Abstract
Bipolar Disorder (BD) mania is a psychiatric disorder with multifaceted symptoms. Development of targeted treatments for BD mania may benefit from animal models that mimic multiple symptoms, as opposed to hyperactivity alone. Using the reverse-translated multivariate exploratory paradigm, the Behavioral Pattern Monitor (BPM), we reported that patients with BD mania exhibit hyperactivity as well as increased specific exploration and more linear movements through space. This abnormal profile is also observed in mice with reduced function of the dopamine transporter (DAT) through either constitutive genetic (knockdown (KD)) or acute pharmacological (GBR12909) means. Here, we assessed the pharmacological predictive validity of these models by administering the BD-treatment valproic acid (VPA) for 28 days. After 28 days of 1.5% VPA- or regular-chow treatment, C57BL/6J mice received GBR12909 (9 mg/kg) or saline and were tested in the BPM. Similarly, DAT KD and WT littermates were treated with VPA-chow and tested in the BPM. GBR12909-treated and DAT KD mice on regular chow were hyperactive, exhibited increased specific exploration, and moved in straighter patterns compared to saline-treated and WT mice respectively. Chronic 1.5% VPA-chow treatment resulted in therapeutic concentrations of VPA and ameliorated hyperactivity in both models, while specific exploration and behavioral organization remained unaffected. Hence, the mania-like profile of mice with reduced functional DAT was partially attenuated by chronic VPA treatment, consistent with the incomplete symptomatic effect of VPA treatment in BD patients. Both DAT models may help to identify therapeutics that impact the full spectrum of BD mania.
Keywords: bipolar mania, dopamine transporter, chronic treatment, valproate, mice
INTRODUCTION
Patients with Bipolar Disorder (BD) mania are characterized primarily by symptoms of overactive behavior as described in the DSM-IV (APA, 1994). There are many facets to the overactive behavior exhibited by patients with BD (Grunze et al., 2009). There is a need for therapeutics targeting the multifaceted nature of the disorder, which affects approximately 1% of the worldwide population for BD type-I (Merikangas et al., 2011). Current treatments have been found serendipitously, likely in part due to the limited availability of predictive preclinical animal models of BD mania that go beyond treating hyperactivity (Gould and Einat, 2007; Young et al., 2011a).
We developed a multivariate approach to quantify the abnormal exploration of patients with BD in a novel environment – the Behavioral Pattern Monitor (BPM) (Perry et al., 2009; Young et al., 2007). Using the BPM, we evaluated mouse models of mania based on reduced dopamine transporter (DAT) functioning. These models include:1) DAT knockdown (KD) mice with approximately 10% expression of the DAT; and 2) selective inhibition of the DAT using GBR12909 treatment (Young et al., 2010a). Both models are based on the putative etiology of BD, in which polymorphisms in the human DAT have been associated with the diagnosis (Greenwood et al., 2006; Pinsonneault et al., 2011). In vitro studies suggest that these polymorphisms may result in reduced cell surface expression of the DAT (Horschitz et al., 2005). Indeed, lower striatal availability of DAT has been observed in unmedicated patients with BD by using positron emission tomography (Anand et al., 2011).
In the BPM, patients with BD mania are hyperactive, interact more with objects, and walk in more direct paths, compared to healthy subjects (Perry et al., 2009). The exploratory profiles of DAT KD and GBR12909-treated mice in the mouse BPM are similarly characterized by hyperactivity, increased specific exploration, and straighter patterns of movement (Perry et al., 2009; Ralph-Williams et al., 2003; Young et al., 2010a). Moreover, the mania-like phenotype in DAT KD mice is attenuated with environmental familiarity but reinstated by environmental novelty and a sub-threshold dose of GBR12909 (Young et al., 2010b), consistent with environmental uncertainty (Sylvia et al., 2009) and stimulants (Wingo and Ghaemi, 2008) deleteriously affecting patients with BD.
One of the core strategies in the development of a valid animal model is the assessment of its pharmacological predictive validity. Lithium and valproic acid (VPA) are among the most commonly used treatments for BD mania, although they are not effective in every subject (Bowden et al., 1994) or in each facet of the disorder (Yatham et al., 2010). Indeed, patients with BD mania remained more active, with increased object interactions and more direct movements in the BPM compared with healthy subjects even when on BD medication for 3 weeks, although effect size differences in activity levels diminished over time (Minassian et al., 2011). Since long-term treatment regimens are required to exert optimal therapeutic effects, chronic treatment regimens should be used when assessing the pharmacological predictive validity of an animal model (Harrison-Read, 2009; Young et al., 2011a).
To assess the pharmacological predictive validity of the DAT mouse model for BD mania, we examined the effects of chronic VPA medication on the mania-like behavior of these mice in the BPM. We hypothesized that (a) DAT KD and GBR12909-treated mice would exhibit a BD mania-like profile in the BPM; and (b) chronic treatment with VPA at therapeutic concentrations would attenuate these mania-like profiles.
METHODS
Animals
DAT KD mice were generated by inserting modified embryonic stem cells of the 129Sv/J mouse strain in C57BL/6J blastocysts (Zhuang et al., 2001). DAT heterozygous breeders were sent to our laboratory from Columbia University and all the subsequent mice resulted from a breeding colony in the vivarium at the University of California San Diego (UCSD). Male and female DAT KD (20-40 g) and WT (20-30 g) littermate mice were generated from heterozygous breeding pairs and tested in the BPM at approximately 6 months of age. Male C57BL/6J mice (20-30 g) were obtained from Jackson laboratories and tested at approximately 3 months of age for acute GBR12909 studies. All mice were group housed (where possible, maximum 4/cage), maintained in a temperature-controlled vivarium (21±1°C) on a reversed day-night cycle (lights on at 7.00 PM, off at 7.00 AM), and were tested during the dark phase of the cycle. Mice were brought to the laboratory 60 min before testing between 8.00 AM and 5.00 PM. Food (normal or VPA chow; Harlan Teklab, Madison, WI) and water were provided ad libitum, except during behavioral testing. During chronic treatment, all mice were inspected 2 times a week to check for possible drug-induced adverse effects. All procedures were approved by the UCSD Institutional Animal Care and Use Committee. The UCSD animal facility meets all federal and state requirements for animal care and was approved by the American Association for Accreditation of Laboratory Animal Care.
Drug Treatment
Sodium valproate and GBR12909 dihydrochloride were purchased from Sigma (St Louis, MO). Rodent chow with VPA was custom produced by Harlan Laboratories (Harlan Teklab, Madison, WI). The VPA and vehicle chows were identical with the exception of the added drug in the former, which was produced with a concentration of 15 g VPA/kg chow. This dose was chosen based on a pilot dose-response study indicating that blood concentrations of VPA were within the therapeutic concentrations for patients with BD (Fig. 1). Mice were treated with 4 weeks of VPA- or vehicle-chow and then tested in the BPM.GBR12909 was dissolved in saline (0.9%) vehicle after sonicating for 2-4 h at 40°C, injection volume was 10 ml/kg. Free-base drug weights were used in all drug calculations. GBR12909 at 9.0 mg/kg was administered by intraperitoneal injection immediately before the mouse was placed in the testing chamber and data recording started.
Fig. 1.
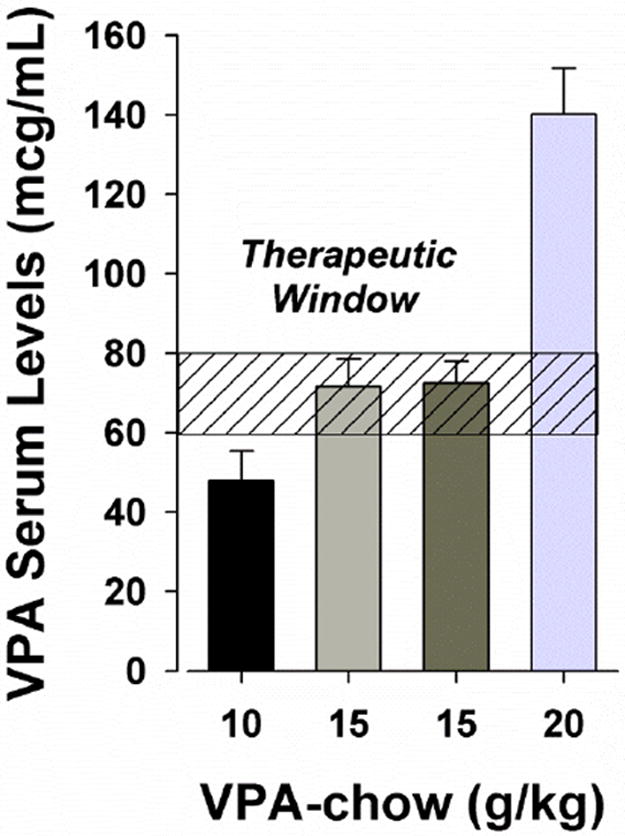
Serum concentrations of VPA (mcg/mL) after 28 days of different doses of VPA-chow treatment. Dose-dependent effects of chronic VPA treatment concentrations were observed on serum concentrations in mice. 1.0 % VPA-chow resulted in low, while 2.0 % VPA-chow resulted in high/toxic VPA concentrations. The current experiments with 1.5 % VPA chow resulted in optimal therapeutic serum concentrations for Bipolar Disorder (between 60-80 mcg/mL). Data are presented as the mean + SEM.
Chemical analysis of VPA blood concentrations
All mice were decapitated and trunk blood collected. Blood was left to clot for approximately 15 min. Samples were then centrifuged at 3000 rpm for 10 min and serum was taken to determine drug concentrations. Valproic acid concentrations were quantified with a glucose-6-phosphate dehydrogenase-based enzyme immunoassay technique and were performed by UCSD Medical Center (San Diego, CA).
Mouse Behavioral Pattern Monitor
Spontaneous locomotor behavior and specific exploration were examined in nine mouse BPM chambers (BPM; San Diego Instruments, San Diego, CA) as described previously (Risbrough et al., 2006). In brief, each Plexiglas chamber consists of a 30.5×61×38-cm area, with three floor holes and eight wall holes (three on each long and one on each short wall; 1.25 cm diameter, 1.9 cm from the floor). Each hole contains an infrared photobeam to detect holepoking. Each chamber is enclosed in an outer box that minimizes outside light and noise, with an internal white house-light above the arena (350 lux in the center and 92 lux in the four corners). Activity was obtained from a grid of 12×24 infrared photobeams 1 cm above the floor (2.5 cm apart along the length and the width of the chamber; 24×12 X-Y array), recording the location of the mouse every 0.1 s. Rearing behavior was detected by another set of 16 photobeams, located on the Y-axis only and placed 2.5 cm above the floor. Position was defined across nine unequal regions (four corners, four walls and center (Geyer et al., 1986)).
Exploratory Assessment
At the start of each session, the mouse was placed in the bottom left-hand corner of the arena and the test session started immediately for 60 min. The primary outcome measures were: locomotor activity as measured by transitions across the defined regions and center entries (cumulative entries into the center region); specific exploration as measured by holepoking and rearing; and locomotor pattern as measured by spatial d. Spatial d quantifies the geometric structure of the locomotor path, where a value of 1 represents a path in a straight distance-covering line, and 2 highly circumscribed small-scale movements (Paulus and Geyer, 1991).
Experiment 1: Effects of chronic vehicle- or VPA-chow treatment on vehicle- or GBR12909-induced exploration in the mouse BPM
Male C57BL/6J mice (n=60) were tested first without drug in the BPM for 60 min to baseline-match their behavior. Using baseline measures of activity, specific exploration, and spatial d, mice were then counter-balanced into groups that received vehicle- (n=28) or VPA-chow (n=32). After 28 days of treatment, mice in the vehicle-chow group received saline (n=14) or GBR12909 at 9 mg/kg (n=14). Mice in the VPA-chow group received saline (n=14) or GBR12909 at 9 mg/kg (n=15).During this experiment, 3 mice that received VPA-chow died of unknown causes; all other mice appeared healthy during treatment and testing. The locomotor and behavioral activity of the mice was assessed for 60 min in the BPM.
Experiment 2: Effects of chronic vehicle- or VPA-chow treatment on the exploratory profile of WT and DAT KD mice in the mouse BPM
Female (n=13) and male DAT KD mice (n=11) and their female (n=13) and male WT littermates (n=15) were tested in the BPM for 60 min to baseline-match their behavior. Using baseline measures of activity, specific exploration, and spatial d, mice were then counter-balanced into groups that received vehicle- (female KD (n=9), male KD (n=6), female WT (n=8), male WT (n=8)) or VPA-chow (female KD (n=4), male KD (n=5), female WT (n=5), male WT (n=7)). After 28 days of treatment, the locomotor and behavioral activity of the mice was assessed for 60 min in the BPM.
Statistics
The outcome measures for each experiment were analyzed using two- or three-way ANOVAs, with sex, genotype, chow treatment, or drug as between-subject variables. Significant interactions and main effects were analyzed using Tukey’s post hoc analyses. The data were analyzed for 60 min testing period using the Biomedical Data Programs statistical software (Statistical Solutions Inc., Saugus, MA, USA). The alpha level was set to 0.05.
RESULTS
Experiment 1: Exploratory profile of vehicle- or GBR12909-treated C57BL/6J mice after chronic vehicle- or VPA-chow treatment
Locomotor activity
Significant GBR12909 effects were observed for transitions (F(1,53)=122.53, p<0.0001) and center entries (F(1,53)=33.65, p<0.0001). Significant VPA effects were also observed for transitions (F(1,53)=5.06, p<0.05; Fig. 2a) and center entries (F(1,53)=9.46, p<0.005; Fig. 2b). A VPA by GBR12909 interaction was found for center entries (F(1,53)=4.74, p<0.05), but not for transitions. Post hoc analyses revealed that GBR12909 increased transitions compared to saline in both vehicle- and VPA-chow treated mice (p<0.0001). Mice administered GBR12909 after VPA-chow treatment exhibited fewer transitions compared to mice administered GBR12909 after vehicle-chow treatment (p<0.05). VPA-chow treatment did not affect transition levels in the saline-administered mice (p>0.1). Analyses revealed that GBR12909 increased center entries compared to saline in both the vehicle- and VPA-chow treated mice (p<0.005). Mice administered GBR12909 after VPA-chow treatment exhibited fewer center entries compared to vehicle-chow treated mice administered GBR12909 (p<0.005). VPA-chow treatment did not affect the amount of center entries in the saline-treated mice (p>0.1).
Fig. 2.
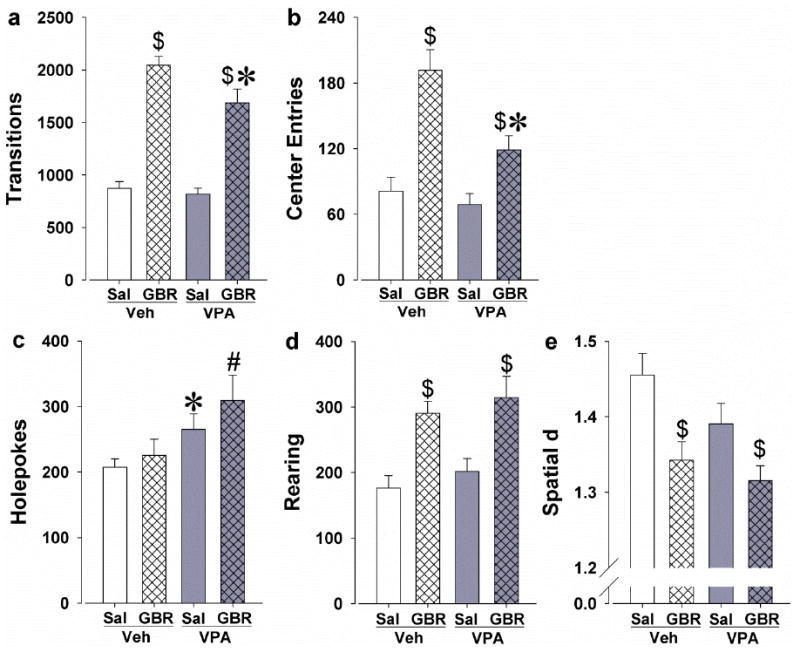
Effects of chronic treatment with the mood-stabilizer VPA on the exploratory profile of C57BL/6J mice administered acute GBR12909 at 9 mg/kg. GBR12909 increased activity as measured by transitions, which was attenuated by VPA (a). VPA also attenuated GBR12909-induced increases in center entries (b). Chronic VPA-chow treatment did not affect activity alone (a-b). GBR12909 did not affect holepoking, while VPA increased holepoking (c). GBR12909 increased specific exploration as measured by rearing, which was not affected by VPA treatment (d). GBR12909 induced more linear patterns of movement (reduced spatial d), which also were unaffected by VPA (e). Data are presented as the mean + SEM. * Denotes p<0.05 and # denotes p<0.1 when compared with the vehicle-chow treatment group and $ denotes p<0.05 when compared with saline.
Exploratory behavior
A VPA effect was observed for holepoking (F(1,53)=6.70, p<0.05; Fig. 2c) with no main effect of or interaction with GBR12909.A significant GBR12909 effect was observed for rearing (F(1,53)=23.26, p<0.0001; Fig. 2d) with no main effect of or interaction with VPA. Post hoc analyses revealed that GBR12909 increased rearing compared to saline in both the vehicle-(p<0.0005) and VPA-chow treated mice (p<0.01). Analyses revealed that VPA-chow treatment increased holepoking in the saline-treated mice compared to vehicle-chow (p<0.05). Mice administered GBR12909 after VPA-chow treatment exhibited a trend towards increased holepoking compared to vehicle-chow treated mice administered GBR12909 (p<0.1).
Locomotor patterns
A significant GBR12909 effect was observed for spatial d (F(1,53)=14.07, p<0.0005; Fig. 2e) as well as a trend effect of VPA (F(1,53)=3.36, p<0.1) with no GBR12909 by VPA interaction. Post hoc analyses revealed that GBR12909 decreased spatial d compared to saline in both the vehicle- (p<0.01) and VPA-chow treated mice (p<0.05).
Experiment 2: Exploratory profile of WT and DAT KD mice after chronic vehicle- or VPA-chow treatment
Locomotor activity
Significant genotype effects were observed for transitions (F(1,44)=33.3, p<0.0001) and center entries (F(1,44)=13.1, p<0.001). Significant main effects of VPA were also observed for transitions (F(1,44)=8.4, p<0.01; Fig. 3a) and center entries (F(1,44)=4.2, p<0.05; Fig. 3b). There was a genotype by VPA interaction for transitions (F(1,44)=4.3, p<0.05), but not for center entries. Since no sex by genotype, sex by VPA, or sex by genotype by VPA interactions were observed, male and female data were pooled for post hoc analyses. These analyses revealed that both the vehicle- (p<0.0001) and VPA-chow treated DAT KD mice (p<0.01) exhibited increased transitions compared to WT mice. DAT KD mice treated with VPA-chow exhibited fewer transitions compared to DAT KD mice treated with vehicle-chow (p<0.05) however. VPA-chow treatment did not affect transitions in the WT mice (p>0.1). DAT KD mice made more center entries compared to WT mice in both the vehicle- (p<0.005) and VPA-chow treated mice (p<0.005). Post hoc analyses did not reveal any significant effect of VPA on center entries in the DAT KD or WT mice (p>0.1).
Fig. 3.
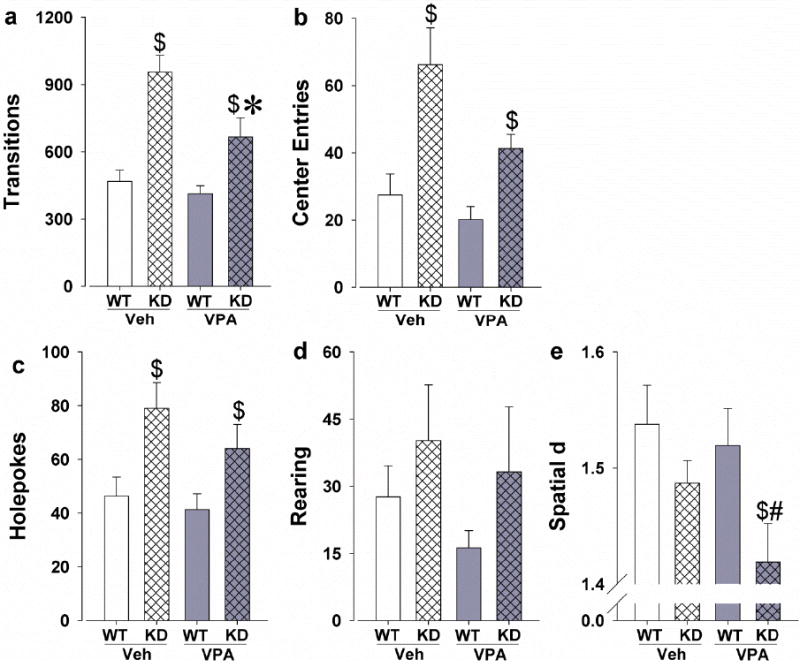
Effects of chronic treatment with VPA on the mania-like profile of DAT KD mice in the BPM. The DAT KD mice were significantly more active than the WT mice as measured by transitions, behavior that was attenuated by VPA treatment (a). DAT KD mice also exhibited more center entries compared with WT mice, which was unaffected by VPA treatment (b). DAT KD mice exhibited increased specific exploration as measured by the amount of holepokes, which was not affected by VPA (c). No genotype or treatment effects were observed on rearing behavior (d). DAT KD mice exhibited straighter paths of movement than the WT mice (reduced spatial d), which were further reduced by VPA treatment (e). Data are presented as the mean + SEM. * Denotes p<0.05 and # denotes p<0.1 when compared with the vehicle-chow treatment group and $denotes p<0.05 when compared with WT mice.
Exploratory behavior
A significant genotype effect was observed for holepoking (F(1,44)=10.3, p<0.005; Fig. 3c) and a trend toward KD mice exhibiting more rearings compared with WT mice (F(1,44)=3.2, p<0.1; Fig. 3d). There was no main effect of VPA or genotype by VPA interaction for holepoking or rearing. Post hoc analyses on holepoking revealed that DAT KD mice exhibited increased holepoking compared to WT mice in both the vehicle- (p<0.05) and VPA-chow treated mice (p<0.05).
Locomotor patterns
DAT KD mice exhibited straighter path movements compared with WT mice as measured by spatial d (F(1,44)=5.1, p<0.05; Fig. 3e). Although no main effect of sex was observed, there was a sex by genotype by VPA interaction (F(1,44)=5.0, p<0.05). Given the lack of sex effects throughout this study and previous studies, male and female data were combined for post hoc analyses. These analyses revealed that VPA-chow treated DAT KD mice exhibited lower spatial d compared to VPA-chow treated WT mice (p<0.05). There was a trend towards lower spatial d in the VPA-chow treated DAT KD mice compared to the vehicle-chow treated DAT KD mice (p<0.1).
DISCUSSION
We examined the pharmacological predictive validity of reduced DAT functioning mouse models of BD mania. We observed that VPA attenuated the hyperactivity seen in these models, but did not affect other mania-like behavioral characteristics such as heightened specific exploration and more linear movements. The selectivity of VPA effects could be a reflection of the fact that VPA does not treat every aspect of BD. Thus, these data support the need of measuring beyond hyperactivity alone when developing novel treatments for BD mania (Gould and Einat, 2007).
Consistent with previous reports (Ralph-Williams et al., 2003; Young et al., 2010a; Young et al., 2011c), we observed that both DAT KD mice and GBR12909-treated mice exhibit behavioral profiles in the BPM that are similar to those of patients with BD mania (Perry et al., 2009). The rationale for using a 9 mg/kg dose of GBR12909 came from other studies such as GBR12909-induced increases in motor impulsivity (Loos et al., 2010) in a 5-choice serial reaction time task, increased risk preference in a mouse Iowa Gambling Task (van Enkhuizen et al., accepted), and accelerated choice latency and increased motivation in mice in a progressive ratio breakpoint test (Young and Geyer, 2010). Mice administered 9 mg/kg of the specific DAT inhibitor GBR12909 exhibited increased activity, increased specific exploration (as measured by rearing), and straighter patterns of movement. A similar pattern was observed in the DAT KD mice, with levels of holepoking being the increased measure of specific exploration. Consistently, DAT KD mice on a 129Sv/J background exhibited increased holepoking, while GBR12909 administration to C57BL/6J mice resulted in more prominent effects on rearing behavior (Perry et al., 2009). The divergence in specific exploration is thus likely caused by a difference in background strain and may be in part due to the more active baseline phenotype of the C57 mouse strain (Paulus et al., 1999; Peeler and Nowakowski, 1987). Consistent with such baseline differences, 129 mouse strains also exhibit a higher spatial d and fewer center entries compared to the C57BL/6 strains (Paulus et al., 1999). These background strain variations are consistent with earlier studies demonstrating that such variations change the effects of the DAT inhibitor modafinil (Young et al., 2011b).
After pilot studies in which 1.0 and 2.0% VPA-chow treatment for 4 weeks resulted in low-end (≈50 mcg/mL) and high-end (≈150 mcg/mL) therapeutic serum concentrations respectively (Figure 1), 1.5% VPA-chow was chosen for investigation. Others have found that 2.5% VPA-chow resulted in therapeutic blood concentrations in mice from N171-82Q and YAC128 strains (≈80 mcg/mL) (Chiu et al., 2011). In rats, chronic dietary VPA treatment at 2.0% resulted in low-end blood concentrations (40 mcg/mL) in one study (Einat et al., 2003) and in therapeutic concentrations (≈80 mcg/mL) in another (Du et al., 2008). Importantly, in the current chronic study, treatment with 1.5% VPA in the animals’ chow for 28 days led to optimal therapeutic mood-stabilizing concentrations for the treatment of BD mania (60-80 mcg/mL) (Grunze et al., 2009). In both models of DAT inhibition, this treatment attenuated their hyperactivity and the frequency of center entries (Fig. 4 & 5). VPA-induced amelioration of center entries was significantly selective in GBR12909- and not saline-treated mice, with a greater effect visually in the DAT KD than WT mice, although a main effect of VPA was observed. Such differences in the models could relate to sample size or background differences described above. VPA modestly increased holepoking in the C57BL/6J mice, while VPA led to slightly reduced levels of spatial d in the DAT KD mice. The slight variation of effect could reflect background strain differences described above.
Fig. 4.
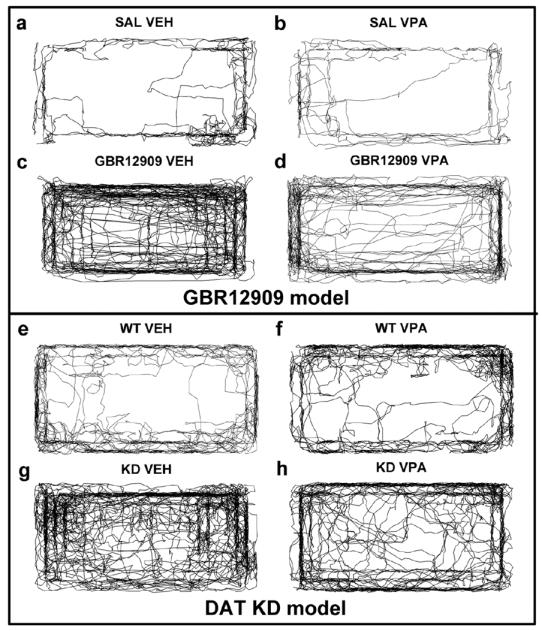
Representative X-Y plots from each study are displayed. These figures are representative behavioral patterns from mice of each study. As shown, VPA treatment did not affect the patterns of movement of saline-administered mice (a-b). Chronic VPA treatment attenuated GBR12909-induced hyperactivity (c-d), but did not affect specific exploration or sequential organization. VPA treatment did not affect the behavioral patterns of WT mice (e-f). VPA treatment attenuated the hyperactive behavioral pattern of DAT KD mice (g-h), but did not affect specific exploration or sequential organization.
Fig. 5.
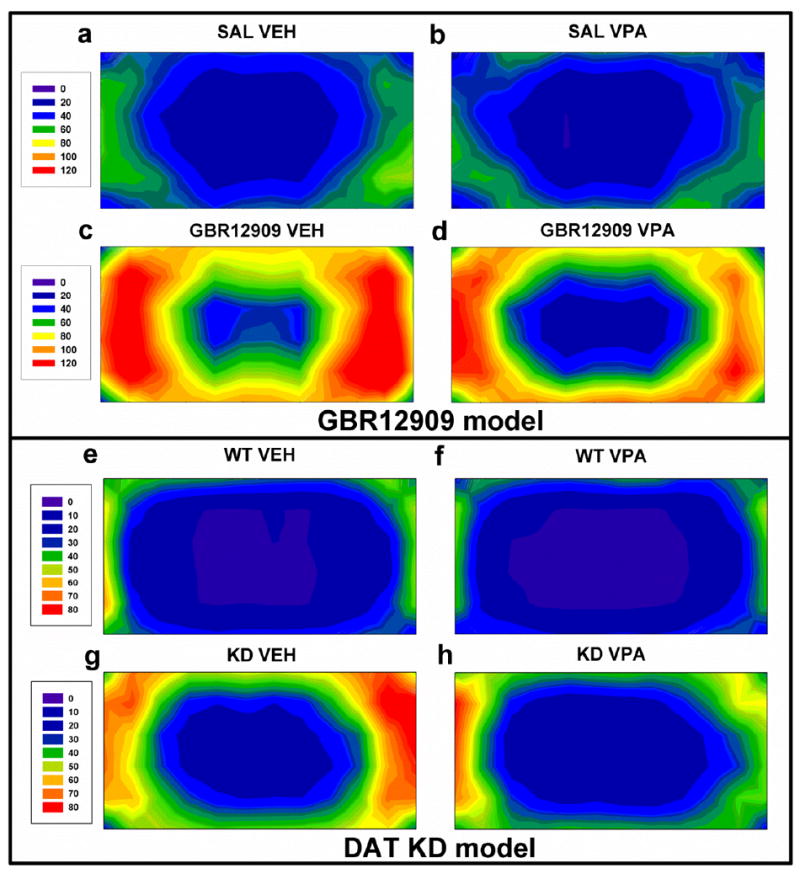
Heat maps from each study are displayed. These figures represent the average group data and are based on 72 evenly distributed sector entries. As displayed, VPA treatment did not affect activity levels of saline-treated mice (a-b). Chronic VPA treatment attenuated GBR12909-induced hyperactivity (c-d). VPA treatment did not affect activity levels of WT mice (e-f), but attenuated the hyperactive behavioral pattern of DAT KD mice (g-h). The scale ranges from 0 – 120 for (a-d) and from 0 – 80 for (e-h), because of the higher baseline activity of the mice on a C57BL/6J background.
Longitudinal VPA treatment studies in the human BPM would assist direct comparisons with the present studies. Similar to the current findings in both DAT models however, 3-week treatment with various medications reduced effect size differences of activity but not spatial d or object interactions in BD patients compared with healthy subjects (Minassian et al., 2011).
Direct cross-species comparisons of treatment effects using similar measures are rarely available. Despite such limitations however, the anticonvulsant drug VPA has been used frequently to test the predictive validity of animal models of BD mania. Several studies have observed that acute VPA treatment attenuated hyperactivity in animals treated with amphetamine (Dencker and Husum, 2010; Flaisher-Grinberg and Einat, 2010; Kalinichev and Dawson, 2011) or a combination of amphetamine and chlordiazepoxide (Kozikowski et al., 2007). Acute VPA also reversed hyperactivity induced by sleep deprivation in D-box binding protein (DBP) knockout (KO) mice (Le-Niculescu et al., 2008). It is important to note however, that acute VPA can also lower basal activity levels in saline-treated animals (Dencker and Husum, 2010), confounding the interpretation of the ‘therapeutic’ effects as being specific to the manipulation-induced hyperactivity. Acute VPA attenuated hyperactivity in DAT KD mice while not affecting the activity of WT mice however (Ralph-Williams et al., 2003). Acute VPA treatment did not remediate GBR12909-induced hyperactivity in mice, leading to suggestions that the predictive validity of the GBR12909 model for BD mania should be examined using chronic treatment (Douma et al., 2011). In support of this point, treatment of BD mania requires chronic administration of a mood-stabilizer, nominally at least 3 weeks in clinical trials (Cipriani et al., 2011). When chronic treatment is used in animal models to eliminate the risk of false-positive or false-negative effects, the activity-attenuating effects are less striking. Eleven days of oral treatment with 1.2% VPA in drinking water non-significantly attenuated the dopamine D2/D3 agonist quinpirole-induced hyperactivity (Shaldubina et al., 2002), which may have been due to lower end VPA blood concentrations (≈50 mcg/m). Chronic VPA treatment failed to normalize ‘mania-like’ behaviors in a rat model of mania (D’Aquila et al., 2006).Nevertheless, chronic VPA treatment normalized hyperactivity in transgenic CN98 mice (Herzog et al., 2008) and VPA microinjections into the nucleus accumbens attenuated amphetamine-induced hyperactivity in rats (Kim et al., 2008). Thus, positive data for models have been generated using chronic VPA treatment but methodologies have been diverse and few studies examined VPA concentrations to determine whether therapeutic levels were achieved.
In addition to marked hyperactivity in patients with BD mania, other aspects of abnormal exploratory behavior of patients with BD mania were quantified in the human BPM (Perry et al., 2009). Although therapeutic concentrations of VPA attenuated hyperactivity in the present models, specific exploration and locomotor patterns remained unaffected or worsened. One could speculate that the ineffectiveness of VPA on specific exploration may go beyond this behavior and be related to cognitive impairments that currently go untreated in BD mania as well. For example, holepoking behavior in the mouse BPM correlated with risk preference in the mouse Iowa Gambling Task (Young et al., 2011c). Furthermore, greater object interactions in the human BPM correlated with poor human performance in another frontal-mediated task, the Wisconsin Card Sorting Task (Henry et al., 2011). Chronic VPA treatment increased holepoking in C57BL/6J mice in the present studies. This effect is similar to a human BPM study in which subjects receiving VPA exhibited significantly more object interactions than those not receiving VPA (Perry et al., 2009). Although specific pharmacological treatment comparisons would aid such cross-species comparisons, these data reinforce the need for therapeutics that treat the multiple facets of BD (Gould and Einat, 2007; Young et al., 2011a; Fig. 6). Accordingly, a drug that would reduce specific exploration in the BPM may also have beneficial effects on cognitive functions. The models based on reduced functioning DAT provide a robust and practical method of investigating the effects of novel compounds.
Fig. 6. Schematic on the utility of a multivariate approach for developing treatments for Bipolar Disorder (BD) mania.
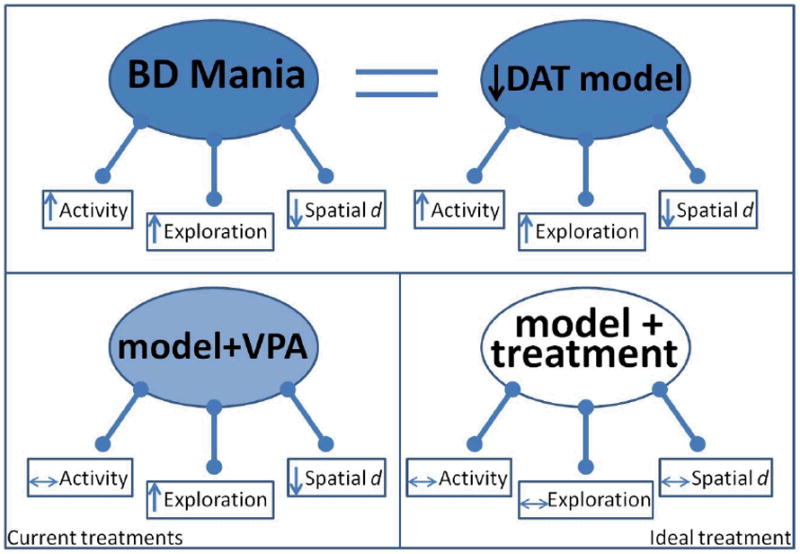
In the top panel (left), patients with BD exhibit hyperactivity, more specific exploration, and straighter line movements through space compared with healthy subjects. Both reduced functioning dopamine transporter (DAT) models (knockdown mice and GBR12909-treated mice (right)) exhibit a similarly altered pattern of exploration to that of BD patients. In the bottom panel (left), the results of the current study are summarized whereby valproate only attenuated the hyperactivity of these models while not affecting other measures of abnormal exploration. Thus, using these models, an ideal treatment (bottom right panel) could be identified that treats each aspect of abnormal exploration.
The mechanisms by which VPA exerts its therapeutic effects in humans are still not fully understood. VPA affects multiple processes, including glycogen synthase kinase-3 (GSK-3) (Sutton and Rushlow, 2011), γ-aminobutyric acid (GABA)ergic neurotransmission (Guidotti et al., 2011), and N-methyl-D-aspartate (NMDA) glutamatergic signaling (Rapoport et al., 2009). One of the biological constructs by which VPA attenuated the mania-like behavior could be by increasing DAT gene expression in the brain (Wang et al., 2007). Importantly, VPA-induced increase in DAT expression continued to rise with the duration of treatment (Wang et al., 2007), underscoring the necessity for chronic treatment. Furthermore, VPA down-regulates dopaminergic D2-like (D2, D3, and D4) receptor signaling (Ramadan et al., 2011), supporting a dopaminergic mechanism for VPA. Future studies could target these putative mechanisms.
One of the limitations of this study and others when assessing the pharmacological predictive validity of an animal model for BD mania is the use of suboptimal therapeutics to validate the models. By using a multivariate approach to quantify exploration however, the effects of a treatment can be assessed on different aspects of exploratory behavior beyond motor hyperactivity alone. Future studies should be performed to assess the effects of other chronic medications such as the mood-stabilizer lithium on the behavioral profile of the DAT KD and GBR12909-treated mice. Additionally, while most treatment studies involve animal models, these data provide a specific testable hypothesis for longitudinal drug testing in humans in the BPM; to wit, VPA treatment for mania would reduce hyperactivity compared to patients in an unmedicated state, but would not affect increased specific exploration or reduced spatial d.
In conclusion, mania-like behavior of mice with reduced functional DAT is partially alleviated by chronic treatment with VPA, mimicking the real-life situation in which BD treatment does not alleviate all symptoms. The data presented here provide predictive validation that selective DAT inhibition enables modeling of BD mania-like behavior, although more explicit human medication studies would provide more specific support. The DAT model can be used to screen better therapeutics treating the complete mania-like behavioral profile including cognitive dysfunction associated with mania.
Acknowledgments
We thank Richard Sharp, Mahalah Buell, and Dr. Berend Olivier for their support, as well as Dr. Xiaoxi Zhuang for supplying us with the original DAT heterozygous breeding pairs. These studies were supported by NIH grants R01-MH071916, and R21-MH091571, as well as by the Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center.
Footnotes
Statement of Interest
Mr. van Enkhuizen and Kooistra report no conflict of interest. Dr. Geyer has received consulting compensation from Abbott, Cerca, Merck, Omeros, Takeda, and Teva, and holds an equity interest in San Diego Instruments. Dr. Geyer also has research grant support from Intracellular Therapeutics, Johnson & Johnson, NIDA, NIMH, and the U.S. Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center. Dr. Young has received research grant support from Cerca Insights and Lundbeck Ltd. The aforementioned support did not direct any of the research presented here.
References
- Anand A, Barkay G, Dzemidzic M, Albrecht D, et al. Striatal dopamine transporter availability in unmedicated bipolar disorder. Bipolar Disorder. 2011;13(4):406–413. doi: 10.1111/j.1399-5618.2011.00936.x. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Bowden CL, Brugger AM, Swann AC, Calabrese JR, et al. Efficacy of divalproex vs lithium and placebo in the treatment of mania. The Depakote Mania Study Group. The Journal of the American Medical Association. 1994;271(12):918–924. [PubMed] [Google Scholar]
- Chiu CT, Liu G, Leeds P, Chuang DM. Combined treatment with the mood stabilizers lithium and valproate produces multiple beneficial effects in transgenic mouse models of Huntington’s disease. Neuropsychopharmacology. 2011;36(12):2406–2421. doi: 10.1038/npp.2011.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani A, Barbui C, Salanti G, Rendell J, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378(9799):1306–1315. doi: 10.1016/S0140-6736(11)60873-8. [DOI] [PubMed] [Google Scholar]
- D’Aquila PS, Panin F, Serra G. Chronic valproate fails to prevent imipramine-induced behavioural sensitization to the dopamine D2-like receptor agonist quinpirole. European Journal of Pharmacology. 2006;535(1-3):208–211. doi: 10.1016/j.ejphar.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Dencker D, Husum H. Antimanic efficacy of retigabine in a proposed mouse model of bipolar disorder. Behavioural Brain Research. 2010;207(1):78–83. doi: 10.1016/j.bbr.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Douma TN, Kolarz A, Postma Y, Olivier B, et al. The amphetamine-chlordiazepoxide mixture, a pharmacological screen for mood stabilizers, does not enhance amphetamine-induced disruption of prepulse inhibition. Behavioural Brain Research. 2011;225(1):377–381. doi: 10.1016/j.bbr.2011.07.032. [DOI] [PubMed] [Google Scholar]
- Du J, Creson TK, Wu LJ, Ren M, et al. The role of hippocampal GluR1 and GluR2 receptors in manic-like behavior. Journal of Neuroscience. 2008;28(1):68–79. doi: 10.1523/JNEUROSCI.3080-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einat H, Yuan P, Gould TD, Li J, et al. The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. Journal of Neuroscience. 2003;23(19):7311–7316. doi: 10.1523/JNEUROSCI.23-19-07311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaisher-Grinberg S, Einat H. Strain-specific battery of tests for domains of mania: effects of valproate, lithium and imipramine. Frontiers in Psychiatry. 2010;1:10. doi: 10.3389/fpsyt.2010.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: pharmacological and behavioral analyses. Pharmacology, Biochemistry and Behavior. 1986;25(1):277–288. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- Gould TD, Einat H. Animal models of bipolar disorder and mood stabilizer efficacy: a critical need for improvement. Neuroscience and Biobehavioral Reviews. 2007;31(6):825–831. doi: 10.1016/j.neubiorev.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Schork NJ, Eskin E, Kelsoe JR. Identification of additional variants within the human dopamine transporter gene provides further evidence for an association with bipolar disorder in two independent samples. Molecular Psychiatry. 2006;11:125–133. doi: 10.1038/sj.mp.4001764. [DOI] [PubMed] [Google Scholar]
- Grunze H, Vieta E, Goodwin GM, Bowden C, et al. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2009 on the treatment of acute mania. The World Journal of Biological Psychiatry. 2009;10(2):85–116. doi: 10.1080/15622970902823202. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Chen Y, Davis JM, et al. Epigenetic GABAergic targets in schizophrenia and bipolar disorder. Neuropharmacology. 2011;60(7-8):1007–1016. doi: 10.1016/j.neuropharm.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison-Read PE. Models of mania and antimanic drug actions: progressing the endophenotype approach. Journal of Psychopharmacology. 2009;23(3):334–337. doi: 10.1177/0269881108089840. [DOI] [PubMed] [Google Scholar]
- Henry BL, Minassian A, van Rhenen M, Young JW, et al. Effect of methamphetamine dependence on inhibitory deficits in a novel human open-field paradigm. Psychopharmacology. 2011;215(4):697–707. doi: 10.1007/s00213-011-2170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog CJ, Miot S, Mansuy IM, Giros B, et al. Chronic valproate normalizes behavior in mice overexpressing calcineurin. European Journal of Pharmacology. 2008;580(1-2):153–160. doi: 10.1016/j.ejphar.2007.10.050. [DOI] [PubMed] [Google Scholar]
- Horschitz S, Hummerich R, Lau T, Rietschel M, et al. A dopamine transporter mutation associated with bipolar affective disorder causes inhibition of transporter cell surface expression. Molecular Psychiatry. 2005;10(12):1104–1109. doi: 10.1038/sj.mp.4001730. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Dawson LA. Evidence for antimanic efficacy of glycogen synthase kinase-3 (GSK3) inhibitors in a strain-specific model of acute mania. International Journal of Neuropsychopharmacology. 2011;14(8):1051–1067. doi: 10.1017/S1461145710001495. [DOI] [PubMed] [Google Scholar]
- Kim WY, Kim S, Kim JH. Chronic microinjection of valproic acid into the nucleus accumbens attenuates amphetamine-induced locomotor activity. Neuroscience Letters. 2008;432(1):54–57. doi: 10.1016/j.neulet.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Kozikowski AP, Gaisina IN, Yuan H, Petukhov PA, et al. Structure-based design leads to the identification of lithium mimetics that block mania-like effects in rodents. possible new GSK-3beta therapies for bipolar disorders. Journal of the American Chemical Society. 2007;129(26):8328–8332. doi: 10.1021/ja068969w. [DOI] [PubMed] [Google Scholar]
- Le-Niculescu H, McFarland MJ, Ogden CA, Balaraman Y, et al. Phenomic, convergent functional genomic, and biomarker studies in a stress-reactive genetic animal model of bipolar disorder and co-morbid alcoholism. American Journal of Medical Genetics Part B Neuropsychiatric Genetics. 2008;147B(2):134–166. doi: 10.1002/ajmg.b.30707. [DOI] [PubMed] [Google Scholar]
- Loos M, Staal J, Schoffelmeer AN, Smit AB, et al. Inhibitory control and response latency differences between C57BL/6J and DBA/2J mice in a Go/No-Go and 5-choice serial reaction time task and strain-specific responsivity to amphetamine. Behavioural Brain Research. 2010;214(2):216–224. doi: 10.1016/j.bbr.2010.05.027. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Jin R, He JP, Kessler RC, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Archives of General Psychiatry. 2011;68(3):241–251. doi: 10.1001/archgenpsychiatry.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Young JW, Masten V, et al. Repeated assessment of exploration and novelty seeking in the human behavioral pattern monitor in bipolar disorder patients and healthy individuals. Public Library of Science One. 2011;6(8):e24185. doi: 10.1371/journal.pone.0024185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Dulawa SC, Ralph RJ, Geyer MA. Behavioral organization is independent of locomotor activity in 129 and C57 mouse strains. Brain Research. 1999;(835):27–36. doi: 10.1016/s0006-8993(99)01137-3. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA. A temporal and spatial scaling hypothesis for the behavioral effects of psychostimulants. Psychopharmacology (Berl) 1991;104(1):6–16. doi: 10.1007/BF02244547. [DOI] [PubMed] [Google Scholar]
- Peeler DF, Nowakowski RS. Genetic factors and the measurement of exploratory activity. Behavioral and Neural Biology. 1987;48(1):90–103. doi: 10.1016/s0163-1047(87)90619-4. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, et al. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Archives of General Psychiatry. 2009;66(10):1072–1080. doi: 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsonneault JK, Han DD, Burdick KE, Kataki M, et al. Dopamine Transporter Gene Variant Affecting Expression in Human Brain is Associated with Bipolar Disorder. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Paulus MP, Zhuang X, Hen R, et al. Valproate attenuates hyperactive and perseverative behaviors in mutant mice with a dysregulated dopamine system. Biological Psychiatry. 2003;53(4):352–359. doi: 10.1016/s0006-3223(02)01489-0. [DOI] [PubMed] [Google Scholar]
- Ramadan E, Basselin M, Taha AY, Cheon Y, et al. Chronic valproate treatment blocks D(2)-like receptor-mediated brain signaling via arachidonic acid in rats. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport SI, Basselin M, Kim HW, Rao JS. Bipolar disorder and mechanisms of action of mood stabilizers. Brain Research Reviews. 2009;61(2):185–209. doi: 10.1016/j.brainresrev.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Masten VL, Caldwell S, Paulus MP, et al. Differential contributions of dopamine D1, D2, and D3 receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology. 2006;31(11):2349–2358. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- Shaldubina A, Einat H, Szechtman H, Shimon H, et al. Preliminary evaluation of oral anticonvulsant treatment in the quinpirole model of bipolar disorder. Journal of Neural Transmission. 2002;109(3):433–440. doi: 10.1007/s007020200035. [DOI] [PubMed] [Google Scholar]
- Sutton LP, Rushlow WJ. The effects of neuropsychiatric drugs on glycogen synthase kinase-3 signaling. Neuroscience. 2011;199:116–124. doi: 10.1016/j.neuroscience.2011.09.056. [DOI] [PubMed] [Google Scholar]
- Sylvia LG, Alloy LB, Hafner JA, Gauger MC, et al. Life events and social rhythms in bipolar spectrum disorders: a prospective study. Behavior Therapy. 2009;40(2):131–141. doi: 10.1016/j.beth.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Geyer MA, Kooistra K, Young JW. Differential effects of dopamine transporter inhibitors in the rodent Iowa Gambling Task: Relevance to mania. Psychopharmacology. doi: 10.1007/s00213-012-2854-2. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Michelhaugh SK, Bannon MJ. Valproate robustly increases Sp transcription factor-mediated expression of the dopamine transporter gene within dopamine cells. European Journal of Neuroscience. 2007;25(7):1982–1986. doi: 10.1111/j.1460-9568.2007.05460.x. [DOI] [PubMed] [Google Scholar]
- Wingo AP, Ghaemi SN. Frequency of stimulant treatment and of stimulant-associated mania/hypomania in bipolar disorder patients. Psychopharmacology Bulletin. 2008;41(4):37–47. [PubMed] [Google Scholar]
- Yatham LN, Torres IJ, Malhi GS, Frangou S, et al. The International Society for Bipolar Disorders-Battery for Assessment of Neurocognition (ISBD-BANC) Bipolar Disorders. 2010;12(4):351–363. doi: 10.1111/j.1399-5618.2010.00830.x. [DOI] [PubMed] [Google Scholar]
- Young JW, Geyer MA. Action of modafinil--increased motivation via the dopamine transporter inhibition and D1 receptors? Biological Psychiatry. 2010;67(8):784–787. doi: 10.1016/j.biopsych.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, et al. GBR 12909 administration as a mouse model of bipolar disorder mania: mimicking quantitative assessment of manic behavior. Psychopharmacology. 2010a;208(3):443–454. doi: 10.1007/s00213-009-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, et al. The mania-like exploratory profile in genetic dopamine transporter mouse models is diminished in a familiar environment and reinstated by subthreshold psychostimulant administration. Pharmacology, Biochemistry and Behavior. 2010b;96(1):7–15. doi: 10.1016/j.pbb.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Henry BL, Geyer MA. Predictive animal models of mania, hits, misses, and future directions. British Journal of Pharmacology. 2011a doi: 10.1111/j.1476-5381.2011.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Kooistra K, Geyer MA. Dopamine receptor mediation of the exploratory/hyperactivity effects of modafinil. Neuropsychopharmacology. 2011b;36(7):1385–1396. doi: 10.1038/npp.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Minassian A, Paulus MP, Geyer MA, et al. A reverse-translational approach to bipolar disorder: rodent and human studies in the Behavioral Pattern Monitor. Neuroscience and Biobehavioral Reviews. 2007;31(6):882–896. doi: 10.1016/j.neubiorev.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, van Enkhuizen J, Winstanley CA, Geyer MA. Increased risk-taking behavior in dopamine transporter knockdown mice: further support for a mouse model of mania. Journal of Psychopharmacology. 2011c;25(7):934–943. doi: 10.1177/0269881111400646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, et al. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(4):1982–1987. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


