Abstract
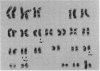
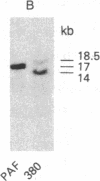
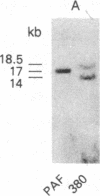
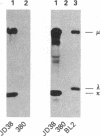
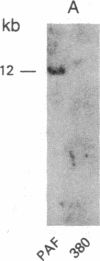
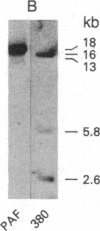
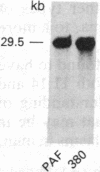
We have established a cell line, which we named 380, from a young male with acute lymphoblastic leukemia (FAB type L2). Karyologic analysis of this cell line indicates that it carries an 8;14 and a 14;18 chromosome translocation, which are characteristic of Burkitt lymphoma and of follicular lymphoma, respectively. This cell line is Epstein-Barr virus antigen-negative, reacts with monoclonal antibodies specific for B cells, and contains rearranged immunoglobulin heavy and light chain genes, but does not express human immunoglobulins. In this cell line, both mu heavy chain constant (C mu) loci are rearranged within the joining (JH) DNA segment. One of the JH segments on one of the 14q+ chromosomes is rearranged with a segment of chromosome 8, where the c-myc oncogene resides, while the other is rearranged with a segment of chromosome 18 where a putative oncogene, which we have called bcl-2, is located. The c-myc oncogene, which is translocated to one of the 14q+ chromosomes, is in its germ-line configuration more than 14 kilobases away from both the JH segment and the heavy chain enhancer that is located between the JH and mu switch region. Based on these findings, we propose a model of some aspects of B-cell oncogenesis according to which B-cell neoplasms carrying translocations involving the heavy chain loci on both human chromosomes 14 are the result of a multiple step process.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bollum F. J. Terminal deoxynucleotidyl transferase as a hematopoietic cell marker. Blood. 1979 Dec;54(6):1203–1215. [PubMed] [Google Scholar]
- Croce C. M., Erikson J., ar-Rushdi A., Aden D., Nishikura K. Translocated c-myc oncogene of Burkitt lymphoma is transcribed in plasma cells and repressed in lymphoblastoid cells. Proc Natl Acad Sci U S A. 1984 May;81(10):3170–3174. doi: 10.1073/pnas.81.10.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M., Thierfelder W., Erikson J., Nishikura K., Finan J., Lenoir G. M., Nowell P. C. Transcriptional activation of an unrearranged and untranslocated c-myc oncogene by translocation of a C lambda locus in Burkitt. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6922–6926. doi: 10.1073/pnas.80.22.6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Bregni M., Erikson J., Patterson D., Gallo R. C., Croce C. M. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Martinotti S., Gallo R. C., Erikson J., Croce C. M. Translocation and rearrangements of the c-myc oncogene locus in human undifferentiated B-cell lymphomas. Science. 1983 Feb 25;219(4587):963–967. doi: 10.1126/science.6401867. [DOI] [PubMed] [Google Scholar]
- Ehlenberger A. G., Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977 Feb 1;145(2):357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel B. S., Selden J. R., Chaganti R. S., Jhanwar S., Nowell P. C., Croce C. M. The 2p breakpoint of a 2;8 translocation in Burkitt lymphoma interrupts the V kappa locus. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2444–2446. doi: 10.1073/pnas.81.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson J., Finan J., Nowell P. C., Croce C. M. Translocation of immunoglobulin VH genes in Burkitt lymphoma. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5611–5615. doi: 10.1073/pnas.79.18.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson J., Finan J., Tsujimoto Y., Nowell P. C., Croce C. M. The chromosome 14 breakpoint in neoplastic B cells with the t(11;14) translocation involves the immunoglobulin heavy chain locus. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4144–4148. doi: 10.1073/pnas.81.13.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson J., Nishikura K., ar-Rushdi A., Finan J., Emanuel B., Lenoir G., Nowell P. C., Croce C. M. Translocation of an immunoglobulin kappa locus to a region 3' of an unrearranged c-myc oncogene enhances c-myc transcription. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7581–7585. doi: 10.1073/pnas.80.24.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson J., ar-Rushdi A., Drwinga H. L., Nowell P. C., Croce C. M. Transcriptional activation of the translocated c-myc oncogene in burkitt lymphoma. Proc Natl Acad Sci U S A. 1983 Feb;80(3):820–824. doi: 10.1073/pnas.80.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebman D., Trucco M., Bottero L., Lange B., Pessano S., Rovera G. A monoclonal antibody that detects expression of transferrin receptor in human erythroid precursor cells. Blood. 1982 Mar;59(3):671–678. [PubMed] [Google Scholar]
- Marcu K. B., Harris L. J., Stanton L. W., Erikson J., Watt R., Croce C. M. Transcriptionally active c-myc oncogene is contained within NIARD, a DNA sequence associated with chromosome translocations in B-cell neoplasia. Proc Natl Acad Sci U S A. 1983 Jan;80(2):519–523. doi: 10.1073/pnas.80.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufti G. J., Hamblin T. J., Oscier D. G., Johnson S. Common ALL with pre-B-cell features showing (8;14) and (14;18) chromosome translocations. Blood. 1983 Nov;62(5):1142–1146. [PubMed] [Google Scholar]
- Neel B. G., Jhanwar S. C., Chaganti R. S., Hayward W. S. Two human c-onc genes are located on the long arm of chromosome 8. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7842–7846. doi: 10.1073/pnas.79.24.7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K., ar-Rushdi A., Erikson J., DeJesus E., Dugan D., Croce C. M. Repression of rearranged mu gene and translocated c-myc in mouse 3T3 cells X Burkitt lymphoma cell hybrids. Science. 1984 Apr 27;224(4647):399–402. doi: 10.1126/science.6424234. [DOI] [PubMed] [Google Scholar]
- Nishikura K., ar-Rushdi A., Erikson J., Watt R., Rovera G., Croce C. M. Differential expression of the normal and of the translocated human c-myc oncogenes in B cells. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4822–4826. doi: 10.1073/pnas.80.15.4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Nowell P., Shankey T. V., Finan J., Guerry D., Besa E. Proliferation, differentiation, and cytogenetics of chronic leukemic B lymphocytes cultured with mitomycin-treated normal cells. Blood. 1981 Mar;57(3):444–451. [PubMed] [Google Scholar]
- Pegoraro L., Matera L., Ritz J., Levis A., Palumbo A., Biagini G. Establishment of a Ph1-positive human cell line (BV173). J Natl Cancer Inst. 1983 Mar;70(3):447–453. [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Ritz J., Nadler L. M., Bhan A. K., Notis-McConarty J., Pesando J. M., Schlossman S. F. Expression of common acute lymphoblastic leukemia antigen (CALLA) by lymphomas of B-cell and T-cell lineage. Blood. 1981 Sep;58(3):648–652. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taub R., Kirsch I., Morton C., Lenoir G., Swan D., Tronick S., Aaronson S., Leder P. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7837–7841. doi: 10.1073/pnas.79.24.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Berghe H., Parloir C., David G., Michaux J. L., Sokal G. A new characteristic karyotypic anomaly in lymphoproliferative disorders. Cancer. 1979 Jul;44(1):188–195. doi: 10.1002/1097-0142(197907)44:1<188::aid-cncr2820440131>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Van den Berghe H., Vermaelen K., Louwagie A., Criel A., Mecucci C., Vaerman J. P. High incidence of chromosome abnormalities in IgG3 myeloma. Cancer Genet Cytogenet. 1984 Apr;11(4):381–387. doi: 10.1016/0165-4608(84)90017-7. [DOI] [PubMed] [Google Scholar]
- Yunis J. J., Oken M. M., Kaplan M. E., Ensrud K. M., Howe R. R., Theologides A. Distinctive chromosomal abnormalities in histologic subtypes of non-Hodgkin's lymphoma. N Engl J Med. 1982 Nov 11;307(20):1231–1236. doi: 10.1056/NEJM198211113072002. [DOI] [PubMed] [Google Scholar]
- Zech L., Haglund U., Nilsson K., Klein G. Characteristic chromosomal abnormalities in biopsies and lymphoid-cell lines from patients with Burkitt and non-Burkitt lymphomas. Int J Cancer. 1976 Jan 15;17(1):47–56. doi: 10.1002/ijc.2910170108. [DOI] [PubMed] [Google Scholar]
- ar-Rushdi A., Nishikura K., Erikson J., Watt R., Rovera G., Croce C. M. Differential expression of the translocated and the untranslocated c-myc oncogene in Burkitt lymphoma. Science. 1983 Oct 28;222(4622):390–393. doi: 10.1126/science.6414084. [DOI] [PubMed] [Google Scholar]