Abstract
Higher levels of cognitive reserve (CR) can be protective against the neuropsychological manifestation of neural injury across a variety of clinical disorders. However, the role of CR in the expression of neurocognitive deficits among persons infected with the hepatitis C virus (HCV) is not well understood. Thirty-nine HCV-infected participants were classified as having either high (n=19) or low (n=20) CR based on educational attainment, oral word reading, and IQ scores. A sample of 40 demographically comparable healthy adults (HA) was also included. All participants completed the Neuropsychological Assessment Battery (NAB), Delis-Kaplan Executive Function System (D-KEFS), and Behavioral Rating Inventory of Executive Function, Adult Version (BRIEF-A). Linear regression analyses, controlling for gender, depression and lifetime substance use disorders, found significant effects of HCV/CR group on verbal fluency, executive functions, and daily functioning T-scores, but not in learning or the BRIEF-A. Pairwise comparisons revealed that the HCV group with low CR performed significantly below the HCV high CR and HA cohorts, who did not differ from one another. Findings indicate that higher levels of CR may be a protective factor in the neurocognitive and real-world manifestation of neural injury commonly associated with HCV infection.
Keywords: Hepatitis C, Cognitive reserve, Neuropsychological assessment, Daily functioning
The hepatitis C virus (HCV) infection is a serious global and national public health issue, with 130 million people worldwide (Global Burden of Hepatitis C Working Group, 2004) and 1.6 million people in the U.S. (Shepard et al, 2005) living with chronic HCV. Infection with HCV is a leading cause of chronic liver disease, cirrhosis, primary hepatocellular carcinoma (HCC), and liver transplantation (Alter, 2007; El-Serag, 2002; Poynard et al, 2003). Additionally, HCV is neurovirulent and can affect the structure and function of the central nervous system (CNS) function. The “trojan horse” theory suggests that HCV likely crosses the blood brain barrier (BBB) via infected peripheral circulating macrophages and monocytes (Laskus et al, 2005). HCV RNA is thus detectable in cerebrospinal fluid (Laskus et al, 2002) and brain parenchyma (Forton et al, 2004; Letendre et al, 2007). Elevations in metabolic markers of neuronal injury/loss and neuroinflammation in basal ganglia and frontal cerebral white matter and occipital gray matter have been observed in HCV-infected individuals (Forton et al, 2005; Taylor et al, 2004; Taylor-Robinson, 2001; Weissenborn et al, 2004). These findings suggest that HCV itself may be a direct pathway for neurocognitive impairment (NCI), independent of the severity of liver involvement, viral replication rate (Monaco et al, 2012), or common comorbidities, such as substance use disorders (Huckans et al, 2009).
NCI is evident in approximately 30% of HCV-infected individuals and it is generally of mild severity (Bieliauskas et al, 2006; Forton et al, 2006; Perry et al, 2008). HCV-associated NCI is most commonly witnessed in domains reliant on frontostriatal systems, such as learning (Posada et al, 2009), executive functions (Cherner et al, 2005; Huckans et al, 2011; Weissenborn et al, 2004), complex attention (Hilsabeck et al, 2002), speed of information processing (Hilsabeck et al, 2003b; Hilsabeck et al, 2002), and motor skills (Cherner et al, 2005; Letendre et al, 2005). Despite being of broadly mild severity, HCV-associated NCI can also impact everyday functioning, including increasing risk of unemployment (Jacobs et al, 2003; Morgan et al, 2012a) and declines in both basic and instrumental activities of daily living (Vigil et al, 2008).
Given the clinical relevance of NCI in HCV, it is essential to understand additional clinical risk factors that may affect its expression. Although this literature is still emerging and these factors do not entirely account for HCV associated NCI, several clinical factors have consistently been associated with increased NCI in HCV. For example, higher NCI is reportedly associated with liver-related conditions (i.e., fibrosis and HE) (Bajaj, 2010; Cordoba, 2011; Hilsabeck et al, 2003a). Fatigue and depression have also been associated with higher NCI, possibly due to viral factors and/or antiviral therapy with interferon-alpha (Forton et al, 2006; Kraus et al, 2005; Perry et al, 2008). Other medical and psychiatric comorbidities, such as co-infection with human immunodeficiency virus (HIV) (Devlin et al, 2012; Vivithanaporn et al, 2012) and substance use disorders (Huckans et al, 2009; Martin-Thormeyer and Paul, 2009) may also contribute to increased risk for and severity of NCI observed among HCV-infected individuals (Letendre et al, 2005).
It is also important to consider possible “protective factors” that may increase the threshold for the expression of HCV-associated NCI. One such protective factor is cognitive reserve (CR), which refers to “the ability to optimize or maximize performance through differential recruitment of brain networks, which perhaps reflect the use of alternate cognitive strategies”(Stern, 2002). Originally described by Satz (Satz et al, 1993), CR is believed to modify the severity and trajectory of neurobehavioral deterioration secondary to a variety of central nervous system injuries (e.g., Alzheimer’s disease; (Alexander et al, 1997; Wilson et al, 2004). The literature supports the existence of two types of reserve - “brain reserve” including intracranial volume, brain weight and neuroplasticity, which reflect biological differences in the brain itself, and “cognitive reserve” including education, estimated premorbid intellectual function, and occupational complexity, which reflect acquired brain functioning differences (Richards and Deary, 2005; Stern, 2009).
The concept of CR is used to explain individual susceptibility to neurodegenerative diseases, such as Alzheimer’s disease (Alexander et al, 1997; Wilson et al, 2004). Also, CR plays a role in the expression of neurobehavioral deficits associated with another commonly studied neurovirulant illness, HIV infection (Basso and Bornstein, 2000; Stern et al, 1996). For example, CR has been identified as a protective factor in the expression of HIV-associated neurocognitive disorders (Basso and Bornstein, 2000), especially among vulnerable older adults (Foley et al, 2012). CR also plays a role in the expression of functional disability (e.g., medication non-adherence, unemployment, and declines in instrumental activities of daily living) among persons with HIV-associated neurocognitive impairment (Morgan et al, 2012b). This latter finding suggested that HIV-infected persons with higher CR were better able to compensate for neurocognitive impairment to perform everyday tasks, perhaps as a function of more efficient cognitive and behavioral strategy utilization.
To our knowledge, there is but one prior study examining CR in HCV. In 2007, Bieliauskas and colleagues examined the relationship between CR and cognitive functions in HCV-infected individuals with advanced liver fibrosis. The authors created a CR score modeled after Stern’s (1996) method, which included indices of educational and occupational achievement, as well as performance on measures of crystalized intelligence (e.g., vocabulary). This study revealed that, despite similar severity of liver disease, HCV-infected persons with NCI showed lower CR as compared to their HCV seropositive counterparts whose neurocognitive profiles were within normal limits. Findings suggested that HCV-infected individuals with low CR might be more susceptible to cognitive impairment, particularly on tests of memory, attention, motor speed, and executive function, and raise several interesting possibilities for future work. For instance, participants in this study had advanced liver disease (e.g., fibrosis scores ranging 3 to 6, detectable HCV RNA in serum); therefore, it is unknown whether or not their findings can be generalized in HCV patients with mild liver disease. Given that HIV studies demonstrated that more vulnerable cohorts (Foley et al, 2012; Morgan et al, 2012b) likely showed stronger CR effects, it is possible that smaller CR effects may be seen in HCV-infected individuals with milder liver disease, who represent 70–90% of the HCV population (Rosen, 2011; Wilkins et al, 2010) and may be at reduced risk of NCI. Furthermore, the Bieliauskas et al. study did not include a sample of healthy adults, who would provide a normative anchor for the extent to which CR is protective against HCV-associated NCI. Finally, given prior research showing that CR was closely associated with real-world outcomes in HIV (Morgan et al, 2012b), it may be relevant to examine the impact of CR on everyday functioning in individuals infected with HCV.
The present study extends the single prior study on this topic by assessing the role of CR on neurocognitive and everyday functioning outcomes in HCV-infected individuals with mild liver disease as compared to seronegative comparison subjects. It was hypothesized that HCV-infected persons with low CR would perform significantly more poorly than HCV-infected persons with high CR and healthy seronegatives, across a battery of neuropsychological tests, especially in the areas of memory, executive function, attention, and speed of information processing. Additionally, it was expected that HCV-infected individuals with low CR would demonstrate worse performance on measures of everyday functioning as compared to HCV-infected persons with high CR and healthy seronegatives.
Methods
Participants
A total of 79 participants [40 healthy adults (HA) and 39 HCV-infected individuals] were recruited from Portland, Oregon area hepatology clinics and the community via advertisements posted in clinics and hospitals, mailings to patients who had previously participated in HCV research, announcements at HCV education classes, or word of mouth. This study was approved by the Institutional Review Board of the Portland Veterans Affairs Medical Center (PVAMC). HCV status was determined by polymerase chain reaction tests. Participants were excluded if they met any of the following criteria: 1) History of antiviral therapy or chemotherapy for any purpose. 2) History of a major medical condition, or currently unstable medical condition, that is likely to be associated with severe neurological, cognitive, or immune dysfunction currently [e.g., stroke, seizures, brain tumors, Parkinson’s disease, neurodegenerative dementia, mental retardation, hepatic encephalopathy, human immunodeficiency virus (HIV)]. In the interest of generalizability to typical HCV+ populations, participants with common well-controlled or stable conditions were included as long as severe cognitive or immunological effects were not currently suspected (e.g., well-controlled diabetes, hypertension, or asthma). 3) History of traumatic brain injury with known loss of consciousness ≥ 30 minutes. 4) Use of alcohol, illicit substances, or medications with acute cognitive effects such as sedation or intoxication (e.g., benzodiazepines, opiates, muscle relaxants, psychostimulants) on the day of testing, or chronic use of medications with long-term cognitive or immune effects (e.g., topiramate, remicade, anticholinergics, steroids). 5) Decompensated liver cirrhosis, clinically determined by a hepatologist (AS) based on clinical indicators, medical record, biopsy results (if available), and a battery of standard medical laboratory tests [liver panel, complete blood count (CBC), International Normalized Ratio (INR), ammonia]. All standard medical laboratory tests were conducted prospectively during the study visits through PVAMC’s medical laboratory, and results were then reviewed with the study hepatologist along with available medical records and biopsy results to assess for decompensated liver cirrhosis. 6) Current pregnancy. 7) History of schizophrenia or schizoaffective disorder, OR, current psychotic or manic episode, OR currently unstable and severe psychiatric disorder. In the interest of generalizability to typical HCV+ populations, patients with mild but stable depression, anxiety, or post-traumatic stress disorder (PTSD) were included as long as present symptoms did not preclude valid participation. 8) Alcohol or drug dependence within the past year (except nicotine or caffeine), based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria (American Psychiatric Association, 1994), confirmed with the Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al, 1998).
Participants were classified into one of three study groups: HCV seropositive high cognitive reserve (HCV+ High CR), HCV seropositive low cognitive reserve (HCV+ Low CR), or HCV seronegative healthy adults (HA). We took a battery approach to determine the level of Cognitive Reserve (CR) using; 1) years of education, 2) estimated verbal IQ as measured by the Wechsler Test of Adult Reading (Psychological Corporation, 2001), and 3) the current intellectual functioning assessed by the Reynolds Intelligence Screening Test (Kamphaus and Reynolds, 2003). Sample-based z-scores were calculated in the HCV group and were averaged across the three metrics. Using a median split (z = −0.1), the HCV cohort was divided into low CR (n = 20, z < − 0.1) and high CR (n = 19, z ≥ − 0.1) groups. Note that, we used the median split approach based on a previous study examining the relationship between CR and neurocognitive functions in HCV (Bieliauskas et al, 2007) and to enhance the clinical relevance of the study findings. Nevertheless, it is also important to mention that the CR score was significantly associated with NP and everyday functioning outcome variables, except the self-report questionnaire, the BRIEF-A, in a similar pattern in the HCV group when used as a continuous rather than dichotomous variable, as well.
Table 1 displays demographic, psychiatric, and medical characteristics of the three study groups. As expected based on group definitions, the HCV high CR group had significantly higher education than the HCV low CR group, and both HA and HCV high CR groups obtained significantly higher scores on RIST and WTAR as compared to HCV low CR group (Table 1). The study groups were comparable in terms of age and ethnicity; however, there were more men in the HCV low CR group than HA or HCV high CR groups. The HCV seropositive groups showed higher history of lifetime substance use disorder than the HA group. In terms of liver biomarkers, AST, ALT, and APRI values were significantly higher in HCV groups than HA group while there were no between-group differences in ammonia and bilirubin levels.
Table 1.
Demographics, and Clinical Characteristics of Study Participants (N = 79)
| Variable | HA (n= 40) | HCV+ High CR (n= 19) | HCV+ Low CR (n= 20) | p | Group comparisons |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 47.9 (13.4) | 54.5 (7.7) | 50.6 (7.9) | .12 | |
| Ethnicity (% Caucasian) | 70.0 | 79.0 | 75.0 | .75 | |
| Sex (% Male) | 72.5 | 57.9 | 95.0 | .01 | HCV low CR > HA, HCV high CR |
| Cognitive Reserve | |||||
| Education (years) | 13.8 (2.3) | 15.1 (1.7) | 12.6 (1.5) | .001 | HCV high CR > HCV low CR |
| RIST (Index Score) | 107.6 (11.1) | 108.7 (7.8) | 95.7 (7.6) | < .0001 | HCV low CR < HCV high CR, HA |
| WTAR (Standard Score) | 106.8 (12.4) | 109.7 (7.5) | 94.4 (12.7) | < .0001 | HCV low CR < HCV high CR, HA |
| CR score (z-score) | .15 (.80) | .45 (.53) | .73 (.46) | < .0001 | HCV low CR < HCV high CR, HA |
| Psychiatric | |||||
| BDI-II | 4.5 (5.1) | 6.4 (6.9) | 8.5 (7.6) | .07 | |
| Substance Use Disorder (% Lifetime) | 35.0 | 73.7 | 80.0 | .0006 | HA < HCV high CR, HCV low CR |
| Liver Biomarkers | |||||
| AST | 22.8 (6.7) | 59.2 (42.9) | 52.4 (41.6) | < .0001 | HCV high CR, HCV low CR > HA |
| ALT | 25.1 (13.6) | 77.1 (47.1) | 79.5 (62.3) | < .0001 | HCV high CR, HCV low CR > HA |
| APRI | .25 (.13) | .88 (.98) | .72 (.94) | .002 | HCV high, CR HCV low CR > HA |
| Ammonia | 42.8 (17.4) | 38.6 (13.3) | 44.9 (18.7) | .57 | |
| Bilirubin | .48 (.20) | .53 (.22) | .50 (.31) | .76 | |
| Albumin | 4.4 (.32) | 4.2 (.26) | 4.3 (.40) | .04 | HA > HCV high CR |
Note: HA = Healthy Adults; HCV = Hepatitis C virus; CR = Cognitive Reserve; RIST = Reynolds Intelligence Screening Test; WTAR = Wechsler Test of Adult Reading; BDI-II = Beck Depression Inventory-II; AST = Aspartate aminotransferase; ALT = Alanine Aminotransferase; APRI = Aspartate aminotransferase-to-Platelet Ration Index.
Measures and Procedure
All participants completed a battery of subtests from the Neuropsychological Assessment Battery (NAB) (Stern and White, 2003) and the Delis-Kaplan Executive Function System (D-KEFS) (Delis et al, 2001) to evaluate performance across six cognitive domains that are commonly affected by HCV infection; Attention/Working Memory [NAB Digits Forward, Digits Backward, Dots, Number and Letters Efficiency Part A, B, C, and D subtests]; Learning [NAB List Learning Immediate Recall, Shape Learning Immediate Recognition, and Story Learning Immediate Recall (Stern and White, 2003)]; Memory [NAB List Learning Long Delayed Recall, Shape Learning Delayed Recognition, and Story Learning Delayed Recall (Stern and White, 2003)]; Verbal Fluency [NAB Word Generation and D-KEFS Letter Fluency]; Executive Function [NAB Mazes and Categories (Stern and White, 2003) and D-KEFS Categories Confirmed Correct Sorts]; and Daily Function [NAB Driving Scenes, Judgment, Daily Living Memory Immediate Recall and Delayed Recall]. All raw scores were converted into demographically-corrected T-scores in order to minimize the influence of age, education, sex and ethnicity whenever appropriate. The mean domain T-scores and Global T-scores were then calculated based on the individual tests described above.
All participants also completed the Beck Depression Inventory-II (BDI-II) (Beck et al, 1996) and the Behavioral Rating Inventory of Executive Function, Adult Version (BRIEF-A) (Gioia et al, 2000). The BDI-II comprises 21 questions measuring current depression. The BRIEF-A is a self-report questionnaire assessing nine theoretically and statistically derived subdomains of executive function. Based on the nine sub-domain T-scores, the global executive composite T-score was calculated and represents the extent to which a person reports subjective problems with executive function in their daily life.
Statistical Analysis
Linear multivariable regression models were conducted to examine whether group status (i.e., HCV high CR vs. HCV low CR- vs. HA) would predict performance in various cognitive domains and daily functioning. Given differences in gender, BDI-II and lifetime substance use disorder across the three study groups, these variables were included in these regression models as predictors along with the CR variable. For all models, all assumptions for a regression analysis were checked and met. Critical alpha was set at .05.
Results
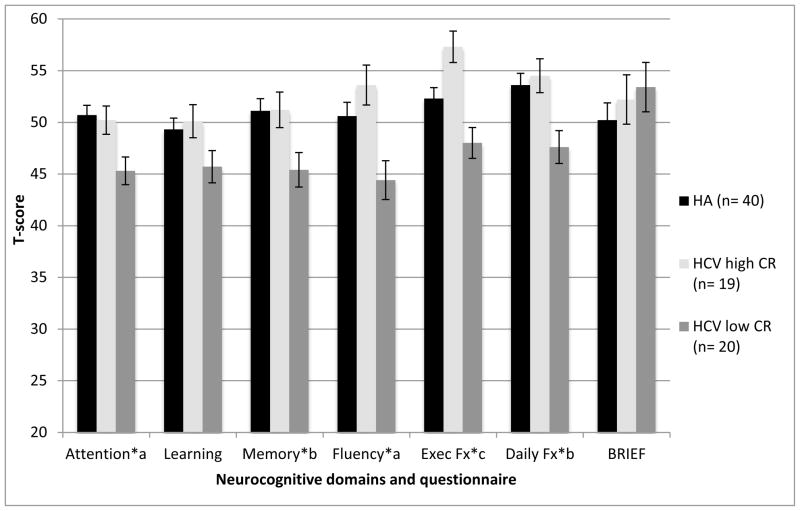
Table 2 displays the results of linear multivariable regression analyses examining effects of CR on NP performance, while controlling for gender, depression, and lifetime substance use disorders. Group status was a significant predictor for all of the NP domains (ps < .05), with the exception of learning (p > .10). The mean T-scores of the study groups across cognitive domains can be found in Figure 1. Pairwise comparisons showed that both HA and HCV-infected individuals with high CR performed significantly better than the HCV-infected persons with low CR in the domains of attention and fluency (ps < .05). The HCV+ group with high CR showed significantly better executive functions and memory as compared to the low CR sample (ps < .05); however, there were no differences between the HCV low CR and HA groups (ps > .10). Interestingly, in the domain of executive functions, HCV-infected persons with high CR performed significantly better than not only HCV-infected individuals with low CR but also healthy adults (β= 0.55, p = 0.0002, Cohen’s d = 0.77).
Table 2.
Multiple Linear Regression Analyses Showing Effects of Cognitive Reserve, Gender, Depression and Lifetime History of Substance Use Disorders on Cognitive Functioning.
| Adjusted R2 | F | Standardized β | Effect Size (Cohen’s d) | p | |
|---|---|---|---|---|---|
| Attention | 0.12 | 3.01 | 0.02* | ||
| CR statusa | 0.02* | ||||
| (HA) | 0.38 | 0.83 | 0.01* | ||
| (HCV High CR) | 0.34 | 0.95 | 0.02* | ||
| Genderb (female) | −0.08 | 0.48 | |||
| BDI-II | −0.21 | 0.07 | |||
| LT SUD historyc | 0.02 | 0.88 | |||
|
| |||||
| Learning | 0.05 | 1.76 | 0.13 | ||
| CR statusa | 0.19 | ||||
| (HA) | 0.26 | 0.11 | 0.83 | ||
| (HCV High CR) | 1.82 | 0.75 | 0.09 | ||
| Genderb (female) | −0.05 | 0.68 | |||
| BDI-II | −0.15 | 0.20 | |||
| LT SUD historyc | −0.16 | 0.22 | |||
|
| |||||
| Memory | 0.15 | 3.67 | 0.005* | ||
| CR statusa | 0.06 | ||||
| (HA) | 0.25 | 0.80 | 0.07 | ||
| (HCV High CR) | 0.31 | 0.78 | 0.02* | ||
| Genderb (female) | −0.10 | 0.37 | |||
| BDI-II | −0.31 | 0.008 | |||
| LT SUD historyc | −0.06 | 0.62 | |||
|
| |||||
| Fluency | 0.09 | 2.55 | 0.035* | ||
| CR statusa | 0.01* | ||||
| (HA) | 0.31 | 0.72 | 0.04* | ||
| (HCV High CR) | 0.43 | 1.11 | 0.003* | ||
| Genderb (female) | 0.05 | 0.69 | |||
| BDI-II | 0.03 | 0.82 | |||
| LT SUD historyc | −0.08 | 0.55 | |||
|
| |||||
| Executive Function | 0.17 | 4.12 | 0.002* | ||
| CR statusa | 0.0007* | ||||
| (HA) | 0.23 | 0.67 | 0.10 | ||
| (HCV High CR) | 0.53 | 1.32 | 0.0002* | ||
| Gender b (female) | 0.01 | 0.92 | |||
| BDI-II | −0.12 | 0.28 | |||
| LT SUD historyc | 0.01 | 0.90 | |||
Note: CR = cognitive reserve; HA = healthy adults; BDI-II = Beck Depression Inventory-II, LT SUD = Lifetime substance use disorders.
HCV Low CR group is the reference.
Male is the reference.
Presence of LT SUD history is the reference.
Figure 1.
Cognitive Reserve Group Differences in Neuropsychological Performance and Self-Report Questionnaire
Note: HCV = Hepatitis C virus; CR = cognitive reserve; HA = healthy adults; BDI = Beck Depression Inventory-II; LT SUD = Lifetime substance use disorders.
Superscript letters reflect results of linear regressions controlling for gender, depression and lifetime substance disorders. Bars indicate standard errors.
*p< .05
a HCV Low CR < HA, HCV High CR, bHCV Low CR < HCV High CR, cHCV High CR > HA, HCV Low CR.
An effect of HCV/CR group was also observed on the NAB performance-based measures of daily functioning but not on the BRIEF-A, which is a self-report measure of daily executive function (Table 3). Specifically, the HCV high CR group performed significantly better in daily function than the HCV low CR group, but there were no group differences between HCV high CR group and HA, nor between HCV low CR group and HA (ps > .10). No between-group effects were found on the BRIEF-A (ps > .10), which was instead most strongly associated with the BDI-II and lifetime substance use histories (ps < .05).
Table 3.
Multiple Linear Regression Analyses Showing Effects of Cognitive Reserve, Gender, Depression and Lifetime History of Substance Use Disorders on Performance-Based Measures of Daily Functioning (Daily Function) and a Self-Report Measure of Everyday Executive Function (BRIEF-A).
| Adjusted R2 | F | Standardized β | Effect Size (Cohen’s d) | p | |
|---|---|---|---|---|---|
| Daily Function | 0.16 | 3.99 | 0.003* | ||
| CR statusa | 0.04* | ||||
| (HA) | 0.25 | 0.86 | 0.07 | ||
| (HCV High CR) | 0.33 | 0.95 | 0.01* | ||
| Genderb (female) | 0.04 | 0.69 | |||
| BDI | −0.28 | 0.01* | |||
| LT SUD historyc | −0.07 | 0.56 | |||
|
| |||||
| BRIEF-A | 0.47 | 14.50 | <0.001* | ||
| CR statusa | 0.63 | ||||
| (HA) | 0.11 | 0.32 | 0.35 | ||
| (HCV High CR) | 0.04 | 0.12 | 0.72 | ||
| Genderb (female) | −0.02 | 0.82 | |||
| BDI | 0.63 | <0.001* | |||
| LT SUD historyc | 0.24 | 0.01* | |||
Note: CR = cognitive reserve, HA = healthy adults, BDI-II = Beck Depression Inventory-II, LT SUD = Lifetime substance use disorders.
HCV Low CR group is the reference.
Male is the reference.
Presence of LT SUD history is the reference.
Discussion
It has been reported that higher levels of cognitive reserve (CR) may be protective against the manifestation of neuropsychological deficits secondary to neuropathology across a variety of clinical disorders (See Stern (2002) for review); however, the role of CR among persons infected with HCV is not fully understood. The findings of this study supported the hypothesis that CR may also play a role in the expression of neurocognitive deficits in HCV-infected individuals. Specifically, HCV+ individuals with low CR performed significantly worse in domains of attention and fluency, as compared to HCV+ persons with high CR and healthy adults. Within the HCV+ groups, lower CR was also associated with memory deficits, executive dysfunction, and lower scores on performance-based measures of daily functioning, although the magnitude of these latter CR effects is modest as the HCV+ Low CR group did not differ from healthy adults. Nevertheless, these findings underscore the potential protective benefits of high CR for neurocognitive functioning among persons with HCV and are unlikely to be attributable to confounding factors, which were relatively well matched between the study groups (e.g., liver markers in the HCV groups) or controlled in the statistical models (e.g., gender, substance use, depression).
Our findings are broadly consistent with those of Bielauskas et al. (2007), who observed that low CR was associated with worse performance on tests of memory, attention, motor speed, and executive function among persons infected with HCV. These results extend that prior study by including: 1) a sample of healthy adults, 2) assessments of daily functioning, and 3) an HCV-infected cohort with generally mild liver disease. With regard to the first point, the present study demonstrated that the HCV+ High CR group performed similarly to (and occasionally better than) the healthy adult group across the battery of NP tests. In this way, our data suggest that high CR indeed serves as a neurocognitive protective factor for individuals living with HCV infection. Whether this observation reflects the prophylactic effects of greater engagement in various cognitively enriching activities and/or premorbid advantages in the resilience of neural systems to the chronic neuroinflammatory processes of HCV infection among the high CR group remains to be determined. Future studies might investigate the possible interactions between cognitive and brain reserve in HCV using neuroimaging and relevant biomarkers of neural injury and protective factors.
To our knowledge, this is the first study to document the impact of CR on daily functioning in HCV infection. Findings converge with recent data from Morgan et al. (2012), who reported HIV-infected persons with low CR exhibited more difficulties in daily functioning than individuals with high CR (Morgan et al, 2012). In this case, the daily functioning domain assessed performance-based skills (i.e., learning and recall of ecologically relevant information, and attention related to automobile driving) rather than manifest aspects of everyday living (e.g., actual employment status). Thus, individuals with low CR may be at particular risk for disability and lower health-related quality of life by way of a “double hit” to both neurocognitive status and functional skills, both of which independently contribute to declines in instrumental activities of daily living (e.g., Heaton et al., 2004). To confirm, future studies may wish to examine the role of CR in HCV using other performance-based (e.g., Valpar, medication management tasks) and manifest (e.g., employment and medication non-adherence) measures of everyday functioning across different aspects of daily living. It is important to note, however, that the self-report questionnaire, the BRIEF-A, used in the present study did not show any associations with HCV or CR. There could be several possible explanations for this null finding. First, the self-report data may be subject to bias whereby HCV-infected persons with low CR may not be keenly aware of their cognitive difficulties. Indeed, limited awareness of NCI and/or everyday dysfunction has been reported in various illnesses, including HIV infection (e.g., (Hannesdottir and Morris, 2007; Morgan et al, 2012b). Second, it is possible that the executive deficits measured by the NP tests may not be sufficiently severe to affect real-world functioning as measured by the BRIEF-A and/or individuals with lower CR may be able to compensate for such deficits in their everyday environments. A third possibility is that the study was underpowered in terms of its sample size and severity of HCV disease to detect the subtle effects of CR on self-reported executive functions in everyday life. However, the BRIEF-A did not relate to CR even when it was used as a continuous variable in the entire HCV+ sample. It nevertheless remains possible that CR is associated with other aspects of cognitive (e.g., memory, attention) and/or functional (e.g., medication management) symptoms experienced in the daily lives of persons infected with HCV.
Another implication of this study is that CR may play a critical role in HCV-associated NCI across various severities of liver disease. CR effects were observed in the present study in participants with generally mild liver disease (see Table 1) and those of Bieliauskas et al., who included participants with more advanced liver disease. The HCV groups in the current study did not differ in terms of liver functioning, which suggests that CR can affect NCI independent of liver disease severity, which also plays a role in the expression of NCI. Given that 70–90% of the HCV population has broadly mild liver disease (i.e., Wilkins et al., 2010; Rosen, 2011), our findings may be useful to understand the NP and everyday functioning of a broad cross-section of the HCV-infected population.
The current study has several limitations. First, sample sizes of HCV groups were small; therefore, type II errors, especially for the HCV high CR and healthy adults comparisons were of concern. However, standard errors for those analyses were fairly small and CR signals with medium-to-large effect sizes were detected. This finding was consistent with previous studies as well. Second, the average scores of our HCV cohort were broadly within normal limits; as seen in Figure 1, even though the low CR group showed significantly lower performance as compared to the high CR and healthy adults groups, their performance was not in the impaired range (i.e., Ts > 40). In fact, even our “low” CR sample had CR that fell generally within the average range, so questions remain regarding the impact of CR among HCV-infected cohorts who evidence below average scores on measures of IQ and word reading. As a result, no significant performance difference might be found in a key cognitive domain, such as verbal learning, between HCV+ low CR group and HA. Additionally, the meaning of significant T-score differences “within normal limits” performance could be arguable; however, it is also important to consider that such differences in the normal range can still have important implications for everyday functioning (e.g., Morgan et al., 2012) and could become important factors to future cognitive and/or real-world functions, particularly when other clinical and psychiatric events occur (e.g., decline in liver function, depressive symptoms, etc.). A third issue is that of shared method variance; i.e., CR was measured by NP tasks, and based on those CR scores, NP performance was compared across groups, which raises a circulatory problem in which measures of NP functioning are included as both the independent and dependent variables. Arguing against this critique, however, are null CR findings across a few cognitive domains (e.g., learning). Fourth, this study is cross-sectional and it does not describe the relationship of CR to potential decline in NP and daily functions over time. As a result, it is unclear whether or not CR delays the onset of deficits in NP and/or daily functions. Therefore, a longitudinal study would help us understand how CR plays a role in protecting against the NP manifestation of neuropathology in HCV infection, while examining other crucial factors including age, liver disease severity, and other potential comorbidities (e.g., depression, substance use disorders).
In summary, the present study demonstrated that CR plays an important role in the expression of NP and everyday functioning among individuals with HCV infection. Although HCV infection may increase the risk of NP impairment, higher levels of CR may be protective of the neurobehavioral manifestation of neural injury associated with the infection. Based on CR levels, clinicians may be able to identify individuals at greater risk for NP impairments as well as daily dysfunction, including difficulties in Instrumental Activities of Daily Living (IADLs) and employment. Future studies on the longitudinal protective value of CR on incident HCV-associated neurocognitive decline and everyday functioning outcomes are warranted.
Acknowledgments
Acknowledgements/Source of Funding: This study was supported in part by R25-MH081482 to MS as well as by career development awards to MH and JML from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research and Development. This material is the result of work supported with resources and the use of facilities at the Portland Veterans Affairs Medical Center, Portland, Oregon. The authors thank the study participants and staff at each of the recruitment sites, especially Betsy Zucker and Janice Voukidis. The authors also acknowledge Peter Hauser, William Hoffman, Diane Howieson, Daniel Storzbach, and Alexander Stevens for study design consultation.
Footnotes
Conflicts of Interest: The authors declare that they have no conflict of interest.
References
- Alexander GE, Furey ML, Grady CL, Pietrini P, Brady DR, Mentis MJ, Schapiro MB. Association of premorbid intellectual function with cerebral metabolism in Alzheimer’s disease: implications for the cognitive reserve hypothesis. Am J Psychiatry. 1997;154:165–72. doi: 10.1176/ajp.154.2.165. [DOI] [PubMed] [Google Scholar]
- Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13:2436–41. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. diagnostic and statistical manual of mental disorders – 4th edition. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual for mental disorders. 4. American Psychiatric Association; Washington, DC: 2000. Text Revision edn. [Google Scholar]
- Bajaj JS. Review article: the modern management of hepatic encephalopathy. Aliment Pharmacol Ther. 2010;31:537–47. doi: 10.1111/j.1365-2036.2009.04211.x. [DOI] [PubMed] [Google Scholar]
- Basso MR, Bornstein RA. Estimated premorbid intelligence mediates neurobehavioral change in individuals infected with HIV across 12 months. J Clin Exp Neuropsychol. 2000;22:208–18. doi: 10.1076/1380-3395(200004)22:2;1-1;FT208. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory manual. 2. Psychological Corporation; New York: 1996. [Google Scholar]
- Bieliauskas LA, Back-Madruga C, Lindsay KL, Snow KK, Kronfol Z, Lok AS, Padmanabhan L, Fontana RJ. Clinical relevance of cognitive scores in hepatitis C patients with advanced fibrosis. J Clin Exp Neuropsychol. 2006;28:1346–61. doi: 10.1080/13803390500473720. [DOI] [PubMed] [Google Scholar]
- Cherner M, Letendre S, Heaton RK, Durelle J, Marquie-Beck J, Gragg B, Grant I, Grp HNRC. Hepatitis C augments cognitive deficits associated with HIV infection and methamphetamine. Neurology. 2005;64:1343–1347. doi: 10.1212/01.WNL.0000158328.26897.0D. [DOI] [PubMed] [Google Scholar]
- Cordoba J. New assessment of hepatic encephalopathy. J Hepatol. 2011;54:1030–40. doi: 10.1016/j.jhep.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Devlin KN, Gongvatana A, Clark US, Chasman JD, Westbrook ML, Tashima KT, Navia B, Cohen RA. Neurocognitive effects of HIV, hepatitis C, and substance use history. J Int Neuropsychol Soc. 2012;18:68–78. doi: 10.1017/S1355617711001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35:S72–8. doi: 10.1097/00004836-200211002-00002. [DOI] [PubMed] [Google Scholar]
- Foley JM, Ettenhofer ML, Kim MS, Behdin N, Castellon SA, Hinkin CH. Cognitive reserve as a protective factor in older HIV-positive patients at risk for cognitive decline. Appl Neuropsychol Adult. 2012;19:16–25. doi: 10.1080/09084282.2011.595601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forton DM, Allsop JM, Cox IJ, Hamilton G, Wesnes K, Thomas HC, Taylor-Robinson SD. A review of cognitive impairment and cerebral metabolite abnormalities in patients with hepatitis C infection. AIDS. 2005;19(Suppl 3):S53–63. doi: 10.1097/01.aids.0000192071.72948.77. [DOI] [PubMed] [Google Scholar]
- Forton DM, Karayiannis P, Mahmud N, Taylor-Robinson SD, Thomas HC. Identification of unique hepatitis C virus quasispecies in the central nervous system and comparative analysis of internal translational efficiency of brain, liver, and serum variants. J Virol. 2004;78:5170–83. doi: 10.1128/JVI.78.10.5170-5183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forton DM, Taylor-Robinson SD, Thomas HC. Central nervous system changes in hepatitis C virus infection. Eur J Gastroenterol Hepatol. 2006;18:333–8. doi: 10.1097/00042737-200604000-00005. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive Function. Psychological Assessment Resources, Inc; Odessa, FL: 2000. [Google Scholar]
- Global Burden of Hepatitis C Working Group. Global burden of disease (GBD) for hepatitis C. Journal of Clinical Pharmacology. 2004;44:20–29. doi: 10.1177/0091270003258669. [DOI] [PubMed] [Google Scholar]
- Hannesdottir K, Morris RG. Primary and secondary anosognosia for memory impairment in patients with Alzheimer’s disease. Cortex. 2007;43:1020–30. doi: 10.1016/s0010-9452(08)70698-1. [DOI] [PubMed] [Google Scholar]
- Hilsabeck RC, Hassanein TI, Carlson MD, Ziegler EA, Perry W. Cognitive functioning and psychiatric symptomatology in patients with chronic hepatitis C. Journal of the International Neuropsychological Society. 2003a;9:847–854. doi: 10.1017/S1355617703960048. [DOI] [PubMed] [Google Scholar]
- Hilsabeck RC, Hassanein TI, Carlson MD, Ziegler EA, Perry W. Cognitive functioning and psychiatric symptomatology in patients with chronic hepatitis C. J Int Neuropsychol Soc. 2003b;9:847–54. doi: 10.1017/S1355617703960048. [DOI] [PubMed] [Google Scholar]
- Hilsabeck RC, Perry W, Hassanein TI. Neuropsychological impairment in patients with chronic hepatitis C. Hepatology. 2002;35:440–6. doi: 10.1053/jhep.2002.31257. [DOI] [PubMed] [Google Scholar]
- Huckans M, Seelye A, Parcel T, Mull L, Woodhouse J, Bjornson D, Fuller BE, Loftis JM, Morasco BJ, Sasaki AW, Storzbach D, Hauser P. The cognitive effects of hepatitis C in the presence and absence of a history of substance use disorder. J Int Neuropsychol Soc. 2009;15:69–82. doi: 10.1017/S1355617708090085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckans M, Seelye A, Woodhouse J, Parcel T, Mull L, Schwartz D, Mitchell A, Lahna D, Johnson A, Loftis J, Woods SP, Mitchell SH, Hoffman W. Discounting of delayed rewards and executive dysfunction in individuals infected with hepatitis C. J Clin Exp Neuropsychol. 2011;33:176–86. doi: 10.1080/13803395.2010.499355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs P, Ng YC, Stafinski T, Dodd R, Larke R, Wong W. Labor force participation among individuals with hepatitis C in the US. Pharmacoeconomics. 2003;21:565–572. doi: 10.2165/00019053-200321080-00003. [DOI] [PubMed] [Google Scholar]
- Kamphaus RW, Reynolds CR. Reynolds Intellectual Screening Test. Psychological Assessment Resources, Inc; Lutz, FL: 2003. [Google Scholar]
- Kraus MR, Schafer A, Wissmann S, Reimer P, Scheurlen M. Neurocognitive changes in patients with hepatitis C receiving interferon alfa-2b and ribavirin. Clin Pharmacol Ther. 2005;77:90–100. doi: 10.1016/j.clpt.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Laskus T, Radkowski M, Adair DM, Wilkinson J, Scheck AC, Rakela J. Emerging evidence of hepatitis C virus neuroinvasion. AIDS. 2005;19(Suppl 3):S140–4. doi: 10.1097/01.aids.0000192083.41561.00. [DOI] [PubMed] [Google Scholar]
- Laskus T, Radkowski M, Bednarska A, Wilkinson J, Adair D, Nowicki M, Nikolopoulou GB, Vargas H, Rakela J. Detection and analysis of hepatitis C virus sequences in cerebrospinal fluid. J Virol. 2002;76:10064–8. doi: 10.1128/JVI.76.19.10064-10068.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre S, Paulino AD, Rockenstein E, Adame A, Crews L, Cherner M, Heaton R, Ellis R, Everall IP, Grant I, Masliah E. Pathogenesis of hepatitis C virus coinfection in the brains of patients infected with HIV. J Infect Dis. 2007;196:361–70. doi: 10.1086/519285. [DOI] [PubMed] [Google Scholar]
- Letendre SL, Cherner M, Ellis RJ, Marquie-Beck J, Gragg B, Marcotte T, Heaton RK, McCutchan JA, Grant I. The effects of hepatitis C, HIV, and methamphetamine dependence on neuropsychological performance: biological correlates of disease. AIDS. 2005;19(Suppl 3):S72–8. doi: 10.1097/01.aids.0000192073.18691.ff. [DOI] [PubMed] [Google Scholar]
- Martin-Thormeyer EM, Paul RH. Drug abuse and hepatitis C infection as comorbid features of HIV associated neurocognitive disorder: neurocognitive and neuroimaging features. Neuropsychol Rev. 2009;19:215–31. doi: 10.1007/s11065-009-9101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco S, Ferrari S, Gajofatto A, Zanusso G, Mariotto S. HCV-Related Nervous System Disorders. Clin Dev Immunol. 2012;2012:236148. doi: 10.1155/2012/236148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Rooney A, Perry W, Grant I, Letendre SL. Intra-individual variability across neurocognitive domains in chronic hepatitis C infection: elevated dispersion is associated with serostatus and unemployment risk. Clin Neuropsychol. 2012a;26:654–74. doi: 10.1080/13854046.2012.680912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Smith C, Weber E, Scott JC, Grant I. Lower Cognitive Reserve Among Individuals with Syndromic HIV-Associated Neurocognitive Disorders (HAND) AIDS Behav. 2012b doi: 10.1007/s10461-012-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry W, Hilsabeck RC, Hassanein TI. Cognitive dysfunction in chronic hepatitis C: a review. Dig Dis Sci. 2008;53:307–21. doi: 10.1007/s10620-007-9896-z. [DOI] [PubMed] [Google Scholar]
- Posada C, Morgan EE, Moore DJ, Woods SP, Letendre SL, Grant I, The Hnrc G. Neurocognitive effects of the hepatitis C virus. Current Hepatitis Reports. 2009;8:18–26. [Google Scholar]
- Poynard T, Yuen MF, Ratziu V, Lai CL. Viral hepatitis C. Lancet. 2003;362:2095–100. doi: 10.1016/s0140-6736(03)15109-4. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation. Wechsler Test of Adult Reading. Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Richards M, Deary IJ. A life course approach to cognitive reserve: a model for cognitive aging and development? Ann Neurol. 2005;58:617–22. doi: 10.1002/ana.20637. [DOI] [PubMed] [Google Scholar]
- Rosen HR. Clinical practice. Chronic hepatitis C infection. N Engl J Med. 2011;364:2429–38. doi: 10.1056/NEJMcp1006613. [DOI] [PubMed] [Google Scholar]
- Satz P, Morgenstern H, Miller EN, Selnes OA, McArthur JC, Cohen BA, Wesch J, Becker JT, Jacobson L, D’Elia LF, et al. Low education as a possible risk factor for cognitive abnormalities in HIV-1: findings from the multicenter AIDS Cohort Study (MACS) J Acquir Immune Defic Syndr. 1993;6:503–11. [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Shepard CW, Finelli L, Alter M. Global epidemiology of hepatitis C virus infection. Lancet Infectious Diseases. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- Stern RA, Silva SG, Chaisson N, Evans DL. Influence of cognitive reserve on neuropsychological functioning in asymptomatic human immunodeficiency virus-1 infection. Arch Neurol. 1996;53:148–53. doi: 10.1001/archneur.1996.00550020052015. [DOI] [PubMed] [Google Scholar]
- Stern RA, White T. Neuropsychological Assessment Battery. Psychological Assessment Resources, Inc; Lutz, FL: 2003. [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–60. [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–28. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, Letendre SL, Schweinsburg BC, Alhassoon OM, Brown GG, Gongvatana A, Grant I. Hepatitis C virus infection is associated with reduced white matter N-acetylaspartate in abstinent methamphetamine users. J Int Neuropsychol Soc. 2004;10:110–3. doi: 10.1017/S1355617704101161. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson SD. Applications of magnetic resonance spectroscopy to chronic liver disease. Clin Med. 2001;1:54–60. doi: 10.7861/clinmedicine.1-1-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil O, Posada C, Woods SP, Atkinson JH, Heaton RK, Perry W, Hassanein TI, Grant I, Letendre SL. Impairments in fine-motor coordination and speed of information processing predict declines in everyday functioning in hepatitis C infection. J Clin Exp Neuropsychol. 2008;30:805–15. doi: 10.1080/13803390701802354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivithanaporn P, Nelles K, DeBlock L, Newman SC, Gill MJ, Power C. Hepatitis C virus co-infection increases neurocognitive impairment severity and risk of death in treated HIV/AIDS. J Neurol Sci. 2012;312:45–51. doi: 10.1016/j.jns.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Weissenborn K, Krause J, Bokemeyer M, Hecker H, Schuler A, Ennen JC, Ahl B, Manns MP, Boker KW. Hepatitis C virus infection affects the brain-evidence from psychometric studies and magnetic resonance spectroscopy. J Hepatol. 2004;41:845–51. doi: 10.1016/j.jhep.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Wilkins T, Malcolm JK, Raina D, Schade RR. Hepatitis C: diagnosis and treatment. Am Fam Physician. 2010;81:1351–7. [PubMed] [Google Scholar]
- Wilson RS, Li Y, Aggarwal NT, Barnes LL, McCann JJ, Gilley DW, Evans DA. Education and the course of cognitive decline in Alzheimer disease. Neurology. 2004;63:1198–202. doi: 10.1212/01.wnl.0000140488.65299.53. [DOI] [PubMed] [Google Scholar]