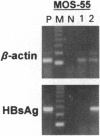
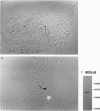
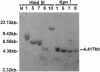
Abstract
The lack of efficient mechanisms for stable genetic transformation of medically important insects, such as anopheline mosquitoes, is the single most important impediment to progress in identifying novel control strategies. Currently available techniques for foreign gene expression in insect cells in culture lack the benefit of stable inheritance conferred by integration. To overcome this problem, a new class of pantropic retroviral vectors has been developed in which the amphotropic envelope is completely replaced by the G glycoprotein of vesicular stomatitis virus. The broadened host cell range of these particles allowed successful entry, integration, and expression of heterologous genes in cultured cells of Anopheles gambiae, the principle mosquito vector responsible for the transmission of over 100 million cases of malaria each year. Mosquito cells in culture infected with a pantropic vector expressing hygromycin phosphotransferase from the Drosophila hsp70 promoter were resistant to the antibiotic hygromycin B. Integrated provirus was detected in infected mosquito cell clones grown in selective media. Thus, pantropic retroviral vectors hold promise as a transformation system for mosquitoes in vivo.
Full text
PDF

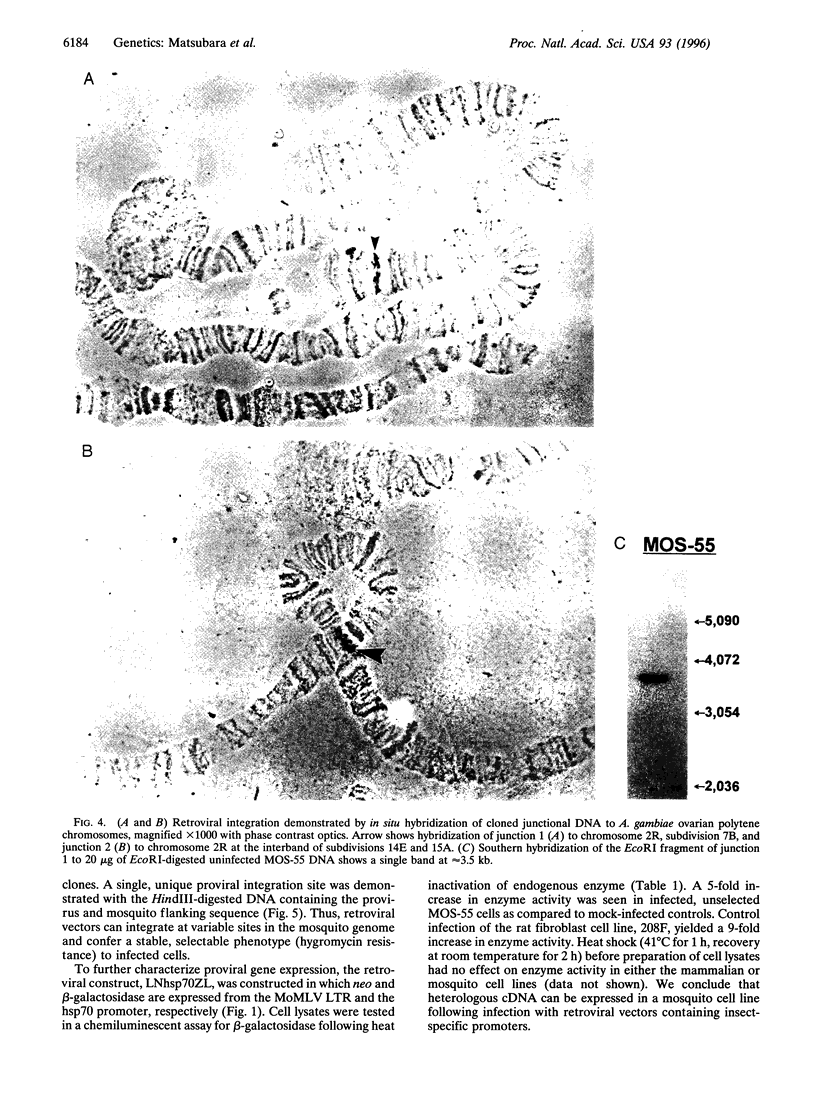

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam M. A., Ramesh N., Miller A. D., Osborne W. R. Internal initiation of translation in retroviral vectors carrying picornavirus 5' nontranslated regions. J Virol. 1991 Sep;65(9):4985–4990. doi: 10.1128/jvi.65.9.4985-4990.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. C., Friedmann T., Driever W., Burrascano M., Yee J. K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. C., Matsubara T., Lozinski G., Yee J. K., Friedmann T., Washabaugh C. H., Tsonis P. A. Pantropic retroviral vector-mediated gene transfer, integration, and expression in cultured newt limb cells. Dev Biol. 1994 Sep;165(1):285–289. doi: 10.1006/dbio.1994.1253. [DOI] [PubMed] [Google Scholar]
- Burns J. C., McNeill L., Shimizu C., Matsubara T., Yee J. K., Friedmann T., Kurdi-Haidar B., Maliwat E., Holt C. E. Retrovirol gene transfer in Xenopus cell lines and embryos. In Vitro Cell Dev Biol Anim. 1996 Feb;32(2):78–84. doi: 10.1007/BF02723038. [DOI] [PubMed] [Google Scholar]
- Emi N., Friedmann T., Yee J. K. Pseudotype formation of murine leukemia virus with the G protein of vesicular stomatitis virus. J Virol. 1991 Mar;65(3):1202–1207. doi: 10.1128/jvi.65.3.1202-1207.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon A. M. DNA-mediated gene transfer: applications to mosquitoes. Nature. 1991 Aug 29;352(6338):828–829. doi: 10.1038/352828a0. [DOI] [PubMed] [Google Scholar]
- Friedmann T. A brief history of gene therapy. Nat Genet. 1992 Oct;2(2):93–98. doi: 10.1038/ng1092-93. [DOI] [PubMed] [Google Scholar]
- Higgs S., Powers A. M., Olson K. E. Alphavirus expression systems: applications to mosquito vector studies. Parasitol Today. 1993 Dec;9(12):444–452. doi: 10.1016/0169-4758(93)90098-z. [DOI] [PubMed] [Google Scholar]
- Kumar V., Collins F. H. A technique for nucleic acid in situ hybridization to polytene chromosomes of mosquitoes in the Anopheles gambiae complex. Insect Mol Biol. 1994 Feb;3(1):41–47. doi: 10.1111/j.1365-2583.1994.tb00149.x. [DOI] [PubMed] [Google Scholar]
- Lin S., Gaiano N., Culp P., Burns J. C., Friedmann T., Yee J. K., Hopkins N. Integration and germ-line transmission of a pseudotyped retroviral vector in zebrafish. Science. 1994 Jul 29;265(5172):666–669. doi: 10.1126/science.8036514. [DOI] [PubMed] [Google Scholar]
- Marhoul Z., Pudney M. A mosquito cell line (MOS. 55) from Anopheles gambiae larva. Trans R Soc Trop Med Hyg. 1972;66(1):183–184. doi: 10.1016/0035-9203(72)90068-5. [DOI] [PubMed] [Google Scholar]
- McGrane V., Carlson J. O., Miller B. R., Beaty B. J. Microinjection of DNA into Aedes triseriatus ova and detection of integration. Am J Trop Med Hyg. 1988 Nov;39(5):502–510. doi: 10.4269/ajtmh.1988.39.502. [DOI] [PubMed] [Google Scholar]
- Mialhe E., Miller L. H. Biolistic techniques for transfection of mosquito embryos (Anopheles gambiae). Biotechniques. 1994 May;16(5):924–931. [PubMed] [Google Scholar]
- Miller L. H., Sakai R. K., Romans P., Gwadz R. W., Kantoff P., Coon H. G. Stable integration and expression of a bacterial gene in the mosquito Anopheles gambiae. Science. 1987 Aug 14;237(4816):779–781. doi: 10.1126/science.3039658. [DOI] [PubMed] [Google Scholar]
- Miyanohara A., Elam R. L., Witztum J. L., Friedmann T. Long-term transgene expression from genetically modified hepatocytes grafted to the rat liver. New Biol. 1992 Mar;4(3):261–267. [PubMed] [Google Scholar]
- Monroe T. J., Muhlmann-Diaz M. C., Kovach M. J., Carlson J. O., Bedford J. S., Beaty B. J. Stable transformation of a mosquito cell line results in extraordinarily high copy numbers of the plasmid. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5725–5729. doi: 10.1073/pnas.89.13.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar G., Turner R. T., Bolander M. E. Restriction-site PCR: a direct method of unknown sequence retrieval adjacent to a known locus by using universal primers. PCR Methods Appl. 1993 May;2(4):318–322. doi: 10.1101/gr.2.4.318. [DOI] [PubMed] [Google Scholar]
- Schloemer R. H., Wagner R. R. Mosquito cells infected with vesicular stomatitis virus yield unsialylated virions of low infectivity. J Virol. 1975 Apr;15(4):1029–1032. doi: 10.1128/jvi.15.4.1029-1032.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. A., Brogdon W. G., Collins F. H. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993 Oct;49(4):520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Boettiger D., Murphy H. M. Pseudotypes of avian sarcoma viruses with the envelope properties of vesicular stomatitis virus. Virology. 1977 Feb;76(2):808–825. doi: 10.1016/0042-6822(77)90261-6. [DOI] [PubMed] [Google Scholar]
- Wyers F., Blondel D., Petitjean A. M., Dezelee S. Restricted expression of viral glycoprotein in vesicular stomatitis virus-infected Drosophila melanogaster cells. J Gen Virol. 1989 Jan;70(Pt 1):213–218. doi: 10.1099/0022-1317-70-1-213. [DOI] [PubMed] [Google Scholar]
- Xiao H., Lis J. T. Germline transformation used to define key features of heat-shock response elements. Science. 1988 Mar 4;239(4844):1139–1142. doi: 10.1126/science.3125608. [DOI] [PubMed] [Google Scholar]
- Yee J. K., Friedmann T., Burns J. C. Generation of high-titer pseudotyped retroviral vectors with very broad host range. Methods Cell Biol. 1994;43(Pt A):99–112. doi: 10.1016/s0091-679x(08)60600-7. [DOI] [PubMed] [Google Scholar]
- Závada J. Pseudotypes of vesicular stomatitis virus with the coat of murine leukaemia and of avian myeloblastosis viruses. J Gen Virol. 1972 Jun;15(3):183–191. doi: 10.1099/0022-1317-15-3-183. [DOI] [PubMed] [Google Scholar]