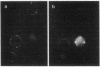
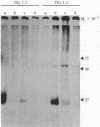
Abstract
We have transferred the gene coding for the Thy-1.2 alloantigen into a Thy-1.1-bearing T-cell lymphoma. BALB/c thymocyte DNA, precipitated with calcium phosphate, was used to effect the transfer. We report the stable transformation of lymphoid cells by total cellular DNA. To our knowledge, this has not been previously reported. Transient expression of the transfected gene could be detected by flow cytofluorometry, and 5% (range, 1.5%-11%) of the recipient cells had Thy-1.2 antigen detectable on their surface 48 hr after transfer. "Stable" transformants were isolated by repeatedly selecting for cells expressing the Thy-1.2 antigen, by use of fluorescence-activated cell sorting or "panning." No metabolic selection was required. The transferred gene, detected by Southern blotting, encoded a product that is indistinguishable from the normal antigen by immunoprecipitation and NaDod-SO4/PAGE.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang L. J., Gamble C. L., Izaguirre C. A., Minden M. D., Mak T. W., McCulloch E. A. Detection of genes coding for human differentiation markers by their transient expression after DNA transfer. Proc Natl Acad Sci U S A. 1982 Jan;79(1):146–150. doi: 10.1073/pnas.79.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao M. V., Mellon P., Charnay P., Maniatis T., Axel R. The regulated expression of beta-globin genes introduced into mouse erythroleukemia cells. Cell. 1983 Feb;32(2):483–493. doi: 10.1016/0092-8674(83)90468-3. [DOI] [PubMed] [Google Scholar]
- Coffino P., Knowles B., Nathenson S. G., Scharff M. D. Suppression of immunoglobulin synthesis by cellular hybridization. Nat New Biol. 1971 May 19;231(20):87–90. doi: 10.1038/newbio231087a0. [DOI] [PubMed] [Google Scholar]
- Corsaro C. M., Pearson M. L. Competence for DNA transfer of ouabain resistance and thymidine kinase: clonal variation in mouse L-cell recipients. Somatic Cell Genet. 1981 Sep;7(5):617–630. doi: 10.1007/BF01549663. [DOI] [PubMed] [Google Scholar]
- Deans R. J., Denis K. A., Taylor A., Wall R. Expression of an immunoglobulin heavy chain gene transfected into lymphocytes. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1292–1296. doi: 10.1073/pnas.81.5.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Goldsby R. A., Osborne B. A., Simpson E., Herzenberg L. A. Hybrid cell lines with T-cell characteristics. Nature. 1977 Jun 23;267(5613):707–708. doi: 10.1038/267707a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guerrero I., Calzada P., Mayer A., Pellicer A. A molecular approach to leukemogenesis: mouse lymphomas contain an activated c-ras oncogene. Proc Natl Acad Sci U S A. 1984 Jan;81(1):202–205. doi: 10.1073/pnas.81.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick S. M., Cohen D. I., Nielsen E. A., Davis M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984 Mar 8;308(5955):149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- Hyman R., Stallings V. Complementation patterns of Thy-1 variants and evidence that antigen loss variants "pre-exist" in the parental population. J Natl Cancer Inst. 1974 Feb;52(2):429–436. doi: 10.1093/jnci/52.2.429. [DOI] [PubMed] [Google Scholar]
- Hyman R., Trowbridge I. Analysis of lymphocyte surface antigen expression by the use of variant cell lines. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):407–415. doi: 10.1101/sqb.1977.041.01.049. [DOI] [PubMed] [Google Scholar]
- Kavathas P., Herzenberg L. A. Stable transformation of mouse L cells for human membrane T-cell differentiation antigens, HLA and beta 2-microglobulin: selection by fluorescence-activated cell sorting. Proc Natl Acad Sci U S A. 1983 Jan;80(2):524–528. doi: 10.1073/pnas.80.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loor F., Forni L., Pernis B. The dynamic state of the lymphocyte membrane. Factors affecting the distribution and turnover of surface immunoglobulins. Eur J Immunol. 1972 Jun;2(3):203–212. doi: 10.1002/eji.1830020304. [DOI] [PubMed] [Google Scholar]
- Lowy I., Pellicer A., Jackson J. F., Sim G. K., Silverstein S., Axel R. Isolation of transforming DNA: cloning the hamster aprt gene. Cell. 1980 Dec;22(3):817–823. doi: 10.1016/0092-8674(80)90558-9. [DOI] [PubMed] [Google Scholar]
- Mage M. G., McHugh L. L., Rothstein T. L. Mouse lymphocytes with and without surface immunoglobulin: preparative scale separation in polystyrene tissue culture dishes coated with specifically purified anti-immunoglobulin. J Immunol Methods. 1977;15(1):47–56. doi: 10.1016/0022-1759(77)90016-3. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Newman R., Domingo D., Trotter J., Trowbridge I. Selection and properties of a mouse L-cell transformant expressing human transferrin receptor. Nature. 1983 Aug 18;304(5927):643–645. doi: 10.1038/304643a0. [DOI] [PubMed] [Google Scholar]
- Pellicer A., Robins D., Wold B., Sweet R., Jackson J., Lowy I., Roberts J. M., Sim G. K., Silverstein S., Axel R. Altering genotype and phenotype by DNA-mediated gene transfer. Science. 1980 Sep 19;209(4463):1414–1422. doi: 10.1126/science.7414320. [DOI] [PubMed] [Google Scholar]
- Pellicer A., Wigler M., Axel R., Silverstein S. The transfer and stable integration of the HSV thymidine kinase gene into mouse cells. Cell. 1978 May;14(1):133–141. doi: 10.1016/0092-8674(78)90308-2. [DOI] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Lipsich L., Wigler M. Isolation of the chicken thymidine kinase gene by plasmid rescue. Nature. 1980 May 22;285(5762):207–210. doi: 10.1038/285207a0. [DOI] [PubMed] [Google Scholar]
- Renkawitz R., Beug H., Graf T., Matthias P., Grez M., Schütz G. Expression of a chicken lysozyme recombinant gene is regulated by progesterone and dexamethasone after microinjection into oviduct cells. Cell. 1982 Nov;31(1):167–176. doi: 10.1016/0092-8674(82)90416-0. [DOI] [PubMed] [Google Scholar]
- Rice D., Baltimore D. Regulated expression of an immunoglobulin kappa gene introduced into a mouse lymphoid cell line. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7862–7865. doi: 10.1073/pnas.79.24.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]