Abstract
Background
Antiretroviral treatment interruptions (TIs) cause suboptimal clinical outcomes. Data on TIs during social disruption are limited.
Methods
We determined effects of unplanned TIs after the 2007–2008 Kenyan postelection violence on virological failure, comparing viral load (VL) outcomes in HIV-infected adults with and without conflict-induced TI.
Results
Two hundred and one patients were enrolled, median 2.2 years after conflict and 4.3 years on treatment. Eighty-eight patients experienced conflict-related TIs and 113 received continuous treatment. After adjusting for preconflict CD4, patients with TIs were more likely to have detectable VL, VL >5,000 and VL >10,000.
Conclusions
Unplanned conflict-related TIs are associated with increased likelihood of virological failure.
Keywords: HIV, treatment interruption, political crisis Kenya, virological failure, drug resistance
INTRODUCTION
After the December 27, 2007 presidential election, Kenya experienced a political crisis resulting in >1200 deaths and displacement of >300,000 Kenyans.1–3 Violence transpired country wide though the epicenter surrounded Eldoret, Western Kenya.1 The Academic Model Providing Access to Healthcare (AMPATH), one of the largest HIV treatment programs in sub-Saharan Africa,4 implemented interventions to resume HIV care for crisis-affected patients. Immediate violence lasted through January 2008, with yet to be documented long-term ramifications, particularly regarding the impact on HIV.
Regimented adherence to antiretrovirals (ARVs) is necessary for successful treatment5,6 and prevention of drug resistance.7,8 Simultaneous cessation of first-line ARVs9 can lead to functional monotherapy and resistance development.8,10–14 Treatment resumption with similar regimens may thus be ineffective.15–17
The association between adherence and clinical outcomes is well established,18 and treatment interruptions (TIs) are not recommended in HIV care.19,20 However, crises can lead to different magnitudes of unplanned TIs21 and their implications on treatment failure and resistance are unknown.18 Understanding conflict consequences on HIV in resource-limited settings, where HIV prevalence is high and political crises may occur more frequently,14 is imperative to avoid preventable complications.
Political crises can lead to unplanned TI due to supply chain disruption, population displacement, unsafe travel, and inability to obtain medications.14,22,23 We evaluate the long-term effects of the Kenyan postelection violence on treatment failure in the hardest-hit region of Western Kenya by comparing HIV outcomes in patients with and without crisis-induced TIs. We hypothesize that patients with postcrisis TIs are at higher risk of failure and development of drug resistance.
METHODS
Study Setting
In December 2007, AMPATH4,24 had 17 clinics in Western Kenya and treated 59,437 HIV-infected patients, 39% on ARVs.4 This retrospective cohort study was conducted between November 2009 and April 2011 in 2 clinics severely affected by the violence: Burnt-Forest (clinic population, 3500) and Turbo (population, 5700). Recruitment began at Burnt-Forest and continued at Turbo after all available Burnt-Forest patients with TI were enrolled.
Charts were screened for eligibility at patients’ scheduled appointments. Potentially eligible patients were interviewed and enrolled if (1) 18 years or older, (2) cared for continuously at clinic on continuous World Health Organization (WHO)–recommended first-line ARVs from at least June 2007 (6 months before crisis) through enrollment, with possible exception of crisis-induced TI, and (3) adherent >50% to all ARVs in the month and week before enrollment. Patients were enrolled into 1 of the 2 groups: (1) “Interruption”: crisis-induced TI for ≥1 week, or (2) “Control”: continuous ARVs with no TIs ≥1 week between 6 months before crisis and enrollment. Patients were enrolled consecutively and simultaneously in the 2 groups to maximize representativeness.
Eligible patients were interviewed for experience of crisis-induced TI, its duration, and ARV adherence in the month and week before enrollment. Charts were reviewed for age, sex, WHO stage at enrollment, ARV history, and CD4 values (first ever and from 6 months before crisis to enrollment). This study was approved by ethics committees in Kenya and the United States.
Laboratory Methods
CD4 (FACSCaliber System; Becton Dickinson, San Jose, CA) and viral load (VL) (Amplicor; Roche Molecular, Pleasanton, CA) were performed at the AMPATH Laboratory. Pol genotyping was performed at the Brown/Lifespan/Tufts Center for AIDS Research Resistance Laboratory. Viral RNA was extracted via Biomerieux's MiniMAG, Durham, NC; reverse transcription and polymerase chain reaction were performed using SuperScript III and High Fidelity Platinum Taq. Products were Sanger sequenced and assembled with Sequencher v4.10.1. Sequence quality control was with SQUAT25 and resistance interpretation was with Stanford Database tools,26 based on the IAS-USA mutation list.27
Statistical Analysis
The primary outcome measure was enrollment VL categorized as (1) detectable (>400 copies per milliliter; assay's threshold); (ii) >5,000 (current WHO threshold28); and (iii) >10,000 (pre-2010 WHO threshold29). Secondary outcomes were enrollment CD4 count (<100, <200, and <350 cells per microliter) and CD4 percentage, and CD4 change from precrisis to enrollment. Logistic, ordinal, and linear regressions were used to asdrugsess outcomes categorically and continuously. Plots and logistic regression models were used to explore relationship between VL and TI duration. Longitudinal mixed-effects models were constructed that included fixed effects for study group, time between crisis and each CD4 test, and their interaction; and random effects for each participant's CD4 at crisis and CD4 change from crisis to enrollment (ie, slope). Covariates assessed as potentially associated with both study selection and virological outcome were disease severity (age, adherence, WHO stage, precrisis CD4, and median CD4 change from first ever to precrisis) and engagement in HIV care (years on ARV precrisis). We compared resistance between groups for participants with detectable VL using linear and ordinal regression of overall and class-specific mutations number and overall class-specific and drug-specific predicted susceptibility. Stata v.10 was used for statistical analysis.
RESULTS
Patient Characteristics
In Burnt-Forest, there were 2315 active patients before crisis (December 2007) and 2334 after crisis (March 2008), with 29 lost to follow-up (LTFU) during these 3 months. Similar figures in Turbo were 3350 in December 2007 and 3453 in March 2008 before and after crisis, respectively, with 56 LTFU. One thousand three hundred nineteen charts were screened in both clinics, 836 in Burnt-Forest and 433 in Turbo, representing 27% and 8%, respectively, of clinic populations at the start of enrollment (November 2009), resulting in 213 interviews. Ineligibility included naive treatment status, ineligible regimens, or time on treatment (specific breakdown not available). Two hundred and one patients were enrolled, median 2.2 years after crisis [interquartile range (IQR), 2.1–2.4], 88 into the Interruption and 113 into the Control group. Five were not enrolled due to blood draw issues, 4 due to <50% adherence, and 3 due to ineligible ARVs. Most participants (197) were enrolled from Burnt-Forest.
Table 1 shows demographic, clinical, and laboratory characteristics of the cohort by interruption status. No history of drug exposure before ARV initiation was recorded; 99% reported >90% adherence. Only median CD4 values before crisis differed between groups, lower in the Interruption group (299 vs. 378 cells per microliter, P = 0.01; 17% vs. 20%, P = 0.01). Change in CD4 count and CD4 percentage from treatment initiation until crisis increased a median of 193 (IQR, 94–322) cells per microliter and 7% (IQR, 4%–12%) in both groups. Median duration of TI was 4.3 weeks (IQR, 1.4–4.6; range, 1 week–1 year). Three patients missed treatment for >3 months due to severe displacement. Median time from ARV initiation to crisis was 1.9 (range, 0.5–4.2) years, from crisis to ARV resumption in the TI group 4.3 (range, 1–52) weeks and from ARV resumption to enrollment 2.2 (range, 1.1–2.9) years.
TABLE 1.
Demographic, Clinical, and Laboratory Characteristics by Interruption Status
| TI Status |
|||
|---|---|---|---|
| Yes (n = 88), n (%) | No (n = 113), n (%) | Total (n = 201), n (%) | |
| Sex | |||
| Male | 30 (34) | 46 (41) | 76 (38) |
| Female | 58 (66) | 67 (59) | 125 (62) |
| Age at enrollment (yrs), median (IQR) | 43 (37–50) | 41 (36–50) | 42 (36–50) |
| Self-reported adherence at enrollment | |||
| Complete (>90%) | 86 (98) | 112 (99) | 198 (99) |
| Partial (50%–90%) | 2 (2) | 1 (1) | 3 (2) |
| WHO stage at enrollment | |||
| 1 | 14 (16) | 22 (20) | 36 (18) |
| 2 | 20 (20) | 15 (13) | 35 (17) |
| 3 | 42 (48) | 52 (46) | 94 (47) |
| 4 | 12 (14) | 24 (21) | 36 (18) |
| ARV regimen at crisis | |||
| 3TC, d4T, NVP | 72 (82) | 91 (81) | 163 (81) |
| 3TC, d4T, EFV | 9 (10) | 11 (10) | 20 (10) |
| 3TC, AZT, NVP | 7 (8) | 7 (6) | 14 (7) |
| 3TC, AZT, EFV | 0 (0) | 4 (4) | 4 (2) |
| First-line regimen switches* | |||
| 0 | 59 (67) | 79 (70) | 138 (69) |
| 1 | 15 (17) | 22 (19) | 37 (18) |
| 2 | 11 (10) | 8 (7) | 19 (10) |
| 3 | 2 (2) | 2 (2) | 4 (2) |
| 4+ | 1 (1) | 2 (2) | 3 (2) |
| ARV regimen at study enrollment | |||
| 3TC, d4T, NVP | 71 (81) | 91 (81) | 162 (81) |
| 3TC, d4T, EFV | 6 (7) | 9 (8) | 15 (7) |
| 3TC, AZT, NVP | 10 (11) | 9 (8) | 19 (9) |
| 3TC, AZT, EFV | 1 (1) | 4 (4) | 5 (2) |
| ARV years of treatment at crisis, median (IQR) | 1.8 (1.2–2.8) | 2.1 (1.4–2.9) | 1.9 (1.3–2.9) |
| ARV years of treatment at enrollment, median (IQR) | 4.1 (3.6–5.0) | 4.4 (3.7–5.1) | 4.3 (3.6–5.1) |
| CD4, most recent Precrisis | |||
| Count (cells/mL), median (IQR) | 299 (182–415) | 378 (248–568) | 336 (221–484) |
| Percent, median (IQR) | 17% (13–23) | 20% (16–26) | 18% (14–24) |
| Change in CD4, first ever to crisis | |||
| Count (cells/mL), median (IQR) | 179 (78–276) | 203 (96–366) | 193 (94–322) |
| Percent, median (IQR) | 7% (4–11) | 8% (4–12) | 7% (4–12) |
Includes all regimen changes from beginning of ARV treatment to study enrollment, both switching to a new and/or reverting to a previous first-line regimen, excludes restarting of same regimen after an interruption.
AZT, zidovudine; d4T, stavudine; EFV, efavirenz; NVP, nevirapine; 3TC, lamivudine.
Virological Failure
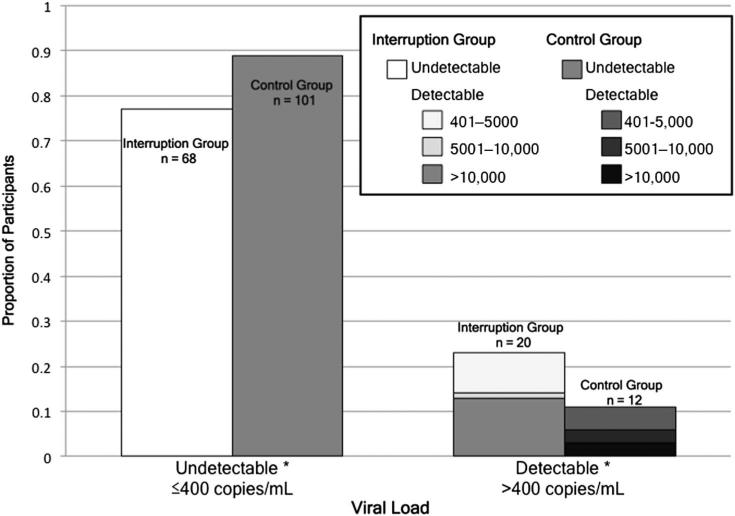
At enrollment, 23% (20/88) of patients with TI had detectable VL, 14% had VL >5000, and 13% had VL >10,000, compared with 11% (12/113) detectable (P = 0.007), 5% VL >5000 (P = 0.04), and 3% VL >10,000 (P = 0.02) in the Control group (Fig. 1). Among those with detectable VL, median VL was 8317 (IQR, 3110–40,517); 14,094 (IQR, 2890–61,367) in the Interruption group compared with 5286 (IQR, 3481–11,651) in the Control group (P < 0.001).
FIGURE 1.
VL at study enrollment according to study group. Light bars represent the Interruption group and dark bars represent the Control group. Study groups are compared by VL undetectability (left bars) and detectability (right bars, by VL threshold categories). Asterisks represent P < 0.05 (undetectable VL, P = 0.02; VL 401–5000 copies per milliliter, P = 0.007; VL 5001–10,000 copies per milliliter, P = 0.04; VL > 10,000 copies per milliliter, P = 0.02); see text for additional details.
In unadjusted analysis, patients with TI were more likely to virologically fail than the Control group at all 3 thresholds [odds ratio (OR), 2.5 for detectable VL, 95% confidence interval (CI): 1.1 to 5.4, P = 0.02; OR, 2.8 for VL >5000, 95% CI: 1.0 to 7.8, P = 0.05; and OR, 5.2 for VL >10,000; 95% CI: 1.4 to 19.4; P = 0.01]. Precrisis CD4 was considered a confounder as it was the only variable to affect relationship between interruption and virological response. Controlling for precrisis CD4, patients with TI were more likely to virologically fail than the Control group at all 3 thresholds [adjusted odds ratio (AOR), 2.4 for detectable VL, 95% CI: 1.1 to 5.4, P = 0.03; AOR, 2.4 for VL >5000, 95% CI: 0.9 to 6.9, P = 0.09; and AOR, 4.5 for VL > 10,000, 95% CI: 1.2 to 17.0, P = 0.03].
Patients with TI were more likely to be in a higher ordinal VL category (≤400, >400 and ≤10,000, and >10,000) compared with those without (AOR, 2.4, adjusted for precrisis CD4; 95% CI: 1.1 to 5.4; P = 0.03). TI duration was not associated with VL threshold. A sensitivity analysis with removal of the 4 patients from Turbo yielded similar results (P = 0.03).
CD4 Values
Mean enrollment CD4 values were 384 and 21% in the Interruption group and 421 and 24% in the Control group (P = 0.18 and 0.04, respectively). The association between CD4 percentage and interruption status disappeared after adjustment for precrisis CD4 count. The odds of having a CD4 count <100, 200, or 350 at enrollment did not differ between groups, regardless of adjustment.
The mean increase in CD4 values from precrisis to enrollment was 44% and 4% in the Interruption group, and 25% and 4% in the Control group (P = ns). Findings from the longitudinal mixed-effects models were similar.
Drug Resistance
Of 32 patients with detectable VL (20 Interruption and 12 Control), genotypes were available for 17 of the 20 and 11 of the 12 patients. Sixty-five percent in the Interruption group and 64% in the Control group had intermediate or high-predicted resistance to first-line ARVs, with class-specific resistance in 53% for nucleoside reverse transcriptase inhibitor and 94% for nonsnucleoside reverse transcriptase inhibitor in the Interruption group, and 36% and 91%, respectively, in the Control group (P = ns for all). Total and class-specific mutation number (range, 0–8) was not different between groups.
DISCUSSION
We evaluated the long-term effects of the Kenyan 2007–2008 postelection violence on HIV treatment failure. Two years after the crisis, patients with crisis-induced TI were up to 5 times more likely to virologically fail compared with those with uninterrupted treatment. In addition to the array of negative effects created by political conflicts in low-income settings, HIV-infected individuals face the additional risk of virological failure, which can lead to disease progression, resistance accumulation, and limited treatment options.
Our results are consistent with studies assessing unplanned TIs,30–33 demonstrating negative association with virological outcomes. However, the context of political crisis-induced TI is unique. Lack of control over one's environment, external factors that may impact adherence during crisis, and abruptness and persistence of crisis-induced interruption are distinctive. These factors may lead to complete interruption, rather than selective nonadherence in noncrisis scenarios. Consequences are augmented by the potential for increased VL, sexual violence, and disruption of family units that may follow crises and increase HIV transmission. Although biological effects of nonadherence may be similar in direction if lesser in magnitude in peaceful settings, implications for actions to correct or mediate the issue differ. Additional studies are needed to externally validate our findings in other populations, conflicts, or unstable settings, where health consequences, both general and HIV related, are often overlooked.
Observed resistance results do not support our hypothesis, explained by 2 aspects. First, the time lapse between interruption and enrollment may have allowed resistance to increase in both groups, rendering similar profiles. Second, a smaller than intended sample size may have resulted in insufficient power to detect a difference.
We note 4 limitations. First, precrisis VL was not available for prospective assessment of virological response to learn the occurrence, duration of, or changes in outcomes over time. Second, due to extraordinary efforts by AMPATH to track patients during crisis to minimize TI, only half of the projected sample size (400 patients) could be recruited, resulting in decreased precision of effect estimates. Therefore, our results may underestimate the impact of crisis-induced TIs. Third, due to the time lapse between the crisis and enrollment, recall bias is possible. Finally, the large crisis-to-enrollment time gap may have resulted in excluded patients due to death, LTFU, or first- to second-line switch. This is unlikely to have had a significant effect on enrollment because monthly mortality, LTFU, and switch rates are consistently <1% in Burnt-Forest.
Aid strategies and medical provider guidance in times of crisis should reflect needs of vulnerable HIV populations, emphasizing the importance of providing continuous ARVs and actively following up patients. Viable options include stocking emergency ARV supplies, implementing innovative follow-up methods, developing guidelines for resumption of care including VL and resistance testing, providing several months of ARVs before controversial events, and providing clinic transfer information for displaced patients, as also reviewed in Mann et al.14 The exemplary care provided by AMPATH during the crisis represented by the difficulty enrolling patients with crisis-induced TI in the hardest-hit region should serve as guidance. Moreover, AMPATH clinicians in Burnt-Forest provided patients with a 2-month ARV supply in December 2007 in preparation for Christmas and the elections, without which the scope of crisis-induced TIs may have been greater.
Increased awareness by policy makers of these long-term often overlooked conflict consequences on lives of HIV-infected patients may provide opportunity for reconsideration in future crises. In countries where postelection violence has occurred or is anticipated, it may be advantageous for leaders to begin a preemptive discourse on violence prevention and contingency planning for vulnerable populations before the imminent conflict. Clinicians and researchers should emphasize linkage between conflict consequences and HIV care, and educate patients, providers, and policy makers. Such awareness may help not only promote peace but also fight the HIV pandemic.
Acknowledgments
Supported by the National Institutes of Health (Grant numbers R25-TW008102, RO1-AI66922, P30AI042853, and 5U01AI069911).
This research was based in part upon work conducted using the Rhode Island Genomics and Sequencing Center, which is supported in part by the National Science Foundation (MRI Grant No. DBI-0215393 and EPSCoR Grant Nos. 0554548 & EPS-1004057), the US Department of Agriculture (Grant Nos. 2002-34438-12688 and 2003-34438-13111), and the University of Rhode Island.
Footnotes
Presented at the Conference on Retroviruses and Opportunistic Infections, March 5–8, 2012, Seattle, WA.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Vreeman R, Nyandiko W, Sang E, et al. Impact of the Kenya post-election crisis on clinic attendance and medication adherence for HIV-infected children in western Kenya. Confl Health. 2009;3:5. doi: 10.1186/1752-1505-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gettleman J. Ethnic violence in rift valley is tearing Kenya Apart. [January 8, 2012];New York Times. 2008 Jan;27 Available at: http://www.nytimes.com/2008/01/27/world/africa/27kenya.html?ref=kenya. [Google Scholar]
- 3.Yoder RB, Nyandiko WM, Vreeman RC, et al. Long-term impact of the Kenya postelection crisis on clinic attendance and medication adherence for HIV-infected children in western Kenya. J Acquir Immune Defic Syndr. 2012;59:199–206. doi: 10.1097/QAI.0b013e31823b4448. [DOI] [PubMed] [Google Scholar]
- 4.USAID Kenya [January 31, 2012];USAID–AMPATH Partnership. 2010 Available at: http://kenya.usaid.gov/programs/health/83.
- 5.Bangsberg DR, Kroetz DL, Deeks SG. Adherence-resistance relationships to combination HIV antiretroviral therapy. Curr HIV/AIDS Rep. 2007;4:65–72. doi: 10.1007/s11904-007-0010-0. [DOI] [PubMed] [Google Scholar]
- 6.Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15:1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 7.Boyer S, Clerc I, Bonono CR, et al. Non-adherence to antiretroviral treatment and unplanned treatment interruption among people living with HIV/AIDS in Cameroon: individual and healthcare supply-related factors. Soc Sci Med. 2011;72:1383–1392. doi: 10.1016/j.socscimed.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 8.Darwich L, Esteve A, Ruiz L, et al. Variability in the plasma concentration of efavirenz and nevirapine is associated with genotypic resistance after treatment interruption. Antivir Ther. 2008;13:945–951. [PubMed] [Google Scholar]
- 9.Taylor S, Boffito M, Khoo S, et al. Stopping antiretroviral therapy. AIDS. 2007;21:1673–1682. doi: 10.1097/QAD.0b013e3281c61394. [DOI] [PubMed] [Google Scholar]
- 10.Hare CB, Mellors J, Krambrink A, et al. Detection of nonnucleoside reverse-transcriptase inhibitor-resistant HIV-1 after discontinuation of virologically suppressive antiretroviral therapy. Clin Infect Dis. 2008;47:421–424. doi: 10.1086/589867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nuesch R, Ananworanich J, Sirivichayakul S, et al. Development of HIV with drug resistance after CD4 cell count-guided structured treatment interruptions in patients treated with highly active antiretroviral therapy after dual-nucleoside analogue treatment. Clin Infect Dis. 2005;40:728–734. doi: 10.1086/427878. [DOI] [PubMed] [Google Scholar]
- 12.Ribaudo HJ, Haas DW, Tierney C, et al. Pharmacogenetics of plasma efavirenz exposure after treatment discontinuation: an adult AIDS Clinical Trials Group Study. Clin Infect Dis. 2006;42:401–407. doi: 10.1086/499364. [DOI] [PubMed] [Google Scholar]
- 13.Trignetti M, Sing T, Svicher V, et al. Dynamics of NRTI resistance mutations during therapy interruption. AIDS Res Hum Retroviruses. 2009;25:57–64. doi: 10.1089/aid.2008.0159. [DOI] [PubMed] [Google Scholar]
- 14.Mann M, Lurie MN, Kimaiyo S, et al. Effects of political conflict-induced treatment interruptions on HIV drug resistance. AIDS Rev. 2013;15:15–24. [PMC free article] [PubMed] [Google Scholar]
- 15.DHHS Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of health and human Services. [March 12, 2012];Panel on antiretroviral guidelines for adults and adolescents. 2011 Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf.
- 16.Luebbert J, Tweya H, Phiri S, et al. Virological failure and drug resistance in patients on antiretroviral therapy after treatment interruption in lilongwe, Malawi. Clin Infect Dis. 2012;55:441–448. doi: 10.1093/cid/cis438. [DOI] [PubMed] [Google Scholar]
- 17.Graham SM, Jalalian-Lechak Z, Shafi J, et al. Antiretroviral treatment interruptions predict female genital shedding of genotypically resistant HIV-1 RNA. J Acquir Immune Defic Syndr. 2012;60:511–518. doi: 10.1097/QAI.0b013e31825bd703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parienti JJ, Das-Douglas M, Massari V, et al. Not all missed doses are the same: sustained NNRTI treatment interruptions predict HIV rebound at low-to-moderate adherence levels. PLoS One. 2008;3:e2783. doi: 10.1371/journal.pone.0002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirschel B, Flanigan T. Is it smart to continue to study treatment interruptions? AIDS. 2009;23:757–759. doi: 10.1097/QAD.0b013e328321b791. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch MS, Gunthard HF, Schapiro JM, et al. Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an International AIDS Society-USA panel. Clin Infect Dis. 2008;47:266–285. doi: 10.1086/589297. [DOI] [PubMed] [Google Scholar]
- 21.Ncaca LN, Kranzer K, Orrell C. Treatment interruption and variation in tablet taking behaviour result in viral failure: a case-control study from Cape Town, South Africa. PLoS One. 2011;6:e23088. doi: 10.1371/journal.pone.0023088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Betsi NA, Koudou BG, Cisse G, et al. Effect of an armed conflict on human resources and health systems in Cote d'Ivoire: prevention of and care for people with HIV/AIDS. AIDS Care. 2006;18:356–365. doi: 10.1080/09540120500200856. [DOI] [PubMed] [Google Scholar]
- 23.Reid T, van Engelgem I, Telfer B, et al. Providing HIV care in the aftermath of Kenya's post-election violence Medecins Sans Frontieres’ lessons learned January–March 2008. Confl Health. 2008;2:15. doi: 10.1186/1752-1505-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kantor R, Diero L, Delong A, et al. Misclassification of first-line anti-retroviral treatment failure based on immunological monitoring of HIV infection in resource-limited settings. Clin Infect Dis. 2009;49:454–462. doi: 10.1086/600396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delong AK, Wu M, Bennett D, et al. Sequence quality analysis tool for HIV Type 1 protease and reverse transcriptase. AIDS Res Hum Retro-viruses. 2012;28:894–901. doi: 10.1089/aid.2011.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. [December 1, 2012];Stanford HIV Sequence Database. Available at: hivdb.stanford.edu.
- 27.Johnson VA, Calvez V, Gunthard HF, et al. 2011 update of the drug resistance mutations in HIV-1. Top Antivir Med. 2011;19:156–164. [PMC free article] [PubMed] [Google Scholar]
- 28.WHO Antiretroviral therapy for HIV infection in adults and adolescents 2010 revision. [March 3, 2012];Recommendations for a public health approach. 2010 Available at: www.who.int/hiv/pub/arv/adult2010/en/index.html. [PubMed]
- 29.WHO Antiretroviral therapy for HIV infection in adults and adolescents 2006 Revision. [March 2, 2012];Recommendations for a public health approach. 2006 Available at: http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf. [PubMed]
- 30.Holkmann Olsen C, Mocroft A, Kirk O, et al. Interruption of combination antiretroviral therapy and risk of clinical disease progression to AIDS or death. HIV Med. 2007;8:96–104. doi: 10.1111/j.1468-1293.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- 31.Kaufmann GR, Elzi L, Weber R, et al. Interruptions of cART limits CD4 T-cell recovery and increases the risk for opportunistic complications and death. AIDS. 2011;25:441–451. doi: 10.1097/QAD.0b013e3283430013. [DOI] [PubMed] [Google Scholar]
- 32.Wolf E, Hoffmann C, Procaccianti M, et al. Long-term consequences of treatment interruptions in chronically HIV-1-infected patients. Eur J Med Res. 2005;10:56–62. [PubMed] [Google Scholar]
- 33.Knobel H, Urbina O, Gonzalez A, et al. Impact of different patterns of nonadherence on the outcome of highly active antiretroviral therapy in patients with long-term follow-up. HIV Med. 2009;10:364–369. doi: 10.1111/j.1468-1293.2009.00696.x. [DOI] [PubMed] [Google Scholar]