Abstract
A major challenge in systems neuroscience is to unravel the complex matrix of connections that characterize functional circuits within the central nervous system. Retrograde transneuronal transport of rabies virus has proven to be especially useful for this purpose. Here we provide specific examples in which transneuronal transport of rabies virus has been used to unravel multi-synaptic pathways within motor, cognitive and autonomic circuits. Tracing with rabies virus defined: 1) the closed-loop organization of cerebellar and basal ganglia circuits with the cerebral cortex; 2) the presence of bidirectional communication between the cerebellum and basal ganglia; 3) the specific cortical areas that have mono- and/or disynaptic connections to spinal motoneurons in non-human primates; and 4) the areas in the cerebral cortex with the most direct influence on the sympathetic innervation of the kidney. These examples demonstrate the power of transneuronal tracing with rabies virus to identify the macroarchitecture of complex neural circuits.
Introduction
One of the major challenges in systems neuroscience is to unravel the complex matrix of connections that characterize functional circuits within the central nervous system. A variety of new techniques have emerged to attack this problem (e.g., functional connectivity with MRI [fcMRI] and diffusion tensor imaging [DTI]). Here we focus on the use of neurotropic viruses as neuroanatomical tracers because of their unique ability to reveal multiple links in a chain of synaptically-connected neurons. Why is this important? A conventional anterograde tracer indicates the site of termination of axons from the injection site, but cannot identify the specific neurons that are the target of these axons. A conventional retrograde tracer can only define the direct inputs to an injection site. FcMRI infers connectivity of brain regions based on correlated fluctuations in blood flow. However, these correlations don’t reveal the underlying basis for these correlations such as the direction, nature or source of the interconnections. In fact, two regions may display correlated fluctuations in blood flow and appear to be “functionally connected” even if no direct or indirect anatomical connections have been shown to exist between them [1]. Thus, there may be substantial disparities between the anatomical connections of a site and its “functional connectivity” with other sites. In contrast, transneuronal transport of selected neurotropic viruses occurs only at sites where neurons are synaptically interconnected. In this brief commentary, we will provide specific examples of the use of rabies virus to decipher the actual network macroarchitecture within motor, cognitive and autonomic circuits. We will focus on recent studies that used transneuronal transport to define circuits of three or more synaptically-linked neurons. The emerging uses of genetically modified viruses to examine local circuits or modify gene expression [e.g. 18, 22] will be addressed elsewhere in this issue.
The use of rabies virus as a tracer
Why use rabies when there are other viruses available as transneuronal tracers such as strains of herpes suis (pseudorabies virus [PRV]) and herpes simplex virus type 1 (HSV1)? PRV has been especially useful for tracing in rodents and ferrets. There are a large number of genetically engineered strains of PRV available which allows targeted analysis of selected neuronal populations [5,8]. Unfortunately, PRV does not infect primate neurons and thus, it cannot be used to trace connections in monkeys. Similarly, specific strains of HSV1 are effective in rodents and New World primates, but the same strains cannot be used as tracers in Old World primates [26, Dum and Strick, unpublished observations]. In addition, the strains of HSV1 that are transported transneuronally in the central nervous system of New World primates do not undergo transneuronal transport when injected at peripheral sites in these animals [31, and unpublished observations].
Rabies virus overcomes many of major shortcomings of other viruses for studies of circuitry in non-human primates. The virus infects a wide range of mammals including Old and New World monkeys [13,19,30]. It is transported transneuronally not only after injections into the central nervous system, but also after injections into peripheral sites such as single muscles [25] and viscera [16].
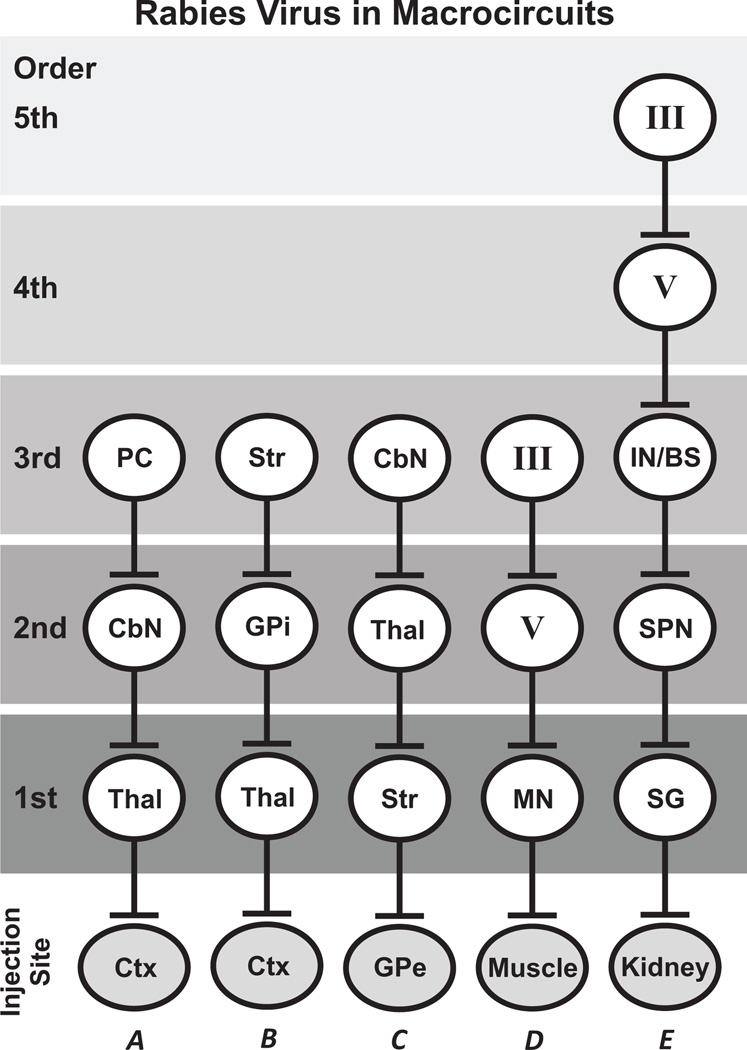
Rabies virus is transported exclusively in the retrograde direction. Transneuronal transport occurs in a time dependent manner from first-order to second-order neurons and then through subsequent stages of synaptically-connected neurons (i.e., from second- to third-order, from third- to fourth-order, … )(Fig. 1) [e.g. 11, 14, 15, 16, 24, 25, 29, 30]. At the survival times used for tracing experiments, rabies infection is restricted to neurons, and infected neurons show no evidence of cell lysis. Rabies virus can be co-injected with weak concentrations of the beta subunit of cholera toxin (CTb), a conventional retrograde tracer [23,28]. This allows improved delineation of the rabies injection sites without compromising the transneuronal transport of rabies. In addition, retrograde transport of the CTb labels many first-order neurons that innervate the injection site.
Figure 1. Examples of retrograde transneuronal transport of rabies virus through circuits composed of 3 or more synaptically-connected neurons.
A- Transport from cerebral cortex to third-order Purkinje cells in cerebellar cortex [14]; B- Transport from cerebral cortex to third-order neurons in the striatum [15]; C- Transport from the external segment of the globus pallidus to third-order neurons in cerebellar nuclei [11]; D- Transport from single muscles to third-order neurons in Layer III of the cerebral cortex [24,25]; E- Transport from the kidney to fifth-order neurons in Layer III of the cerebral cortex [16]. Abbreviations: CbN- cerebellar nuclei; Ctx- cerebral cortex; GPe- external segment of the globus pallidus; GPi- internal segment of the globus pallidus; III- Layer III of the cerebral cortex; IN/BS- spinal interneurons and brainstem neurons; MN- spinal motoneurons; PC- cerebellar Purkinje cells; SG- sympathetic ganglion; SPN- sympathetic preganglionic neurons; Str- striatum; Thal- thalamus; V- Layer V of the cerebral cortex.
Rabies virus grows and replicates in all types of neurons including those that are known to use the major excitatory (glutamate, acetylcholine) and inhibitory neurotransmitters (GABA, glycine) as well as neurotransmitters in specialized systems [2,6]. Transneuronal transport occurs across all types of synapses. There is no evidence that either virus transport or replication are activity dependent. Once first-order neurons become infected, the limiting factors for transneuronal transport are the rates of viral replication and transsynaptic exchange. These rates appear to be the same in all types of neurons. Thus, all second-order neurons are labeled at comparable times and the same is true for the labeling of all third-order and high-order neurons. We have injected rabies virus into various regions of cerebral cortex, cerebellar cortex, the internal and external segments of the globus pallidus (GPi and GPe), dorsal striatum, amygdala, eye muscles, limb muscles and visceral organs [4,11,14,15,16, 24, 25]. The N2c strain of rabies is transported transneuronally in the retrograde direction from all of these injection sites. By carefully adjusting the survival time, it is possible to study circuits composed of from 2 to 5 or more synaptically-connected neurons (Fig. 1). Because rabies virus replicates in infected neurons, there is an on-line amplification of the tracer signal. As a consequence, the quality of labeling in infected neurons at long survival times is comparable to that seen at shorter survival times. Animals display few if any symptoms of rabies infection during the survival times used for tracing experiments.
There are a number of methodological issues that must be considered when designing studies that use rabies as a transneuronal tracer [13]. For example, the various strains of rabies virus are transported and replicate at different rates. Consequently, each strain must be tested to verify its transport properties in a system with known synaptic linkages. However, if the amount and titer of virus injected is kept constant, a particular strain behaves in a consistent fashion. The timing of transneuronal transport in the central nervous system of rats provides a good indication of the timing in non-human primates [16]. Thus, there is little evidence for species differences in rabies transport, at least for the N2c strain of rabies.
Circuit Macroarchitecture
Our initial use of rabies virus focused on examining the topography of cerebro-cerebellar circuits [14](Fig. 1A). Prior neuroanatomical approaches for examining these circuits had been hindered by a number of technical limitations. Chief among these was the multi-synaptic nature of these pathways and the general inability of conventional tracers to label more than the direct inputs and outputs of an area. Cerebro-cerebellar connections represented an ideal circuit to test the transneuronal tracing capabilities of rabies virus in circuits consisting of 3 or more neurons in length. This is because the synaptic linkages at each stage in this circuit are well characterized both physiologically and anatomically.
After injections of rabies virus into either the primary motor cortex (M1) or a region of prefrontal cortex, we saw an orderly progression of infection in the central nervous system [14]. First-order neurons appeared in regions of the ventrolateral thalamus (Thal), second-order neurons appeared in one of the deep cerebellar nuclei (CbN) and third-order neurons appeared as Purkinje cells in regions of cerebellar cortex (PC)(Fig. 1A). In the same animals, we also examined basal ganglia circuits with the cerebral cortex [15]. Here again, we also saw an orderly progression of infection to neurons in basal ganglia nuclei that are synaptically connected to the injection site. First-order neurons appeared in regions of the ventrolateral thalamus (Thal), second-order neurons appeared in GPi and third-order neurons appeared in the striatum (Str), as well as the subthalamic nucleus (STN) and GPe (Fig. 1B). The time course of viral labeling through basal ganglia circuits matched that of cerebellar circuits. This observation is noteworthy because it provides evidence that the time course of transneuronal transport is largely independent of differences in cell types, transmitters and synaptic organization.
The use of rabies to explore the macroarchitecture of cerebro-cerebellar networks resulted in two new observations [14]. First, the areas of cerebellar cortex that influence M1 are separate from the areas of cerebellar cortex that influence prefrontal cortex. Second, the areas of the cerebellar cortex that receive input from M1 are the same as those that influence M1. Similarly, the areas of the cerebellar cortex that receive input from prefrontal cortex are the same as those that influence prefrontal cortex. This means that the macroarchitecture of cerebro-cerebellar networks can be characterized as multiple “closed-loop networks” [14]. To this point, we have identified two such networks– one involved in motor control and another involved in cognitive control [14, 27; see also 9, 17, 23].
These results highlight the unique capacity of transneuronal tracing with rabies virus to unravel the macroarchitecture of multi-synaptic circuits. Below we will highlight three additional examples where this approach has been used. We will focus on instances in which rabies transport has defined networks of three or more synaptically-connected neurons. Other examples of this approach can be found in Moschovakis et al. [20], Miyachi et al. [19] and Iwata et al. [12].
Example 1: In the course of using virus tracing to explore basal ganglia circuits with the cerebral cortex, we placed injections of the N2c strain of rabies into GPe [11]. Retrograde transport of the virus labeled first-order neurons in regions of the dorsal striatum (Str), and then retrograde transneuronal transport of the virus labeled second-order neurons in regions of the thalamus (Thal) and third-order neurons at sites within the cerebellar nuclei (CbN)(Fig. 1C). Most of the third-order neurons were found in the dentate nucleus. Shifts in the virus injection site within GPe resulted in shifts in the location of labeled neurons in the dentate. These results demonstrated that the neural substrate exists for the output stage of cerebellar processing, the cerebellar nuclei to influence the input stage of basal ganglia processing, the striatum. Ultimately this circuit gains access to the so-called “Indirect Pathway” through GPe. In subsequent experiments we examined whether a reciprocal pathway exists between the basal ganglia and cerebellum [4]. Retrograde transport of the N2c strain from different regions of the cerebellar cortex labeled first-order neurons in the pons, and then retrograde transneuronal transport labeled second-order neurons in the STN of the basal ganglia. Thus, the neural substrate exists for the major excitatory nucleus within the basal ganglia, the STN to influence the input stage of cerebellar processing, the cerebellar cortex. Taken together these results indicate that substantial two-way communication exists between the basal ganglia and cerebellum. Furthermore, this communication uses pathways that are independent of the major circuits these subcortical nuclei have with the cerebral cortex. Thus, the basal ganglia and cerebellum appear to be linked together at a subcortical level to form an integrated functional network.
Example 2: Multi-synaptic tracing with rabies virus has revealed new features about the cortical control of single muscles [24,25]. We used retrograde transneuronal transport of rabies virus in macaques to identify the location of cortico-motoneuronal (CM) cells that make monosynaptic connections with the motoneurons of muscles that control movements of the hand, elbow and shoulder. Retrograde transport of virus from single muscles labeled first-order motoneurons (MN) in the spinal cord, and then retrograde transneuronal transport labeled second-order, CM cells in Layer V of the primary motor cortex (M1)(Fig. 1D). We found that CM cells were largely restricted to the caudal portion of M1, which is buried in the central sulcus. We have termed this region “New M1.” Next, in another set of animals we extended the survival time to identify the location of cortical neurons in M1 with disynaptic connections with motoneurons. Transport of virus from single muscles labeled first-order motoneurons (MN) in the spinal cord, second-order, CM cells in Layer V of New M1 and third-order neurons in layers above and below Layer V. The labeling was especially dense in Layer III (Fig. 1D). In addition, a more rostral region of M1, which we have termed “Old M1,” contained labeled neurons only in Layer V at the longer survival time. These results are consistent with Old M1 lacking substantial monosynaptic input to motoneurons, but instead having monosynaptic connections with spinal interneurons that project to motoneurons. In other words, the output cells in Layer V of Old M1 influence motoneurons indirectly through a disynaptic pathway [25].
Example 3: Multi-synaptic tracing with rabies virus has revealed new features about the cortical control of the autonomic nervous system [16]. We used retrograde transneuronal transport of rabies virus from the rat kidney to identify the areas of the cerebral cortex that are potential sources of central commands for the neural regulation of this organ. By careful adjustment of the survival time (78–134 h), multiple stages of replication and transneuronal transport of virus enabled the sequential infection of first-order through fifth-order neurons (Fig. 1E). Infected neurons were first seen in the cerebral cortex at the same time as fourth-order neurons were found at other central sites. The first cortical neurons to be infected were located in Layer V. Two cortical areas in the contralateral hemisphere contained the vast majority (83%) of the infected neurons at this stage, M1 (68%) and a region of premotor cortex, the rostromedial motor area (M2, 15%). By extending the survival time 8–12 h, a subsequent stage of transneuronal transport permitted the infection of fifth-order neurons in other cortical layers (e.g., Layer III) of M1 and M2, and in Layer V of other cortical areas. Thus, we found that multiple motor and nonmotor areas of the cerebral cortex are sources of descending commands to influence kidney function. However, at all survival times, the major cortical origins of these commands are M1 and M2.
Technical Advances
The ability to trace multi-synaptic connections in non-human primates would be enhanced by the development of a virus strain that undergoes anterograde transneuronal transport. The H129 strain of Herpes Simplex Virus, Type 1 (HSV1) is transported transneuronally in the anterograde direction, but only in rodents and New World primates [7,10,31]. In addition, the number of synaptic linkages that can be revealed using this virus is somewhat limited due to the cell lysis and the accompanying adverse neurological symptoms caused by infection with this strain of HSV1. A recent report suggests that vesicular stomatitis virus can be genetically engineered to transport in the retrograde or anterograde direction depending on the nature of the inserted glycoprotein [3]. Both versions of this virus are efficiently transported in mice. Whether this virus will be suitable as a transneuronal tracer in non-human primates is as yet unclear.
Additional technological developments may further enhance the ability of rabies virus to trace macrocircuits within the nervous system of non-human primates. For example, new strains of rabies virus have been genetically engineered that express different reporter proteins [21]. As a consequence, it will be possible to perform “double labeling” experiments with different strains of rabies virus. Although the use of multiple strains of virus to examine more than one pathway simultaneously in the same animal is an important advance, several caveats should be highlighted. The insertion of new genetic material may alter the transport and replication properties of a virus. Consequently, each recombinant virus must be considered a new strain that requires validation of its transport properties. Even more essential is verification that the two strains are able to co-infect neurons with equal efficiency and transport at similar rates. Alterations in titer or the temporal sequence of viral injections may be necessary to counter slight variations in the ability of various strains to co-infect single neurons. With the use of recombinant viruses and the extension of survival times, the opportunity exists to reveal circuits of ever increasing length and complexity.
Highlights.
Retrograde transneuronal transport of rabies virus can trace circuits of three or more neurons.
Rabies travels through multi-synaptic pathways within motor, cognitive and autonomic circuits.
Tracing with rabies has revealed that the cerebellum and basal ganglia are interconnected.
ACKNOWLEDGMENTS
This work was supported in part by funds from the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, by National Institutes of Health Grants R01 NS24328 (P.L.S.), R01 MH56661 (P.L.S.), P40 RR018604 (P.L.S.), P30 NS076405 (P.L.S) and by a grant from the Pennsylvania Department of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1. Adachi Y, Osada T, Sporns O, Watanabe T, Matsui T, Miyamoto K, Miyashita Y. Functional connectivity between anatomically unconnected areas is shaped by collective network-level effects in the macaque cortex. Cerebral Cortex. 2012;22:1586–1592. doi: 10.1093/cercor/bhr234. [PMID: 21893683] This paper compared MRI based functional connectivity in macaque monkeys with their neuroanatomically based connectivity as reported in the CoCoMac Database. Surprisingly, some cortical areas exhibited enhanced functional connectivity without any direct or indirect anatomical connections.
- 2. Badami VM, Rice CD, Lois JH, Madrecha J, Yates BJ. Distribution of hypothalamic neurons with orexin (hypocretin) or melanin concentrating hormone (MCH) immunoreactivity and multisynaptic connections with diaphragm motoneurons. Brain Res. 2010;1323:119–126. doi: 10.1016/j.brainres.2010.02.002. [PubMed :20144885] The authors used immunohistochemistry to identify neurons dually labeled with rabies virus (injected into the diaphragm) and with orexin-A or melanin concentrating hormone. These peptides are involved in energy metabolism and the physiological changes that occur between sleep and wakefulness. This study provides evidence that hypothalamic neurons containing orexin and MCH are involved in the regulation of respiration through multisynaptic linkages.
- 3. Beier KT, Saunders A, Oldenburg IA, Miyamichi K, Akhtar N, Luo L, Whelan SP, Sabatini B, Cepko CL. Anterograde or retrograde transsynaptic labeling of CNS neurons with vesicular stomatitis virus vectors. Proc Natl Acad Sci USA. 2011;108:15414–15419. doi: 10.1073/pnas.1110854108. Erratum in: Proc Natl Acad Sci USA. 2012; 109:9219. [PubMed: 21825165] This study provides strong evidence that the directionality of virus transport is a property of the viral glycoprotein (G) protein. Anterograde transneuronal transport was conferred by addition of the G protein from lymphocytic choriomeningitis virus whereas retrograde transneuronal transport was conferred by the addition rabies virus glycoprotein.
- 4.Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci USA. 2010;107:8452–8456. doi: 10.1073/pnas.1000496107. [PubMed: 20404184] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Card JP, Kobiler O, Ludmir EB, Desai V, Sved AF, Enquist LW. A dual infection pseudorabies virus conditional reporter approach to identify projections to collateralized neurons in complex neural circuits. PLoS One. 2011;6:e21141. doi: 10.1371/journal.pone.0021141. Epub 2011 Jun 16. [PubMed: 21698154] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coulon P, Bras H, Vinay L. Characterization of last-order premotor interneurons by transneuronal tracing with rabies virus in the neonatal mouse spinal cord. J Comp Neurol. 2011;519:3470–3487. doi: 10.1002/cne.22717. [PubMed: 21800300] [DOI] [PubMed] [Google Scholar]
- 7.Dum RP, Levinthal DJ, Strick PL. The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. J. Neurosci. 2009;29:14223–14235. doi: 10.1523/JNEUROSCI.3398-09.2009. [PubMed: 19906970] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enquist LW, Card JP. Recent advances in the use of neurotropic viruses for circuit analysis. Curr Opin Neurobiol. 2003;13:603–606. doi: 10.1016/j.conb.2003.08.001. [PubMed:14630225] [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto M, Takahara D, Hirata Y, Inoue K, Miyachi S, Nambu A, Tanji J, Takada M, Hoshi E. Motor and non-motor projections from the cerebellum to rostrocaudally distinct sectors of the dorsal premotor cortex in macaques. Eur J Neurosci. 2010;31:1402–1413. doi: 10.1111/j.1460-9568.2010.07151.x. [PubMed: 20384784] [DOI] [PubMed] [Google Scholar]
- 10.Hoover JE, Strick PL. The organization of cerebellar and basal ganglia outputs to primary motor cortex as revealed by retrograde transneuronal transport of herpes simplex virus type 1. J Neurosci. 1999;19:1446–1463. doi: 10.1523/JNEUROSCI.19-04-01446.1999. [PubMed: 9952421] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoshi E, Tremblay L, Féger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8:1491–1493. doi: 10.1038/nn1544. [PubMed: 16205719] [DOI] [PubMed] [Google Scholar]
- 12. Iwata K, Miyachi S, Imanishi M, Tsuboi Y, Kitagawa J, Teramoto K, Hitomi S, Shinoda M, Kondo M, Takada M. Ascending multisynaptic pathways from the trigeminal ganglion to the anterior cingulate cortex. Exp Neurol. 2011;227:69–78. doi: 10.1016/j.expneurol.2010.09.013. [PubMed: 20854814] The authors use retrograde transneuronal transport of rabies virus from the nociceptive responsive region of the anterior cingulate cortex to label third order neurons in the trigeminal ganglion. This method has the potential, when combined with immunohistochemistry, to identify the specific afferent fiber types that send information to specific regions of the cerebral cortex.
- 13.Kelly RM, Strick PL. Rabies as a transneuronal tracer of circuits in the central nervous system. J Neurosci Methods. 2000;103:63–71. doi: 10.1016/s0165-0270(00)00296-x. [PubMed: 11074096] [DOI] [PubMed] [Google Scholar]
- 14.Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [PubMed: 12968006] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly RM, Strick PL. Macro-architecture of basal ganglia loops with the cerebral cortex: use of rabies virus to reveal multisynaptic circuits. Prog Brain Res. 2004;143:449–459. doi: 10.1016/s0079-6123(03)43042-2. [PubMed: 14653187] [DOI] [PubMed] [Google Scholar]
- 16. Levinthal DJ, Strick PL. The motor cortex communicates with the kidney. J Neurosci. 2012;32:6726–6731. doi: 10.1523/JNEUROSCI.0406-12.2012. [PubMed: 22573695] These authors used retrograde transneuronal transport of rabies virus to trace up to five synaptically-connected neurons of the circuit connecting the cerebral cortex to the kidney. Surprisingly, the heaviest labeling in the cerebral cortex originated from the primary and secondary motor areas.
- 17.Lu X, Miyachi S, Ito Y, Nambu A, Takada M. Topographic distribution of output neurons in cerebellar nuclei and cortex to somatotopic map of primary motor cortex. Eur J Neurosci. 2007 Apr;25(8):2374–2382. doi: 10.1111/j.1460-9568.2007.05482.x. [PubMed: 17445235] [DOI] [PubMed] [Google Scholar]
- 18.Marshel JH, Mori T, Nielsen KJ, Callaway EM. Targeting single neuronal networks for gene expression and cell labeling in vivo. Neuron. 2010;67:562–574. doi: 10.1016/j.neuron.2010.08.001. [PubMed: 20797534] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyachi S, Lu X, Inoue S, Iwasaki T, Koike S, Nambu A, Takada M. Organization of multisynaptic inputs from prefrontal cortex to primary motor cortex as revealed by retrograde transneuronal transport of rabies virus. J Neurosci. 2005;25:2547–2556. doi: 10.1523/JNEUROSCI.4186-04.2005. [PubMed: 15758164] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moschovakis AK, Gregoriou GG, Ugolini G, Doldan M, Graf W, Guldin W, Hadjidimitrakis K, Savaki HE. Oculomotor areas of the primate frontal lobes: a transneuronal transfer of rabies virus and [14C]-2-deoxyglucose functional imaging study. J Neurosci. 2004;24:5726–5740. doi: 10.1523/JNEUROSCI.1223-04.2004. [PubMed: 15215295] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohara S, Inoue K, Yamada M, Yamawaki T, Koganezawa N, Tsutsui K, Witter MP, Iijima T. Dual transneuronal tracing in the rat entorhinal-hippocampal circuit by intracerebral injection of recombinant rabies virus vectors. Front Neuroanat. 2009;3:1. doi: 10.3389/neuro.05.001.2009. [PubMed: 19169410] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osakada F, Mori T, Cetin AH, Marshel JH, Virgen B, Callaway EM. New rabies virus variants for monitoring and manipulating activity and gene expression in defined neural circuits. Neuron. 2011 Aug 25;71(4):617–631. doi: 10.1016/j.neuron.2011.07.005. Erratum in: Neuron. 2012;74:206. [PubMed: 21867879] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prevosto V, Graf W, Ugolini G. Cerebellar inputs to intraparietal cortex areas LIP and MIP: functional frameworks for adaptive control of eye movements, reaching, and arm/eye/head movement coordination. Cereb Cortex. 2010;20:214–228. doi: 10.1093/cercor/bhp091. [PubMed: 19465740] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rathelot JA, Strick PL. Muscle representation in the macaque motor cortex: an anatomical perspective. Proc Natl Acad Sci USA. 2006;103:8257–8262. doi: 10.1073/pnas.0602933103. [PubMed: 16702556] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rathelot JA, Strick PL. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc Natl Acad Sci USA. 2009;106:918–923. doi: 10.1073/pnas.0808362106. [PubMed: 19139417] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strick PL, Card JP. Transneuronal mapping of neural circuits with alpha herpesviruses. In: Bolam JP, editor. Experimental neuroanatomy: a practical approach. Oxford: Oxford University Press; 1992. pp. 81–101. [Google Scholar]
- 27.Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [PubMed: 19555291] [DOI] [PubMed] [Google Scholar]
- 28. Suzuki L, Coulon P, Sabel-Goedknegt EH, Ruigrok TJ. Organization of cerebral projections to identified cerebellar zones in the posterior cerebellum of the rat. J Neurosci. 2012 Aug 8;32(32):10854–10869. doi: 10.1523/JNEUROSCI.0857-12.2012. [PubMed: 22875920] This paper combined transneuronal rabies virus tracing and conventional retrograde tracing with beta subunit of cholera toxin to examine the projections of the cerebral cortex to specific climbing fiber zones of the cerebellar cortex.
- 29.Ugolini G. Specificity of rabies virus as a transneuronal tracer of motor networks: transfer from hypoglossal motoneurons to connected second-order and higher order central nervous system cell groups. J Comp Neurol. 1995;356:457–480. doi: 10.1002/cne.903560312. [PubMed: 7642806] [DOI] [PubMed] [Google Scholar]
- 30.Ugolini G. Advances in viral transneuronal tracing. J Neurosci Methods. 2010;194:2–20. doi: 10.1016/j.jneumeth.2009.12.001. [PubMed: 20004688] [DOI] [PubMed] [Google Scholar]
- 31.Zemanick MC, Strick PL, Dix RD. Direction of transneuronal transport of herpes simplex virus 1 in the primate motor system is strain-dependent. Proc Natl Acad Sci USA. 1991;88:8048–8051. doi: 10.1073/pnas.88.18.8048. [PubMed: 1654557] [DOI] [PMC free article] [PubMed] [Google Scholar]