibitor bafilomycin A1 were purchased from Tocris Bioscience (Bristol, UK). Transcriptional inhibitors DRB and ActD, p53 inhibitor PFT-α, caspase inhibitor z-VAD-fmk and ER stress inducers Tg and Tun were purchased from Sigma (Lyon, France). Bcl-2 and Bcl-xl inhibitor ABT-263 was purchased from Selleck Chemicals (Houston, TX, USA). GFP siRNA, p53 siRNA, AMPKα siRNA and antibodies against eIF2α and β-actin were purchased from Santa Cruz Biotechnology (Heidelberg, Germany). Antibodies against phospho-eIF2α (Ser51), phospho-p53 (Ser15), AMPKα, p53, Bcl-2, Bcl-xl, PARP, cleaved caspase-3 and LC3A/B were purchased from Cell Signaling Technology (Danvers, MA, USA). eIF2αS51A plasmid was purchased from Addgene (Cambridge, MA, USA).
Cell culture and treatments
Human lung adenocarcinoma epithelial cell line A549, human hepatocellular carcinoma cell line SMMC-7721 and human cervical cancer cell line HeLa were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin in a humidified incubator containing 5% CO2 and 95% ambient air at 37 °C. The protocol used for p53 and AMPKα knockdown has been previously described.59 Transfection of vectors for the expression of GFP and eIF2αS51A, which lacks the phosphorylation site, were performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's manual.
Western blot analysis
Cells were lysed in Triton lysis buffer (20 mM Tris, pH 7.4, 137 mM NaCl, 10% glycerol, 1% Triton X-100, 2 mM EDTA, 1 mM PMSF, 10 mM sodium fluoride, 5 mg/ml of aprotinin, 20 mM leupeptin and 1 mM sodium orthovanadate) and centrifuged at 12 000 × g for 15 min. Protein concentrations were measured using the BCA assay. Protein samples were denatured with 4 × SDS-loading buffer (200 mM Tris, pH 6.8, 8% SDS, 400 mM DTT, 0.4% bromphenol blue and 40% glycerol) at 100 °C for 5 min and subjected to standard SDS-PAGE and western blot analysis as previously described.60
Apoptosis analysis
Apoptosis was detected using the annexin V-FITC apoptosis detection kit (Invitrogen) according to the manufacturer's manual. Annexin V staining was analyzed by flow cytometry within 1 h. The experiments were repeated three times.
Reverse transcription reaction and real-time PCR
Total RNA was isolated with TRIzol reagent (Invitrogen) according to the manufacturer's instructions. The reverse transcription reactions were carried out using the M-MLV reverse transcriptase (Promega, Madison, WI, USA) according to the manufacturer's protocol. Real-time PCR analyses were performed using SYBR Premix Ex Taq (TaKaRa, Tokyo, Japan). Results were normalized with 18S. The primers used in this study are shown in Supplementary Table 1.
Thymidine incorporation
Cells (1–2 × 104 per well) were grown overnight in 24-well plates and treated with 10 μM/l compound C for the experimental wells or physiological saline for the control wells in medium containing 0.5% FBS. After 24 h, the cells were pulsed with [3H]thymidine (1 μCi per well) for 6 h, and then fixed with 5% trichloroacetic acid and solubilized before scintillation counting. Experiments were repeated three times.
RNA isolation and microarray analysis
Total RNA was extracted from cells using the RNeasy Mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer's protocol. Fluorescent aRNA targets were prepared from 2.5 μg total RNA samples using OneArray Amino Allyl aRNA Amplification Kit (Phalanx Biotech Group, Hsinchu, Taiwan) and Cy5 dyes (Amersham Pharmacia, Piscataway, NJ, USA). Fluorescent targets were hybridized to the Human Whole Genome OneArray with Phalanx hybridization buffer using Phalanx Hybridization System. After 16 h hybridization at 50 °C, nonspecific binding targets were washed away by three different washing steps (wash I, 42 °C for 5 min; Wash II, 42 °C for 5 min, 25 °C for 5 min; Wash III, rinse 20 times) and the slides were dried by centrifugation and scanned by Axon 4000B scanner (Molecular Devices, Sunnyvale, CA, USA). The intensities of each probe were obtained by GenePix 4.1 software (Molecular Devices).
Tumor xenograft experiments
The use of animals in present study has been approved by the local committee on animal care. Six-week-old NOD/SCID nude mice were purchased from the Shanghai Experimental Center (CSA, Shanghai, China). Mice were subcutaneously inoculated with A549 or SMMC-7721 cells. Approximately 1 × 107 cells in 0.2 ml culture medium containing phosphate-buffered saline were injected subcutaneously into the right flank of the mice, which were then observed daily for signs of tumor development. Tumor volume was calculated as below: V (cm3)=width2 (cm2) × length (cm)/2. Seven days after inoculation, mice were randomly divided into two groups (n=6). Intratumoral injection was performed to the experimental group mice with 15 mg/kg compound C (compound C were dissolved in physiological saline) twice weekly. The control group mice were treated by direct intratumoral injection of physiological saline twice weekly.
Immunofluorescence staining and confocal microscopy analysis
Cells were replated on chamber slides. When cultured to 60% confluence, cells that were incubated with anti-LC3A/B antibodies conjugated to CY3 (Invitrogen) for immunofluorescence and confocal microscopy assay.
Statistical analysis
Results were expressed as the mean±S.D. Statistical analysis was performed using Student's t-test. P<0.05 was considered statistically significant.
Acknowledgments
This work was supported by grants from Sichuan Province Science Foundation for Youths (2013JQ0045), the National Natural Science Foundation of China (81000886, 81221061, 81101714, 91029732, 91229201), New Century Excellent Talents in University Grant (NCET-11-1058), State Key Project for Infectious Diseases (2012ZX10002-009, 011), and Key Project of Army (BWS11J030).
Glossary
- AMPK
AMP-activated protein kinase
- ActD
actinomycin D
- c-Met
hepatocyte growth factor receptor
- DRB
5,6-dichloro-1-β-D-ribobenzimidazole
- EGFR
epidermal growth factor receptor
- eIF2α
eukaryotic initiation factor-alpha
- GRP78
78 kDa glucose-regulated protein
- Tg
thapsigargin
- Tun
tunicamycin
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by G Raschellá
Supplementary Material
References
- Brown JM, Attardi LD. The role of apoptosis in cancer development and treatment response. Nat Rev Cancer. 2005;5:231–237. doi: 10.1038/nrc1560. [DOI] [PubMed] [Google Scholar]
- Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaravadi RK, Thompson CB. The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin Cancer Res. 2007;13:7271–7279. doi: 10.1158/1078-0432.CCR-07-1595. [DOI] [PubMed] [Google Scholar]
- Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Kono K, Mimura K, Kiessling R. Immunogenic tumor cell death induced by chemoradiotherapy: molecular mechanisms and a clinical translation. Cell Death Dis. 2013;4:e688. doi: 10.1038/cddis.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger I, Blanco AG, Boelens R, Cavarelli J, Coll M, Folkers GE, et al. Structural insights into transcription complexes. J Struct Biol. 2011;175:135–146. doi: 10.1016/j.jsb.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Kornberg RD. The molecular basis of eucaryotic transcription. Cell Death Differ. 2007;14:1989–1997. doi: 10.1038/sj.cdd.4402251. [DOI] [PubMed] [Google Scholar]
- Boeger H, Bushnell DA, Davis R, Griesenbeck J, Lorch Y, Strattan JS, et al. Structural basis of eukaryotic gene transcription. FEBS Lett. 2005;579:899–903. doi: 10.1016/j.febslet.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Chen R, Keating MJ, Gandhi V, Plunkett W. Transcription inhibition by flavopiridol: mechanism of chronic lymphocytic leukemia cell death. Blood. 2005;106:2513–2519. doi: 10.1182/blood-2005-04-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan SK, Gartel AL. A novel transcriptional inhibitor induces apoptosis in tumor cells and exhibits antiangiogenic activity. Cancer Res. 2006;66:3264–3270. doi: 10.1158/0008-5472.CAN-05-3940. [DOI] [PubMed] [Google Scholar]
- Gartel AL. Transcriptional inhibitors, p53 and apoptoss. Biochim Biophys Acta. 2008;1786:83–86. doi: 10.1016/j.bbcan.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Derheimer FA, Chang CW, Ljungman M. Transcription inhibition: a potential strategy for cancer therapeutics. Eur J Cancer. 2005;41:2569–2576. doi: 10.1016/j.ejca.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Flavopiridol, an inhibitor of transcription: implications, problems and solutions. Cell Cycle. 2004;3:1537–1542. doi: 10.4161/cc.3.12.1278. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan SK, Halasi M, Bhat UG, Kurmasheva RT, Houghton PJ, Gartel AL. Proapoptotic compound ARC targets Akt and N-myc in neuroblastoma cells. Oncogene. 2008;27:694–699. doi: 10.1038/sj.onc.1210692. [DOI] [PubMed] [Google Scholar]
- Rosato RR, Almenara JA, Kolla SS, Maggio SC, Coe S, Gimenez MS, et al. Mechanism and functional role of XIAP and Mcl-1 down-regulation in flavopiridol/vorinostat antileukemic interactions. Mol Cancer Ther. 2007;6:692–702. doi: 10.1158/1535-7163.MCT-06-0562. [DOI] [PubMed] [Google Scholar]
- Koumenis C, Giaccia A. Transformed cells require continuous activity of RNA polymerase II to resist oncogene-induced apoptosis. Mol Cell Biol. 1997;17:7306–7316. doi: 10.1128/mcb.17.12.7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao SH, Fujinaga K, Marion JE, Taube R, Sausville EA, Senderowicz AM, et al. Flavopiridol inhibits P-TEFb and blocks HIV-1 replication. J Biol Chem. 2000;275:28345–28348. doi: 10.1074/jbc.C000446200. [DOI] [PubMed] [Google Scholar]
- Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006;24:1770–1783. doi: 10.1200/JCO.2005.03.7689. [DOI] [PubMed] [Google Scholar]
- Benson C, White J, De Bono J, O'Donnell A, Raynaud F, Cruickshank C, et al. A phase I trial of the selective oral cyclin-dependent kinase inhibitor seliciclib (CYC202; R-Roscovitine), administered twice daily for 7 days every 21 days. Br J Cancer. 2007;96:29–37. doi: 10.1038/sj.bjc.6603509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TS, Ruppert AS, Johnson AJ, Fischer B, Heerema NA, Andritsos LA, et al. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. J Clin Oncol. 2009;27:6012–6018. doi: 10.1200/JCO.2009.22.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dispenzieri A, Gertz MA, Lacy MQ, Geyer SM, Fitch TR, Fenton RG, et al. Flavopiridol in patients with relapsed or refractory multiple myeloma: a phase 2 trial with clinical and pharmacodynamic end-points. Haematologica. 2006;91:390–393. [PubMed] [Google Scholar]
- MacCallum DE, Melville J, Frame S, Watt K, Anderson S, Gianella-Borradori A, et al. Seliciclib (CYC202, R-Roscovitine) induces cell death in multiple myeloma cells by inhibition of RNA polymerase II-dependent transcription and down-regulation of Mcl-1. Cancer Res. 2005;65:5399–5407. doi: 10.1158/0008-5472.CAN-05-0233. [DOI] [PubMed] [Google Scholar]
- Byrd JC, Lin TS, Dalton JT, Wu D, Phelps MA, Fischer B, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109:399–404. doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Zang M, Guo W. AMPK as a metabolic tumor suppressor: control of metabolism and cell growth. Future Oncol. 2010;6:457–470. doi: 10.2217/fon.09.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano K, Ikeda Y, Handa M, Kamata T, Anbo H, Araki Y, et al. Enhancing effect by heparin on shear-induced platelet aggregation. Semin Thromb Hemost. 1990;16 (Suppl:60–65. [PubMed] [Google Scholar]
- Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, et al. 5'-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol. 2006;26:5336–5347. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Pinedo C, El Mjiyad N, Ricci JE. Cancer metabolism: current perspectives and future directions. Cell Death Dis. 2012;3:e248. doi: 10.1038/cddis.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YK, Park KG.Metabolic roles of AMPK and metformin in cancer cells Mol Cells 2013. e-pub ahead of print 19 June 2013doi: 10.1007/s10059-013-0169-8 [DOI] [PMC free article] [PubMed]
- Pimentel GD, Ropelle ER, Rocha GZ, Carvalheira JB. The role of neuronal AMPK as a mediator of nutritional regulation of food intake and energy homeostasis. Metabolism. 2013;62:171–178. doi: 10.1016/j.metabol.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q, Feng L, Behrens C, Bekele BN, Wistuba II, Hong WK, et al. Implication of AMP-activated protein kinase and Akt-regulated survivin in lung cancer chemopreventive activities of deguelin. Cancer Res. 2007;67:11630–11639. doi: 10.1158/0008-5472.CAN-07-2401. [DOI] [PubMed] [Google Scholar]
- Park HU, Suy S, Danner M, Dailey V, Zhang Y, Li H, et al. AMP-activated protein kinase promotes human prostate cancer cell growth and survival. Mol Cancer Ther. 2009;8:733–741. doi: 10.1158/1535-7163.MCT-08-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZX, Liang J, Haridas V, Gaikwad A, Connolly FP, Mills GB, et al. A plant triterpenoid, avicin D, induces autophagy by activation of AMP-activated protein kinase. Cell Death Differ. 2007;14:1948–1957. doi: 10.1038/sj.cdd.4402207. [DOI] [PubMed] [Google Scholar]
- Zhou W, Han WF, Landree LE, Thupari JN, Pinn ML, Bililign T, et al. Fatty acid synthase inhibition activates AMP-activated protein kinase in SKOV3 human ovarian cancer cells. Cancer Res. 2007;67:2964–2971. doi: 10.1158/0008-5472.CAN-06-3439. [DOI] [PubMed] [Google Scholar]
- Langelueddecke C, Jakab M, Ketterl N, Lehner L, Hufnagl C, Schmidt S, et al. Effect of the AMP-kinase modulators AICAR, metformin and compound C on insulin secretion of INS-1E rat insulinoma cells under standard cell culture conditions. Cell Physiol Biochem. 2012;29:75–86. doi: 10.1159/000337589. [DOI] [PubMed] [Google Scholar]
- Hsu YC, Meng X, Ou L, Ip MM. Activation of the AMP-activated protein kinase-p38 MAP kinase pathway mediates apoptosis induced by conjugated linoleic acid in p53-mutant mouse mammary tumor cells. Cell Signal. 2010;22:590–599. doi: 10.1016/j.cellsig.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P, Mandl-Weber S, Emmerich B, Straka C, Schmidmaier R. Inhibition of adenosine monophosphate-activated protein kinase induces apoptosis in multiple myeloma cells. Anticancer Drugs. 2007;18:405–410. doi: 10.1097/CAD.0b013e32801416b6. [DOI] [PubMed] [Google Scholar]
- Jin J, Mullen TD, Hou Q, Bielawski J, Bielawska A, Zhang X, et al. AMPK inhibitor Compound C stimulates ceramide production and promotes Bax redistribution and apoptosis in MCF7 breast carcinoma cells. J Lipid Res. 2009;50:2389–2397. doi: 10.1194/jlr.M900119-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucicevic L, Misirkic M, Janjetovic K, Harhaji-Trajkovic L, Prica M, Stevanovic D, et al. AMP-activated protein kinase-dependent and -independent mechanisms underlying in vitro antiglioma action of compound C. Biochem Pharmacol. 2009;77:1684–1693. doi: 10.1016/j.bcp.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Huang SW, Wu CY, Wang YT, Kao JK, Lin CC, Chang CC, et al. p53 modulates the AMPK inhibitor compound C induced apoptosis in human skin cancer cells. Toxicol Appl Pharmacol. 2013;267:113–124. doi: 10.1016/j.taap.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Yang WL, Perillo W, Liou D, Marambaud P, Wang P. AMPK inhibitor compound C suppresses cell proliferation by induction of apoptosis and autophagy in human colorectal cancer cells. J Surg Oncol. 2012;106:680–688. doi: 10.1002/jso.23184. [DOI] [PubMed] [Google Scholar]
- Lai E, Teodoro T, Volchuk A. Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology. 2007;22:193–201. doi: 10.1152/physiol.00050.2006. [DOI] [PubMed] [Google Scholar]
- Hsiao JR, Chang KC, Chen CW, Wu SY, Su IJ, Hsu MC, et al. Endoplasmic reticulum stress triggers XBP-1-mediated up-regulation of an EBV oncoprotein in nasopharyngeal carcinoma. Cancer Res. 2009;69:4461–4467. doi: 10.1158/0008-5472.CAN-09-0277. [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Arima Y, Nitta M, Kuninaka S, Zhang D, Fujiwara T, Taya Y, et al. Transcriptional blockade induces p53-dependent apoptosis associated with translocation of p53 to mitochondria. J Biol Chem. 2005;280:19166–19176. doi: 10.1074/jbc.M410691200. [DOI] [PubMed] [Google Scholar]
- Demidenko ZN, Blagosklonny MV. Flavopiridol induces p53 via initial inhibition of Mdm2 and p21 and, independently of p53, sensitizes apoptosis-reluctant cells to tumor necrosis factor. Cancer Res. 2004;64:3653–3660. doi: 10.1158/0008-5472.CAN-04-0204. [DOI] [PubMed] [Google Scholar]
- Lu W, Chen L, Peng Y, Chen J. Activation of p53 by roscovitine-mediated suppression of MDM2 expression. Oncogene. 2001;20:3206–3216. doi: 10.1038/sj.onc.1204412. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang L. The transcriptional targets of p53 in apoptosis control. Biochem Biophys Res Commun. 2005;331:851–858. doi: 10.1016/j.bbrc.2005.03.189. [DOI] [PubMed] [Google Scholar]
- Sun XM, Bratton SB, Butterworth M, MacFarlane M, Cohen GM. Bcl-2 and Bcl-xL inhibit CD95-mediated apoptosis by preventing mitochondrial release of Smac/DIABLO and subsequent inactivation of X-linked inhibitor-of-apoptosis protein. J Biol Chem. 2002;277:11345–11351. doi: 10.1074/jbc.M109893200. [DOI] [PubMed] [Google Scholar]
- Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Murphy KM, Ranganathan V, Farnsworth ML, Kavallaris M, Lock RB. Bcl-2 inhibits Bax translocation from cytosol to mitochondria during drug-induced apoptosis of human tumor cells. Cell Death Differ. 2000;7:102–111. doi: 10.1038/sj.cdd.4400597. [DOI] [PubMed] [Google Scholar]
- Sassone J, Maraschi A, Sassone F, Silani V, Ciammola A. Defining the role of the Bcl-2 family proteins in Huntington's disease. Cell Death Dis. 2013;4:e772. doi: 10.1038/cddis.2013.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34 (Pt 1:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- Vucicevic L, Misirkic M, Janjetovic K, Vilimanovich U, Sudar E, Isenovic E, et al. Compound C induces protective autophagy in cancer cells through AMPK inhibition-independent blockade of Akt/mTOR pathway. Autophagy. 2011;7:40–50. doi: 10.4161/auto.7.1.13883. [DOI] [PubMed] [Google Scholar]
- Saito S, Furuno A, Sakurai J, Park HR, Shin-ya K, Tomida A. Compound C prevents the unfolded protein response during glucose deprivation through a mechanism independent of AMPK and BMP signaling. PLoS One. 2012;7:e45845. doi: 10.1371/journal.pone.0045845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Poele RH, Okorokov AL, Joel SP. RNA synthesis block by 5, 6-dichloro-1-beta-D-ribofuranosylbenzimidazole (DRB) triggers p53-dependent apoptosis in human colon carcinoma cells. Oncogene. 1999;18:5765–5772. doi: 10.1038/sj.onc.1202961. [DOI] [PubMed] [Google Scholar]
- Dai R, Li J, Fu J, Chen Y, Yu L, Zhao X, et al. Disturbance of Ca2+ homeostasis converts pro-Met into non-canonical tyrosine kinase p190MetNC in response to endoplasmic reticulum stress in MHCC97 cells. J Biol Chem. 2012;287:14586–14597. doi: 10.1074/jbc.M111.333435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai R, Li J, Fu J, Chen Y, Wang R, Zhao X, et al. The tyrosine kinase c-Met contributes to the pro-tumorigenic function of the p38 kinase in human bile duct cholangiocarcinoma cells. J Biol Chem. 2012;287:39812–39823. doi: 10.1074/jbc.M112.406520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.