Abstract
We have previously shown that tolerance of kidney allografts across a full major histocompatibility complex (MHC) barrier can be induced in miniature swine by a 12-day course of high-dose tacrolimus. However, that treatment did not prolong survival of heart allografts across the same barrier. We have now tested the effect of cotransplanting an allogeneic heart and kidney from the same MHC-mismatched donor using the same treatment regimen. Heart allografts (n = 3) or heart plus kidney allografts (n = 5) were transplanted into MHC-mismatched recipients treated with high-dose tacrolimus for 12 days. As expected, all isolated heart allografts rejected by postoperative day 40. In contrast, heart and kidney allografts survived for >200 days with no evidence of rejection on serial cardiac biopsies. Heart/kidney recipients lost donor-specific responsiveness in cell-mediated lympholysis and mixed-lymphocyte reaction assays, were free of alloantibody and exhibited prolonged survival of donor, but not third-party skin grafts. Late (>100 days) removal of the kidney allografts did not cause acute rejection of the heart allografts (n = 2) and did not abrogate donor-specific unresponsiveness in vitro. While kidney-induced cardiac allograft tolerance (KI-CAT) has previously been demonstrated across a Class I disparity, these data demonstrate that this phenomenon can also be observed across the more clinically relevant full MHC mismatch. Elucidating the renal element(s) responsible for KICAT could provide mechanistic information relevant to the induction of tolerance in recipients of isolated heart allografts as well as other tolerance-resistant organs.
Keywords: Heart transplantation, kidney transplantation, miniature swine, tolerance
Introduction
Despite improvements in early posttransplant survival over the last two decades, a relentless annual attrition continues to plague recipients of previously successful heart allografts. Registry data for the International Society for Heart and Lung Transplantation (ISHLT) show that the graft half-life is only 11 years for heart recipients (1). Infection accounts for 33% of cardiac transplant recipient death 1-year posttransplant while after 5 years, cardiac allograft vasculopathy (CAV) (>30%) and malignancy (23%) account for most cardiac recipient deaths (1). These sobering statistics emphasize the limitations of chronically administered immunosuppression and make clear the need for tolerance strategies that achieve long-term graft survival and prevent chronic rejection without the use of long-term immunosuppression.
Using the preclinical, large animal model of major histocompatibility complex (MHC)-inbred miniature swine (2), we have previously shown that isolated, Class I-disparate hearts transplanted without immunosuppression all rejected within 8 days. The addition of a 12-day course of cyclosporine A (CsA) prolonged Class I-mismatched heart allograft survival slightly but all grafts rejected by 55 days. In contrast, hearts in recipients cotransplanted with a kidney allograft from the same Class I MHC-disparate donor and treated with the same 12-day course of CsA all developed long-term and stable tolerance of both the heart and kidney allografts (3,4).
Although kidney-induced cardiac allograft tolerance (KICAT) was successfully achieved across a Class I MHC barrier, it was unclear whether the same effect could be achieved across a more clinically relevant full MHC barrier. Here we show a dramatic difference in outcomes between recipients of isolated, MHC-mismatched hearts, which rejected their grafts by Day 40, and recipients of cotransplanted, MHC-mismatched heart and kidneys whose cardiac allografts continued to contract strongly for over 200 days without signs of rejection or CAV on serial biopsies.
Materials and Methods
Animals
Transplant donors and recipients were selected from our herd of partially inbred miniature swine (age, 3–6 months; weight, 15–30 kg). The immuno-genetic characteristics of this herd have been described previously (2). SLAdd (Class Id/IId) donor organs were transplanted into SLAcc (Class Ic/IIc) recipients to achieve a 2-haplotype full MHC Class I and Class II mismatch. All recipients demonstrated significant in vitro anti-donor cytotoxic activity (>20% specific lysis) before organ transplantation. Institutional review board approval was obtained for this study. All animal care and procedures were in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources, National Research Council, and published by the National Academy Press, revised 2011.
Surgery
The surgical procedures used for heart transplantation, combined heart/kidney transplantation and skin grafting have been described in detail previously (3,5,6). Briefly, the recipients underwent bilateral nephrectomy. The aorta and inferior vena cava were used for end-to-side arterial and venous anastomoses for both the heart and kidney, with the heart placed at least 1 cm caudad to the kidney. The kidney transplantation was completed by performing a vesicoureteral anastomosis. Two indwelling silastic central venous catheters were placed surgically into the external or internal jugular veins. The catheters facilitated tacrolimus administration and frequent blood sampling for in vitro assays and for monitoring of renal function and whole-blood tacrolimus levels.
Kidney allograft graftectomy was performed in two long-term tolerant animals. Briefly, the kidney allograft was removed and the recipient underwent self-matched (SLAcc) kidney transplant.
Skin grafting was performed by placing split-thickness skin grafts on the dorsum of long-term tolerant recipients. Animals received fresh (self) or frozen (donor, third party) skin grafts, which were recovered using a Zimmer dermatome. Graft beds were prepared with a single pass of the dermatome. An occlusive compression dressing was applied and removed on postoperative day (POD) 4 after skin grafting. Day of rejection was defined as the point at which the skin graft became necrotic and was confirmed by biopsy.
Rejection monitoring
Kidney function was monitored by serial serum creatinine levels. Heart function was monitored by daily palpation and electrocardiogram using the AliveCor Veterinary Heart Monitor (AliveCor, Inc., San Francisco, CA). Routine biopsies were performed on all transplant recipients via flank incisions at predetermined time intervals (PODs 20–30, 50–60, 90–100) or whenever a decrease in palpation or QRS-wave amplitude occurred. Cardiac allograft rejection (heart survival time) was defined by either loss of a ventricular impulse on palpation, and/or QRS-wave amplitude of less than 0.3 mV, and/or the lack of ventricular contraction on echocardiography (7). Renal allograft rejection was defined as sustained rise in serum creatinine to >10 mg/dL and/or anuria. Allograft rejection was confirmed histologically in all cases.
Immunosuppression
Tacrolimus (Haorui Pharma-Chem, Inc., Irvine, CA) was mixed and administered as an intravenous suspension according to the specifications of the manufacturer. Tacrolimus was given as a continuous infusion at a dose of 0.10–0.20 mg/kg (adjusted to maintain a whole blood level of 30–50 ng/mL) for 12 consecutive days, starting on the day of transplantation (Day 0).
Histopathological examination
Core needle biopsies were performed on cardiac allografts. Scoring of acute rejection in the cardiac allograft was based on the International Society for Heart and Lung Transplantation System (8). Wedge biopsies were performed on kidney allografts. Acute rejection in the kidney allograft was scored according to the Banff classification (9). Heart and kidney biopsy specimens were also evaluated for alloantibody deposition by direct immunofluorescence staining. Frozen tissue sections were stained with a saturating concentration of a fluorescence isothiocyanate (FITC)-labeled goat anti-swine immunoglobulin (IgM or IgG) and evaluated by fluorescence microscopy.
Preparation of peripheral blood leukocytes
Freshly heparinized whole blood was diluted approximately 1:2 with HBSS (Gibco BRL, Grand Island, NY), and the mononuclear cells were obtained by means of gradient centrifugation with Histopaque (Sigma Chemical Co., St. Louis, MO). The mononuclear cells were washed once with HBSS, and contaminating red cells were lysed with ammonium chloride potassium lysing buffer (BioWhittaker, Inc., Walkersville, MD). Cells were then washed with HBSS and resuspended in tissue culture medium. All cell suspensions were kept at 4°C until used in cellular assays.
Cell-mediated lymphocytotoxicity assay
Cell-mediated lympholysis (CML) assays with porcine cells have been described previously (10). The tissue culture media used for the CML assays consisted of RPMI-1640 (Gibco BRL) supplemented with 6% fetal bovine serum (Sigma Chemical Co.), 100 U/mL penicillin, 135 mg/mL streptomycin (Gibco BRL), 50 mg/mL gentamicin (Gibco BRL), 10 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES; Fisher Scientific, Pittsburgh, PA), 2 mmol/L L-glutamine (Gibco BRL), 1 mmol/L sodium pyruvate (BioWhittaker, Inc.), nonessential amino acids (BioWhittaker, Inc.) and 5 × 10−5 mol/L β2-mercaptoethanol (Sigma Chemical Co.). The effector phase of the CML assay was performed with Basal Medium Eagle (Gibco BRL) supplemented with 6% controlled processed serum replacement 3 (Sigma Chemical Co.) and 10 mmol/L HEPES. Briefly, lymphocyte cultures containing 4 × 106/mL responder and 4 × 106/mL stimulator peripheral blood leukocytes (PBLs) (irradiated with 2500 cGy) were incubated for 6 days at 37°C in 7.5% carbon dioxide and 100% humidity in CML medium. Bulk cultures were recovered, and effectors were tested for cytotoxic activity on chromium 51-labeled (Amersham, Arlington Heights, IL) lymphoblast targets generated from phytohemagglutinin (M-form; Life Technologies, Gaithersburg, MD) stimulation. Effector cells were incubated for 5.5 h with target cells at effector/target ratios of 100:1, 50:1, 25:1 and 12.5:1. Two target cells were tested in each assay: (1) PBLs swine lymphocyte antigen (SLA) matched to the donor (SLAdd: Class Idd and Class IIdd), and (2) third-party PBLs. Supernatants were then recovered by using the Skatron Collection System (Skatron, Sterling, VA), and 51Cr release was determined on a gamma counter (Micromedics, Huntsville, AL). The results were expressed as a percentage of specific lysis and calculated as follows:
Mixed-lymphocyte reaction assay
Mixed-lymphocyte reaction (MLR) responses to self, donor and third party were determined in a single assay for each animal. MLR media consisted of RPMI 1640 (Life Technologies) supplemented with 6% fetal pig serum (Sigma Chemical Co.), 100 U/mL penicillin (GIBCO-Invitrogen Corporation, Carlsbad, CA), 135 μg/mL streptomycin (GIBCO-Invitrogen Corporation), 50 μg/mL gentamicin (GIBCO-Invitrogen Corporation), 10 mM HEPES (Cellgro Mediatech, Inc., Manassas, VA), 2 mM L-glutamine (Life Technologies), 1 mM sodium pyruvate (BioWhittaker–Cambrex, East Rutherford, NJ), nonessential amino acids (BioWhittaker–Cambrex) and 5 × 10−5 M β2-mercaptoethanol (Sigma Chemical Co.). Cultures containing 4 × 106 responder and 4 × 106 irradiated (2500 cGy) stimulator peripheral blood mononuclear cells were incubated in 200 μL of media in 96-well flat-bottomed plates (Costar Corning, Lowell, MA) for 5 days at 37°C in 6% CO2 and 100% humidity. After the 5-day incubation, 1 uCi of [3H]-thymidine was added to each well, followed by an additional 5-h incubation under the same conditions. [3H]-thymidine incorporation was determined in triplicate samples by beta-scintillation counting. Absolute counts were compensated for background and then expressed as stimulation indices (SI), calculated as SI = average cpm for a responder–stimulator pair per cpm of the same responder stimulated by an autologous stimulator.
Flow cytometry
The presence of anti-donor immunoglobulin (IgM and IgG) in the serum of experimental swine was examined by indirect flow cytometry using a Becton Dickinson FACScalibur (Sunnyvale, CA) to determine the SLA-binding specificity of the antibody. FITC-labeled goat anti-swine IgM or IgG polyclonal antibodies were used as secondary reagents (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD). For staining, 1 × 106 cells per tube of donor-type PBLs (SLAdd, Class Id and IId) were resuspended in 100 μL of HBSS containing 0.1% bovine serum albumin and 0.05% NaN3 and incubated for 30 min at 4°C with 10 μL of decomplemented test sera (neat). After two washes, a saturating concentration of FITC-labeled goat anti-swine IgM or IgG was added and incubated for 30 min at 4°C. After a final wash, cells were analyzed by means of flow cytometry with propidium iodide gating to exclude dead cells. Both normal pig serum and pretransplant sera from each experimental animal were used as controls for specific binding.
Results
Tolerance was induced in recipients of MHC-disparate heart/kidney allografts
The three recipients that received isolated heart allografts followed by 12 days of tacrolimus all rejected their allografts by POD 40 (Table 1). In contrast, four of five recipients that received combined heart/kidney transplantation accepted both organs for over 200 days (Table 1). The cardiac allografts maintained strong contractions and exhibited no significant rejection or CAV on multiple serial biopsies taken through the recipient’s course (Table 1, Figure 1B–F). Immunohistochemical analyses of kidney allograft biopsy specimens from these heart/kidney recipients showed enrichment for Foxp3+T cells as compared to native kidneys (data not shown). The donor heart in the fifth heart/kidney recipient fibrillated during a biopsy procedure on POD 100 and could not be defibrillated despite multiple attempts. It was sacrificed several days later. Postmortem examination revealed no signs of rejection but did show acute myocyte injury consistent with ischemia, suggesting a technical error at the time of the biopsy.
Table 1.
Histology and survival of cardiac allografts in recipients of MHC-mismatched isolated hearts or combined heart/kidney transplants treated with 12 days of tacrolimus
| Organ(s) transplanted | SLA mismatches
|
Heart allograft histology at week1
|
Graft survival (days) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Donor | Recipient | 3 | 4 | 5 | 8 | 9 | 13 | 14 | 27 | 30 | 32 | 34 | 36 | 38 | 41 | 44 | ||
| Heart | DD | CC | – | – | 3R | – | – | – | – | – | – | – | – | – | – | – | – | 35 |
| Heart | DD | CC | 2R | 3R | – | – | – | – | – | – | – | – | – | – | – | – | – | 32 |
| Heart | DD | CC | 3R | 3R | – | – | – | – | – | – | – | – | – | – | – | – | – | 18 |
| Heart and kidney | DD | CC | – | 0 | – | – | 0 | 0 | – | – | – | – | – | – | – | 0 | – | >295 |
| Heart and kidney | DD | CC | – | – | 0 | 1R | – | – | 0 | – | – | – | 0 | – | 0 | – | 0 | >2842 |
| Heart and kidney | DD | CC | 0 | – | – | 0 | – | – | 0 | 0 | 0 | – | – | 0 | – | – | – | >2723 |
| Heart and kidney | DD | CC | – | 0 | – | 1R | – | – | 0 | – | 0 | 0 | – | – | – | – | – | 2234 |
| Heart and kidney | DD | CC | – | 0 | – | 1R | – | – | 0 | – | – | – | – | – | – | – | – | 1005 |
CAV, cardiac allograft vasculopathy; ISHLT, Society for Heart and Lung Transplantation; MHC, major histocompatibility complex; POD, postoperative day; SLA, swine lymphocyte antigen.
Grading of rejection from 0 (no rejection) to 4 (severe rejection) based on ISHLT scoring system (9).
Underwent donor kidney graftectomy on POD 211.
Underwent donor kidney graftectomy on POD 156.
Animal was sacrificed on POD 223 to examine the heart allograft for cardiac allograft vasculopathy.
Heart allograft fibrillated during POD100 biopsy and could not be defibrillated but no rejection was seen in necropsy specimens.
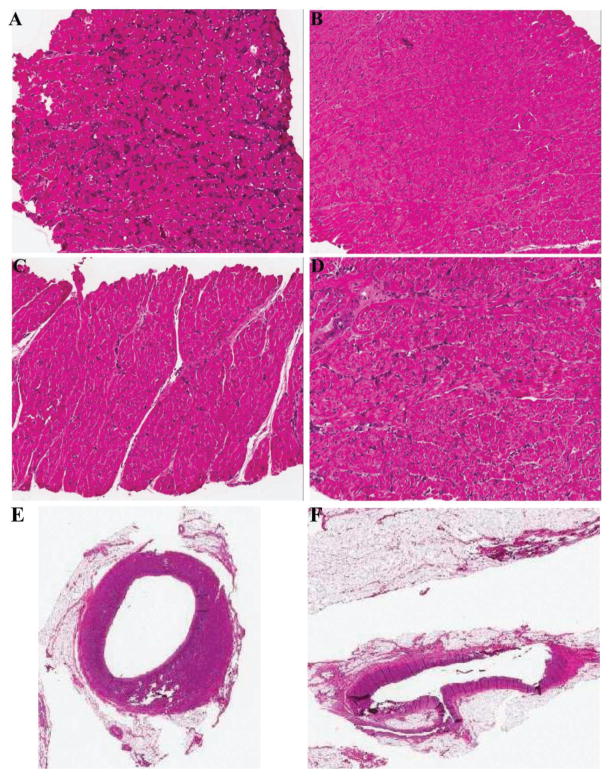
Figure 1. Histology from heart biopsies.
(A) Heart biopsy at POD 29 after heart alone transplant showing ISHLT 3R (animal #21109). (B–D) Serial heart biopsies of tolerant animal, #20977, at POD 25 (B), POD 60 (C) and POD 189 (D). (E and F) Heart biopsy of long-term tolerant animal, #21016, showing no cardiac allograft vasculopathy in the left main coronary artery (E) or in the left anterior descending coronary artery (F). ISHLT, Society for Heart and Lung Transplantation; POD, postoperative day.
Heart/kidney recipients demonstrated suppressed CML and MLR
Serial CML and MLR assays were performed to assess the immune competence in recipients of isolated heart versus heart/kidney allografts. All five heart/kidney recipients demonstrated loss of donor-specific responsiveness in both CML and MLR assays by POD 30 but maintenance of third-party reactivity (Figures 2 and 3). Donor-specific hyporesponsiveness was maintained for the duration of the experiment (>200 days). Pretransplant anti-third-party responses were significantly higher than pretransplant anti-donor responses most likely because the third-party stimulator cells were from an outbred pig line (Yorkshire). One heart-alone recipient (#21109) maintained donor responsiveness in MLR and CML assays while the other two developed donor-specific hyporesponsiveness, which may be due to the localization of cytotoxic T lymphocyte precursors in the rejecting heart allograft.
Figure 2. CML assays from heart/kidney recipients (n = 5).
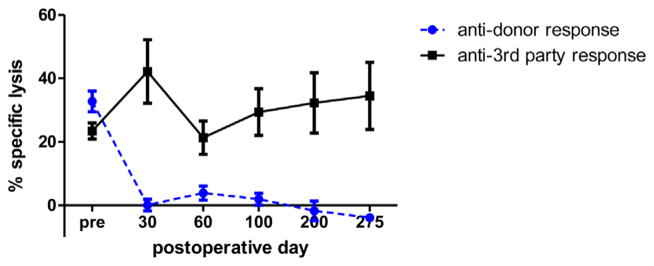
Percent specific lysis at 100:1 effector:target ratio is plotted as a function of postoperative day. Response against donor-type (SLAdd) targets (-●-) and response against third-party (York) targets (-■-). CML, cell-mediated lympholysis.
Figure 3. MLR assays from heart/kidney recipients (n = 5).
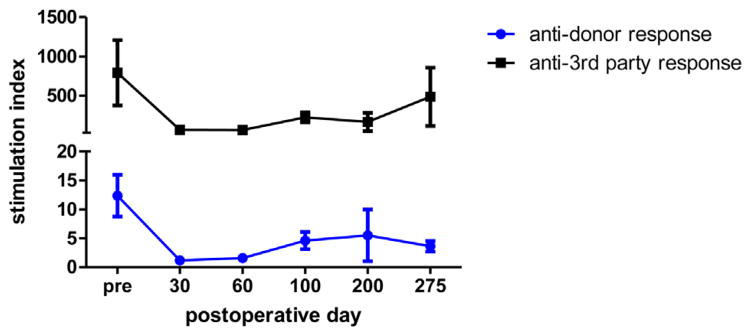
Stimulation indices to donor-type (SLAdd, -●-) and third-party (York, -■-) peripheral blood mononuclear cells are plotted as a function of postoperative day. MLR, mixed-lymphocyte reaction.
Alloantibody production was suppressed in combined heart/kidney recipients
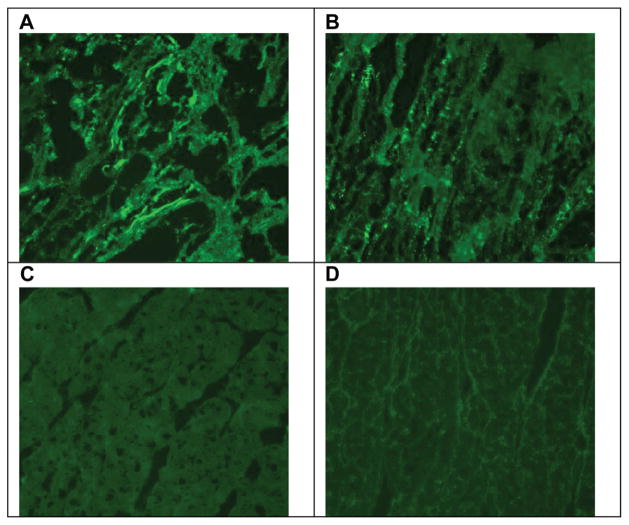
Heart/kidney recipients never showed detectable levels of circulating IgM and IgG alloantibody whereas IgG alloantibody was elevated in some but not all recipients of isolated hearts (Figure 4). However, immunohistochemical analysis of biopsies specimens demonstrated that by POD 40, significant amounts of anti-SLAdd IgM and IgG were deposited in the grafts of isolated heart recipients. In contrast, no detectable anti-SLAdd IgM and IgG deposition was observed in the cardiac allografts of heart/kidney recipients (Figure 5). Thus, the low levels of circulating alloantibody observed in some isolated heart recipients could be explained by their absorption in the allograft.
Figure 4. Alloantibody response.
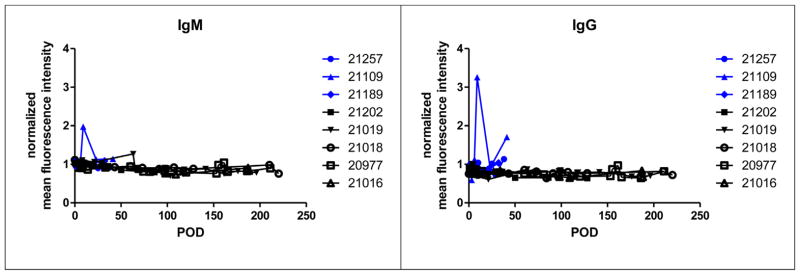
Levels of circulating anti-SLAdd IgM (left) and IgG (right) alloantibody were measured by flow cytometry in heart alone (#s 21257, 21109, 21189) and heart/kidney (#s 21202, 21019, 21018, 20977, 21016) recipients. Data were normalized to the mean fluorescence intensity of negative control values to plot normalized mean fluorescence intensity as a function of postoperative day (POD).
Figure 5. Alloantibody response.
Immunofluorescence staining of POD 40 alloheart biopsies with IgM or IgG alloantibody. In a representative heart alone recipient (#21109), heart grafts have positive IgM and IgG staining (A and B, respectively). In a representative heart/kidney recipient (#21019), heart grafts are negative for IgM and IgG staining (C and D, respectively). POD, postoperative day.
Donor skin graft survival was prolonged in heart/kidney recipients
On POD 162, autologous (SLAcc), donor-specific (SLAdd) and third-party (SLAaa) skin grafts were placed on the long-term tolerant heart/kidney recipient #21019. The autologous skin graft was accepted long term, the third-party skin graft was rejected by POD8 and the donor skin graft remained intact for 95 days (Figure 6). Despite rejecting the donor skin graft, tolerance was maintained as evidenced by lack of cardiac allograft rejection on biopsies and maintenance of donor-specific unresponsiveness in CML and MLR assays (data not shown).
Figure 6. Skin graft.
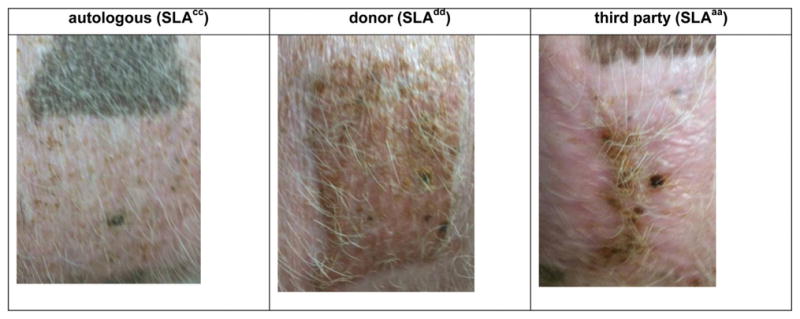
One long-term tolerant heart/kidney animal (#21019) underwent skin grafting on POD 162 with placement of autologous (SLAcc), donor (SLAdd) and third-party (SLAaa) skin grafts. Photos taken on Day 78 after skin grafting. POD, postoperative day.
Donor kidney graftectomy did not induce acute rejection of the cardiac allograft
Two long-term tolerant heart/kidney recipients underwent kidney graftectomy with transplantation of self-matched matched (SLAcc) kidney to maintain renal function. Animal #21018 underwent kidney allograftectomy on POD 211 and animal #20977 underwent kidney allograftectomy on POD 156. Serial biopsies on PODs 30, 60 and 100 after kidney allograft removal show no acute rejection of the heart (Table 1). Furthermore, serial CML and MLR assays showed persistent donor-specific unresponsiveness (data not shown).
Discussion
We have previously shown that long-term cardiac allograft tolerance can be achieved across a Class I MHC mismatch with 12 days of CsA (3). Here, we established that KICAT could be consistently achieved across a full MHC incompatibility in recipients treated with 12 days of tacrolimus. Tolerance was confirmed by indefinite allograft survival, hyporesponsiveness in CML and MLR assays, absence of alloantibody production and prolongation of donor-specific skin grafts. Furthermore, the cardiac allografts maintained strong contraction as assessed by palpation and echocardiography, and exhibited no clinically relevant rejection or CAV on serial biopsies. Together, these findings strongly suggest that these heart allografts would be life-sustaining if they had been transplanted orthotopically.
It has long been recognized that the immune response to a particular organ varies with the organ transplanted and that some organ allografts, especially livers (11), are able to confer a survival advantage upon another organ allograft procured from the same donor and cotransplanted into the same recipient. Although less powerful, kidney allografts also seem to possess a tolerogenic effect as evidenced by the dramatic difference in survival of swine kidney and heart allografts transplanted across the same histoincompatibility into recipients treated with the same short tolerance induction regimen (3).
The reason for these organ-specific differences is unclear. However, our earlier studies suggest that cells or cell products intrinsic to a donor kidney, but not heart allograft, promote a thymic-dependent expansion/activation of host regulatory T cells (Tregs) in the host, which mediates tolerance of the heart graft. We found that removing the recipient thymus, a major source of natural Tregs, prevented the induction of KICAT (12). We also noted that a radiosensitive, lymphohematopoietic cell population intrinsic to the donor kidney but not heart appeared necessary for the development of KICAT (13). Using in vitro suppression assays, we found that primed PBLs from tolerant heart/kidney recipients completely suppressed lysis of Class I-mismatched target cells by naïve cells but that suppression was lost following removal of CD25+ cells from the responder population (14). Finally, we demonstrated that KICAT was not simply due to the additional donor antigen load presented by the cotransplanted kidney as recipients grafted with two Class I-disparate hearts showed early high grade rejection and severe CAV (15). Together these studies and others from this laboratory (16,17) support a role for a regulatory mechanism in the state of tolerance induced, in part, by kidney transplantation.
The relevant clinical question then becomes what is the renal element responsible for promoting the expansion/activation of Tregs. There are two cell populations present in kidney allografts with the capacity to down-regulate alloimmune responses, (1) plasmacytoid dendritic cells (pDCs), which can promote the generation of Tregs and can induce tolerance to heart allografts in mice (18–20), and (2) renal tubular epithelial cells (RTECs), which can promote T cell unresponsiveness to self- and alloantigens in mice and humans (21–27). The hypothesis that pDCs mediate KICAT predicts that donor pDCs transferred with the kidney allograft traffic to the host thymus where they facilitate the activation/expansion of donor-specific Tregs. It is known that abundant CD11c+ conventional DCs are present in normal mouse kidneys (28). There is precedent for the pDC theory as it was recently shown that hepatic stellate cells from liver allografts were able to confer unresponsiveness and long-term survival to islet allografts by inducing Tregs (29) and myeloid-derived suppressor cells (30,31). It is less likely that migrating, kidney-derived pDCs activate deletional mechanisms in the host thymus, because in our earlier studies, circulating anti-donor cytotoxic T lymphocyte precursors were shown to be present in long-term heart/kidney recipients when SLAcc skin grafting resulted in the return of anti-donor responsiveness in CML assays and rejection of donor skin graft without heart or kidney allograft injury (3).
The hypothesis that RTECs mediate KICAT predicts that RTECs, intrinsic to the donor kidney, down-regulate or inactivate effector T cells emigrating from the host thymus or convert them to Tregs, thus shifting the balance of the immune response away from rejection and toward tolerance. There is also precedent for this theory. Foxp3+ cells are enriched in the tubules in human (32) and mouse (33) renal allografts. Frasca et al. (27) have shown that IFNγ-treated human RTECs induce allospecific tolerance. They showed that overnight incubation with IFNγ-treated, antigen-pulsed RTECs induced nonresponsiveness in B7-dependent T cell clones, suggesting that MHC Class II expression on RTEC may contribute to the induction of allospecific tolerance following organ transplantation. IFNγ also induces the T cell inhibitor molecule PD-1 and IDO expressed by RTECs (34,35). Importantly, Amarnath et al. (36) have recently shown that PD-1 signaling results in the conversion of human TH1 cells into Tregs. Finally, RTECs are known to produce and activate TGFβ (37), which is a major inducer of Foxp3+ Treg (38) and tolerogenic pDCs (39) generation. High levels of TGFβ have been documented in spontaneously accepted DBA/2 kidneys (40,41).
With regard to clinical applications, a tolerance induction protocol for heart allograft recipients that requires the simultaneous use of the donor kidney would only be tenable for the small number of patients requiring both organs. Instead, our goal now is to identify the renal component that is responsible for conferring tolerance to cotransplanted heart allografts, in order to use it in tolerance protocols for heart transplant recipients not requiring a kidney allograft. If the effective component should turn out to be a particular cell type, one might envision procuring such cells via a kidney biopsy at the time of organ retrieval and expanding them in vitro for use in a delayed tolerance protocol (42). Finally, it should be noted that a rising number of human heart allograft recipients do also require donor kidney cotransplantation for preestablished medical reasons. For this special subpopulation, KICAT may be directly applicable, using a delayed mixed chimerism strategy (42).
In conclusion, we achieved cardiac allograft tolerance across a full MHC mismatch via a kidney-specific mechanism in a robust and reproducible manner. We also demonstrated in this large animal model that the kidney is necessary for the induction phase but not the maintenance phase of cardiac allograft tolerance. This preclinical model will serve as the basis for further studies to isolate and apply the renal element responsible for KICAT to protocols aimed at inducing tolerance in recipients of tolerance-resistant allografts such as isolated heart, lung, composite tissue and islet allografts.
Acknowledgments
This work was supported in part by grants from the National Heart, Lung, and Blood Institute (P01HL18646) and the National Institute of Allergy and Infectious Disease (U01AI94374) of National Institutes of Health, and the Thoracic Surgery Foundation for Research & Education. M.L.M. is an Edward D. Churchill Surgical Research Fellow, Massachusetts General Hospital and recipient of a fellowship from the International Society for Heart & Lung Transplantation. We acknowledge C06RR020135-01 for construction of the facility utilized for production and maintenance of miniature swine and are indebted to Mr. J. Scott Arn for herd management and quality control typing.
Abbreviations
- ACR
acute cellular rejection
- CAV
cardiac allograft vasculopathy
- CML
cell-mediated lympholysis
- CsA
cyclosporine A
- ISHLT
Society for Heart and Lung Transplantation
- KICAT
kidney-induced cardiac allograft tolerance
- MHC
major histocompatibility complex
- MLR
mixed-lymphocyte reaction
- PBL
peripheral blood leukocytes
- pDCs
plasmacytoid dendritic cells
- POD
postoperative day
- PSL
percent specific lysis
- RTECs
renal tubular epithelial cells
- SLA
swine lymphocyte antigen
- Tregs
regulatory T cells
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th Official Adult Heart Transplant Report—2012. J Heart Lung Transplant. 2012;31:1052–1064. doi: 10.1016/j.healun.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Sachs DH, Leight G, Cone J, Schwarz S, Stuart L, Rosenberg S. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation. 1976;22:559–567. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Madsen JC, Yamada K, Allan JS, et al. Transplantation tolerance prevents cardiac allograft vasculopathy in major histocompatibility complex class I-disparate miniature swine. Transplantation. 1998;65:304–313. doi: 10.1097/00007890-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 4.Utsugi R, Barth RN, Lee RS, et al. Induction of transplantation tolerance with a short course of tacrolimus (FK506): I. Rapid and stable tolerance to two-haplotype fully MHC-mismatched kidney allografts in miniature swine. Transplantation. 2001;71:1368–1379. doi: 10.1097/00007890-200105270-00003. [DOI] [PubMed] [Google Scholar]
- 5.Madsen JC, Sachs DH, Fallon JT, Weissman NJ. Cardiac allograft vasculopathy in partially inbred miniature swine. I. Time course, pathology, and dependence on immune mechanisms. J Thorac Cardiovasc Surg. 1996;111:1230–1239. doi: 10.1016/s0022-5223(96)70226-x. [DOI] [PubMed] [Google Scholar]
- 6.Kirkman RL, Colvin MW, Flye GS, et al. Transplantation in miniature swine. VI. Factors influencing survival of renal allografts. Transplantation. 1979;28:18–23. [PubMed] [Google Scholar]
- 7.Avitall B, Payne DD, Connolly RJ, et al. Heterotopic heart transplantation: Electrophysiologic changes during acute rejection. J Heart Transplant. 1988;7:176–182. [PubMed] [Google Scholar]
- 8.Mehra MR, Crespo-Leiro MG, Dipchand A, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy—2010. J Heart Lung Transplant. 2010;29:717–727. doi: 10.1016/j.healun.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant. 2008;8:753–760. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 10.Kirkman RL, Colvin RB, Flye MW, Williams GM, Sachs DH. Transplantation in miniature swine. VII. Evidence for cellular immune mechanisms in hyperacute rejection of renal allografts. Transplantation. 1979;28:24–30. [PubMed] [Google Scholar]
- 11.Calne R, Davies H. Organ graft tolerance: The liver effect. Lancet. 1994;343:67–68. doi: 10.1016/s0140-6736(94)90809-5. [DOI] [PubMed] [Google Scholar]
- 12.Yamada K, Choo JK, Allan JS, et al. The effect of thymectomy on tolerance induction and cardiac allograft vasculopathy in a miniature swine heart/kidney transplantation model. Transplantation. 1999;68:485–491. doi: 10.1097/00007890-199908270-00007. [DOI] [PubMed] [Google Scholar]
- 13.Mezrich JD, Yamada K, Lee RS, et al. Induction of tolerance to heart transplants by simultaneous co-transplantation of donor kidneys may depend on a radiation-sensitive renal-cell population. Transplantation. 2003;76:625–631. doi: 10.1097/01.TP.0000079926.80833.42. [DOI] [PubMed] [Google Scholar]
- 14.Mezrich JD, Kesselheim JA, Johnston DR, Yamada K, Sachs DH, Madsen JC. The role of regulatory cells in miniature swine rendered tolerant to cardiac allografts by donor kidney co-transplantation. Am J Transplant. 2003;3:1107–1115. doi: 10.1046/j.1600-6143.2003.00202.x. [DOI] [PubMed] [Google Scholar]
- 15.Yamada K, Mawulawde K, Menard MT, et al. Mechanisms of tolerance induction and prevention of cardiac allograft vasculopathy in miniature swine: The effect of augmentation of donor antigen load. J Thorac Cardiovasc Surg. 2000;119:709–719. doi: 10.1016/S0022-5223(00)70005-5. [DOI] [PubMed] [Google Scholar]
- 16.Wu A, Yamada K, Ierino FL, Vagefi PA, Sachs DH. Regulatory mechanism of peripheral tolerance: In vitro evidence for dominant suppression of host responses during the maintenance phase of tolerance to renal allografts in miniature swine. Transpl Immunol. 2003;11:367–374. doi: 10.1016/S0966-3274(03)00006-6. [DOI] [PubMed] [Google Scholar]
- 17.Okumi M, Scalea JR, Gillon BC, et al. The induction of tolerance of renal allografts by adoptive transfer in miniature swine. Am J Transplant. 2013;13:1193–1202. doi: 10.1111/ajt.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gehrie E, Van der Touw W, Bromberg JS, Ochando JC. Plasmacytoid dendritic cells in tolerance. Methods Mol Biol. 2011;677:127–147. doi: 10.1007/978-1-60761-869-0_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ochando JC, Homma C, Yang Y, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 20.Abe M, Wang Z, de Creus A, Thomson AW. Plasmacytoid dendritic cell precursors induce allogeneic T-cell hyporesponsiveness and prolong heart graft survival. Am J Transplant. 2005;5:1808–1819. doi: 10.1111/j.1600-6143.2005.00954.x. [DOI] [PubMed] [Google Scholar]
- 21.Deckers JGM, Boonstr JG, Van der Kooij SW, Daha MR, van der Woude FJ. Tissue-specific characteristics of cytotoxic graft-infiltrating T cells during renal allograft rejection. Transplantation. 1997;64:178–181. doi: 10.1097/00007890-199707150-00034. [DOI] [PubMed] [Google Scholar]
- 22.Hadley GA, Rostapshova EA, Bartlett ST. Dominance of tissue-restricted cytotoxic T lymphocytes in the response to allogeneic renal epithelial cell lines. Transplantation. 1996;62:75–83. doi: 10.1097/00007890-199607150-00016. [DOI] [PubMed] [Google Scholar]
- 23.Hagerty DT, Allen PM. Processing and presentation of self and foreign antigens by the renal proximal tubule. J Immunol. 1992;148:2324–2330. [PubMed] [Google Scholar]
- 24.Kirby JA, Rajasekar MR, Lin Y, Proud G, Taylor RM. Interaction between T lymphocytes and kidney epithelial cells during renal allograft rejection. Kidney Int Suppl. 1993;39:S124–S128. [PubMed] [Google Scholar]
- 25.Neilson EG. Is immunologic tolerance of self modulated through antigen presentation by parenchymal epithelium? Kidney Int. 1993;44:927–931. doi: 10.1038/ki.1993.333. [DOI] [PubMed] [Google Scholar]
- 26.Singer GG, Yokoyama H, Bloom RD, Jevnikar AM, Nabavi N, Kelley VR. Stimulated renal tubular epithelial cells induce anergy in CD4+ T cells. Kidney Int. 1993;44:1030–1035. doi: 10.1038/ki.1993.345. [DOI] [PubMed] [Google Scholar]
- 27.Frasca L, Marelli-Berg F, Imami N, et al. Interferon-gamma-treated renal tubular epithelial cells induce allospecific tolerance. Kidney Int. 1998;53:679–689. doi: 10.1046/j.1523-1755.1998.00800.x. [DOI] [PubMed] [Google Scholar]
- 28.Soos TJ, Sims TN, Barisoni L, et al. CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int. 2006;70:591–596. doi: 10.1038/sj.ki.5001567. [DOI] [PubMed] [Google Scholar]
- 29.Yang HR, Chou HS, Gu X, et al. Mechanistic insights into immunomodulation by hepatic stellate cells in mice: A critical role of interferon-gamma signaling. Hepatology. 2009;50:1981–1991. doi: 10.1002/hep.23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chou HS, Hsieh CC, Yang HR, et al. Hepatic stellate cells regulate immune response by way of induction of myeloid suppressor cells in mice. Hepatology. 2011;53:1007–1019. doi: 10.1002/hep.24162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chou HS, Hsieh CC, Charles R, et al. Myeloid-derived suppressor cells protect islet transplants by B7-H1 mediated enhancement of T regulatory cells. Transplantation. 2012;93:272–282. doi: 10.1097/TP.0b013e31823ffd39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veronese F, Rotman S, Smith RN, et al. Pathological and clinical correlates of Foxp3+ cells in renal allografts during acute rejection. Am J Transplant. 2007;7:914–922. doi: 10.1111/j.1600-6143.2006.01704.x. [DOI] [PubMed] [Google Scholar]
- 33.Brown K, Moxham V, Karegli J, Phillips R, Sacks SH, Wong W. Ultra-localization of Foxp3+ T cells within renal allografts shows infiltration of tubules mimicking rejection. Am J Pathol. 2007;171:1915–1922. doi: 10.2353/ajpath.2007.070396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoop R, Wahl P, Le HM, Heemann U, Wang M, Wuthrich RP. Suppressed T-cell activation by IFN-gamma-induced expression of PD-L1 on renal tubular epithelial cells. Nephrol Dial Transplant. 2004;19:2713–2720. doi: 10.1093/ndt/gfh423. [DOI] [PubMed] [Google Scholar]
- 35.Mohib K, Guan Q, Diao H, Du C, Jevnikar AM. Proapoptotic activity of indoleamine 2,3-dioxygenase expressed in renal tubular epithelial cells. Am J Physiol Renal Physiol. 2007;293:F801–F812. doi: 10.1152/ajprenal.00044.2007. [DOI] [PubMed] [Google Scholar]
- 36.Amarnath S, Mangus CW, Wang JC, et al. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med. 2011;3:111ra120. doi: 10.1126/scitranslmed.3003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robertson H, Wong WK, Talbot D, Burt AD, Kirby JA. Tubulitis after renal transplantation: Demonstration of an association between CD103+ T cells, transforming growth factor beta1 expression and rejection grade. Transplantation. 2001;71:306–313. doi: 10.1097/00007890-200101270-00024. [DOI] [PubMed] [Google Scholar]
- 38.Zheng SG. The critical role of TGF-beta1 in the development of induced Foxp3+ regulatory T cells. Int J Clin Exp Med. 2008;1:192–202. [PMC free article] [PubMed] [Google Scholar]
- 39.Pallotta MT, Orabona C, Volpi C, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12:870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 40.Miyajima M, Chase CM, Alessandrini A, et al. Early acceptance of renal allografts in mice is dependent on foxp3(+) cells. Am J Pathol. 2011;178:1635–1645. doi: 10.1016/j.ajpath.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cook CH, Bickerstaff AA, Wang JJ, et al. Spontaneous renal allograft acceptance associated with “regulatory” dendritic cells and IDO. J Immunol. 2008;180:3103–3112. doi: 10.4049/jimmunol.180.5.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamada Y, Boskovic S, Aoyama A, et al. Overcoming memory T-cell responses for induction of delayed tolerance in nonhuman primates. Am J Transplant. 2012;12:330–340. doi: 10.1111/j.1600-6143.2011.03795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]