Abstract
Background: Since the liver metastases rather than the colorectal cancer itself is the main determinant of patient’s survival, the ‘Liver-First Approach (LFA)’ with upfront chemotherapy followed by a hepatic resection of colorectal liver metastases (CLM) and finally a colorectal cancer resection was proposed. The aim of this review was to analyse the evidence for LFA in patients with colorectal cancer and synchronous CLM.
Methods: A literature search of databases (MEDLINE and EMBASE) to identify published studies of LFA in patients with colorectal cancer and synchronous CLM was undertaken focussing on the peri-operative regimens of LFA and survival outcomes.
Results: Three observational studies and one retrospective cohort study were included for review. A total of 121 patients with colorectal cancer and synchronous CLM were selected for LFA. Pre-operative chemotherapy was used in 99% of patients. One hundred and twelve of the initial 121 patients (93%) underwent a hepatic resection of CLM. In total, 60% had a major liver resection and the R0 resection rate was 93%. Post-operative morbidity and mortality after the hepatic resection were 20% and 1%, respectively. Ultimately, 89 of the initial 121 (74%) patients underwent a colorectal cancer resection. Post-operative morbidity and mortality after a colorectal resection were 50% and 6%, respectively. The median overall survival was 40 months (range 19–50) with a recurrence rate of 52%.
Conclusions: Current evidence suggests that LFA is safe and feasible in selected patients with colorectal cancer and synchronous CLM. Future studies are required to further define patient selection criteria for LFA and the exact role of LFA in the management of synchronous CLM.
Introduction
Colorectal cancer is the third most common cancer worldwide.1 Up to 25% of patients have synchronous colorectal liver metastases (CLM) at presentation2,3 and such synchronous presentation has been associated with poor survival outcomes.4–6 Nevertheless, surgical resection of all tumour sites is considered the only curative therapy enabling long-term survival.4–6 The traditional surgical strategy for patients with resectable synchronous CLM is a two-stage approach that includes colorectal cancer resection followed by chemotherapy and delayed hepatic resection of the CLM. One disadvantage of this approach includes disease progression of CLM between the colorectal and hepatic surgery rendering the CLM unresectable.7 This is of particular concern in patients who develop post-operative complications after colorectal cancer resection, precluding the administration of chemotherapy and hepatic resection of CLM.8 In the last decade, simultaneous resection of colorectal cancer and CLM has been increasingly used in selected patients with comparable peri-operative morbidity and mortality as well as survival outcomes.9,10 This approach however is associated with increased post-operative complications when a major hepatic resection of CLM is performed.9 The benefit of peri-operative chemotherapy in patients undergoing a hepatic resection of CLM has been increasingly apparent. Indeed a recent multicentre randomized controlled trial of 5-fluorouracil (5-FU) monotherapy demonstrated an increased disease-free survival and a trend towards increased overall survival compared with a hepatic resection of CLM alone.11 With the introduction of several effective systematic cytotoxic and targeted agents, recent studies have also demonstrated an improved response rate and survival outcomes in patients with metastatic colorectal cancer.12 In patients with synchronous CLM, the optimal sequence of colorectal cancer resection, hepatic resection of CLM and systematic chemotherapy has not been clearly defined and remains controversial. Since the proposal that liver metastases rather than the colorectal primary may be the most common cause of a patient’s death, Mentha et al. 7 described the ‘Liver-First Approach (LFA)’ with chemotherapy given upfront followed by hepatic resection of CLM and finally colorectal cancer resection. This systematic review has been undertaken to assess the published evidence for the safety and feasibility of LFA in patients with synchronous CLM.
Methods
Literature search strategy
A search was undertaken of MEDLINE (1966 to July 2012) and EMBASE (January 1974 to July 2012) databases. The search terms colorectal cancer or colorectal neoplasm; liver metastases or hepatic metastases; hepatectomy or liver resection or hepatic resection; liver-first or reverse approach were used. These terms were mapped to MEDLINE Subject Headings (MESH) terms as well as being searched for as text items. Reference lists from relevant articles were searched for other potentially relevant studies.
Study selection
The study evaluation was performed by two reviewers (V.L. and J.L.). After the initial search, reviews, case reports, conference abstract, non-human studies or case series including less than 10 patients completing the LFA were excluded. Abstracts of the remaining studies were retrieved and then reviewed for relevance. The full text of the selected articles was thoroughly reviewed. Studies from which a decision could not be made based upon the abstract were also reviewed. Those studies which described the use of the LFA with curative intent in patients with colorectal cancer and synchronous CLM were included for analysis. Studies that adopted a hybrid approach combining liver resection with ablation techniques, or two-stage hepatectomy, or resection of extra-hepatic metastases with the aim of expanding the criteria for resection of CLM, were also included for review. Only studies reporting both short-and long-term outcomes of LFA were included. When multiple publications were identified from the same or overlapping patient series, only the most complete or recent publication was included. Study methodology quality was assessed according to the Newcastle–Ottawa scale.13 A score of four or more was required for inclusion.
Data extraction and critical appraisal
Two reviewers (V.L and J.L.) independently appraised each article using predefined criteria. Data extracted included the methodology, quality criteria, the setting of the use of peri-operative chemotherapy, the response to chemotherapy, the proportion of R0 resections, the overall survival (OS); the morbidity and mortality of this multimodality approach. Discrepancies were resolved by consensus. A major hepatectomy was defined as a resection of three or more Couinaud segments. Owing to the lack of a control group and the heterogeneity present amongst the selected studies, a meta-analysis could not be carried out. According to PRISMA guidelines,14 a systematic review was performed without a comparator group by full tabulation of the results. The level of evidence of each article was scored according to the Hierarchy of Evidence table.15
Results
The literature search using the above-described strategy identified 697 studies. Duplicated studies, non-human studies, review articles, case report and conference abstract were excluded. The manuscripts of the 13 remaining articles were reviewed. Nine articles not fulfilling the inclusion criteria were excluded. The remaining 4 studies were individually reviewed (Fig. 1). No meta-analyses or randomized control trials were identified.
Figure 1.
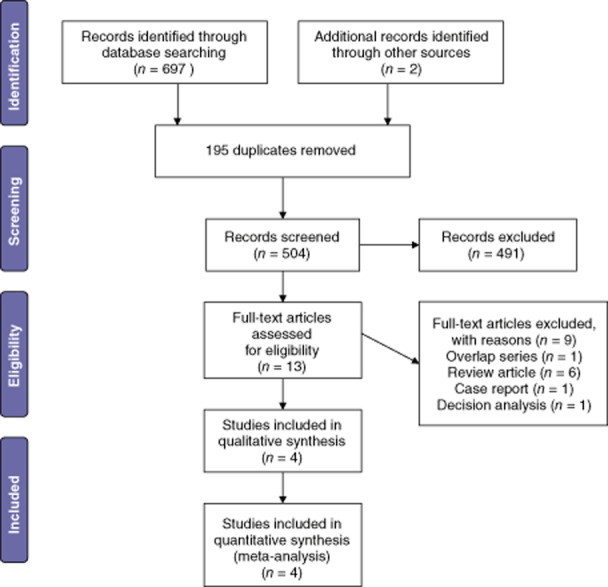
Flow chart showing the search strategy used to identify studies
This review comprised of 3 observational studies (level IV evidence) and 1 retrospective cohort (level III evidence) study. Overall, 121 patients with synchronous colorectal cancer and CLM were selected for the LFA.16–19 Two studies with overlapping patient series7,20 were excluded as well as a case report.21 Selection criteria for the LFA were reported in 4 studies and none of these studies used the same criteria (Table 1).16–19 One study considered all patients with primary rectal cancer with synchronous CLM19 whereas another study included only patients with locally advanced rectal cancer with synchronous CLM.17 Three of the four studies included patients with both colon or rectal cancer16,18,19 while one study included rectal cancer only.17 Three studies reported the proportion of rectal and colon cancer primary sites (Table 2) with a total of 70/86 (81%, range 68–100%) patients having primary rectal cancer. Three studies reported the proportion of patients with colonic symptoms, and a total of 39/40 (98%) were symptomatic at the time of presentation.16,17,19 Two studies defined the criteria of resectability of CLM16,19 according to a previous consensus conference of the surgical management of CLM.22 All four studies used modern pre-operative chemotherapy (Oxaliplatin or Irinotecan-based regimens ± bevacizumab and/or cetuximab) with a total of 99% (120/121) of patients treated.16–19 However, the dosage and duration of the pre-operative chemotherapy regimens were not uniform. One study also included pelvic radiation in the pre-operative chemotherapy phase before hepatic resection of CLM.19 The rate of response of CLM to pre-operative chemotherapy was reported in only one study with a complete response rate of 1/22, a partial response rate of 15/22 and a stable response rate of 6/22.17 All four studies reported colonic complications during pre-operative chemotherapy but only 6/120 (5%) (range, 3–10%) patients required an emergency colorectal cancer resection with no mortality.16–19 Overall, one patient died of fungal sepsis during the pre-operative chemotherapy phase.16
Table 1.
Setting of the studies reporting the results of the liver-first approach (LFA) in patients with synchronous colorectal liver metastases including the selection criteria of LFA
| Reference | Year of publication | Country | Setting | Study period | Selection criteria of ‘LFA’ |
|---|---|---|---|---|---|
| Mentha16 | 2008 | Switzerland | Prospective observational study | 1998–2007 |
|
| Verhoef17 | 2009 | Netherlands | Retrospective observational study | 2003–2007 |
|
| Brouquet18 | 2010 | United States | Retrospective cohort study | 1992–2009 |
|
| De Jong19 | 2011 | Netherlands | Retrospective Observational study | 2005–2010 |
|
Criteria of ‘Advanced Synchronous Colorectal Liver metastases’ was not clearly defined.
Table 2.
Patient characteristics, systemic chemotherapy and response to treatment
| Reference | Total patients selected initially for ‘Liver-first Approach’ | Rectal Primary n (%) | Colonic symptoms at the time of presentation n (%) | Preoperative chemotherapy n (%) | Chemotherapy regimens | Radiological response rate of chemotherapya n (%) | Colonic complications during chemotherapy n (%) |
|---|---|---|---|---|---|---|---|
| Mentha16 | 35 | NR | 0 (0) | 35 (100) | Oxalipatin, Irinotecan, 5-FU, leucovorin ± bevacizumab and/or cetuximab | NR | 1/35b (3) |
| Verhoef17 | 23 | 23 (100) | 23 (100) | 22 (96) | 5-FU/Capecitabine, Oxaliplatin or Irinotecan ± bevacizumab | 16/22 (73) | 1/22c (5) |
| Brouquet18 | 41 | 28 (68) | NR | 41 (100) | 5-FU, Oxaliplatin or Irinotecan ± bevacizumab and/or cetuximab | NR | 2/41d (5) |
| De Jong19 | 22 | 19 (86) | 16 (73) | 22 (100) |
|
NR | 2/22e (10) |
| Total percentage | 70/86 (81) | 39/80 (49) | 120/121 (99) | 6/120 (5) | |||
| Range | 68–100 | 0–100% | 3–10 | ||||
| Total | 121 |
Complete or partial response.
Colonic occlusion requiring an emergency Hartmann’s procedure.
Caecal perforation requiring an emergency colectomy.
Symptomatic requiring an emergency colostomy.
The surgical characteristics and peri-operative outcomes of the first stage (hepatic resection of CLM) of LFA are depicted in Table 3. Two studies reported the proportion of concomitant colorectal resection with a value of 7/31 and 1/22, respectively, in each study.16,17 A liver resection was performed in all four studies with a total of 112/121 (93%, range 83–100%) patients undergoing a liver resection.16,17 The R0 resection rate and proportion of major liver resections were reported in three studies with a total of 62/67 (93%, range 85–100%) and 40/67 (60%, range 32–89%) patients, respectively.17–19 Post-operative morbidity was reported in 3 studies with a total morbidity rate of 13/64 (20%) (range, 9–27%).17–19 Overall, there was one peri-operative mortality after the first stage of LFA.19 Interval chemotherapy ± pelvic radiation was reported in three studies with a total of 39/68 (57%, range 18–100%) patients receiving such treatment.17–19 The interval duration between the first and second stage of LFA was reported in two studies with a median interval duration of 4 weeks and 3 months, respectively, in each study.17,19
Table 3.
Surgical characteristics and peri-operative outcomes of a liver resection stage of the liver-first approach (LFA)
| Reference | Number of patients underwent liver resection n (%) | Synchronous liver and bowel resections n (%) | R0 resection n (%) | Major liver resection n (%) | Perioperative Morbidity n (%) | Perioperative Mortality n (%) | Interval chemotherapy ± radiation n (%) |
|---|---|---|---|---|---|---|---|
| Mentha16 | 31/35 (89) | 7 (23) | NR | NR | 5 (16) | 0 | NR |
| Verhoef17 | 19/23 (83) | 1 (5) | 19 (100) | 6 (32) | 2 (9) | 0 | 19 (100) |
| Brouquet18 | 41/41 (100) | NR | 23/27a (85) | 24/27a (89) | NR | 1/27a (4) | 16/27a (59) |
| De Jong19 | 21/22 (95) | NR | 20 (95) | 10 (48) | 6 (27) | 0 | 4 (18) |
| Total Percentage | 112/121 (93) | 62/67 (93) | 40/67 (60) | 13/64 (20) | 1/91 (1) | 39/68 (57) |
Twenty-seven of the initial 41 patients completed both liver and bowel resection; R0 and major liver resection rates and peri-operative outcomes were reported in these 27 patients.
Thirty of the initial 121 (25%) patients did not progress to the second stage of LFA. Interval disease progression (26/30) was the main reason followed by disappearing primary (2/30) and death before the second stage of LFA (2/30). The surgical characteristics and peri-operative outcomes of the second stage (colorectal cancer resection) of the LFA are depicted in Table 4. Eighty-nine of the initial 121 (74%) patients ultimately underwent a colorectal cancer resection. Post-operative morbidity was reported in two studies with a rate of 1/30 and 8/16, respectively.16,19 Post-operative mortality was reported in 3 studies and there was one [1/62(2%)] post-operative death.16,17,19
Table 4.
Surgical characteristics and peri-operative outcomes of bowel resection stage of the liver-first approach (LFA); and survival outcomes after completion of LFA
| Reference | Patients underwent bowel resection n (%) | Bowel Resection | Survival Outcomes in patients who completed both hepatic and bowel resection stages of the LFA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Perioperative morbidity n (%) | Perioperative mortality n (%) | Number of patients completed both stages of LFA | Median Follow-up (months) | Median OS (months) | Recurrence rate n (%) | 3-year OS (%) | 5-year OS (%) | ||
| Mentha16 | 30/35 (86) | 1/30 (3) | 0 | 30 | NR | 44 | 20/30 (68%) | 60 | 31 |
| Verhoef17 | 16/23 (70) | NR | 0 | 16 | 18 | 19 | 2/16 (13%) | 89 | 89 |
| Brouquet18 | 27/41 (66) | NR | NR | 27 | 25 | 50 | 19/27 (70%) | 79 | 39 |
| De Jong19 | 16/22a (73) | 8/16 (50) | 1/16 (6) | 18b | NR | 36 | 6/18 (33%) | 41 | NR |
| Total Percentage | 89/121 (74) | 91/121 (75) | 47/91 (52%) | ||||||
| Range | 13–70 | ||||||||
Excluding two patients who had a complete response of the rectal cancer after chemoradiation.
Survival outcomes included two patients who had a complete response of rectal cancer after chemoradiation and no bowel resection was thus performed.
OS, overall survival.
Overall, 91 of the initial 121 (75%) patients completed both stages of the LFA including 89 patients who underwent a colorectal cancer resection and 2 patients with disappearing rectal cancer primary after peri-operative chemoradiation. The survival outcomes after completion of LFA are depicted in Table 4. The follow-up time was reported in two studies with a median follow-up of 18 and 25 months, respectively.17,18 Survival outcomes were reported in all four studies. The median OS of patients who completed both stages of the LFA was reported in all 4 studies with values of 19, 36, 44 and 50 months, respectively.16–19 The recurrence rate was reported in all four studies with a total of 47/91 (52%) (range, 13–70%) patients suffering recurrence.16–19 Three-year OS post-completion of the LFA was reported in all 4 studies with values of 41%, 60%, 79% and 89% respectively16–19 whereas 5-year OS post-completion of LFA was reported in 3 studies with the value of 31%, 39% and 89%, respectively.16–18
Discussion
This systematic review demonstrates that LFA in selected patients with synchronous colorectal cancer and CLM is associated with low peri-operative morbidity and mortality and acceptable survival outcomes. The 3-year OS ranged from 41% to 89% whereas the 5-year OS was 31% to 89%. These outcomes are comparable with those of a hepatic resection in selected patients with resectable CLM after the colorectal cancer resection.4–6 Although the LFA has been discussed widely among hepatobiliary surgeons in international and regional hepatobiliary surgery conferences,23,24 only four surgical series with a total of 91 patients who completed the LFA were identified and thus the results should be interpreted with caution.
Although the value of a hepatic resection for CLM has never been demonstrated in a prospective randomized controlled trial, numerous surgical series have demonstrated the possibility of long-term survival. Additionally, no other treatment apart from a liver resection has shown a survival plateau. Recently, a number of case series describing 10-year actual survivors after a liver resection of CLM have also been published.5,6 These results support liver resection as the standard practice as well as the only curative treatment for CLM.
In this systematic review, there were no uniform patient selection criteria for the LFA and it is a significant limitation to the critical evaluation of outcomes of this approach. The majority of the selected patients who had rectal cancer [70/86 (81%) and 39/80 (49%)] had colonic symptoms at the time of presentation. Two of the 4 studies made reference to the recent consensus conference CLM resectability criteria as part of the patient selection criteria. It was unclear whether the remaining two studies included patients with definitely resectable, borderline resectable or unresectable CLM or a combination of these three. Pre-operative chemotherapy with oxaliplatin-or irinotecan-based regimens with bevacizumab and/or cetuximab were administered in 120/121 (99%) of patients and appeared to be an integral part of the LFA. This was followed by a hepatic resection in 112/121 (93%) of patients and ultimately colorectal cancer resection in 89/121 (74%) of patients. Thirty out of 121 (25%) patients did not complete all stages of the LFA with disease progression being the most common reason.
The timing and sequence of therapeutic interventions in patients presenting with synchronous CLM has not been clearly defined and the decision whether to go ‘Bowel-First’, ‘Simultaneous’ or ‘Liver-First’ remains controversial.25 For patients presenting with severe symptoms related to the intact colorectal cancer, the decision to resect the colorectal cancer first is straightforward.26 However, the intact colorectal cancer is asymptomatic in the majority of patients. The traditional ‘Bowel First’ approach is a two-stage procedure that includes colorectal cancer resection followed by delayed hepatic resection of CLM. Arguments for an initial colorectal cancer resection in these patients include: (i) if the tumour obstructs, bleeds or perforates, emergency surgery is required, which is associated with a higher peri-operative morbidity and mortality; and27 (ii) the interval time period between a colorectal resection and hepatic resection may allow occult systematic disease to become detectable.28 Patients with disease progression rendering the disease unresectable can thus be spared the peri-operative morbidity and mortality of futile hepatic surgery.
Several arguments however have been posited against upfront colorectal resection: (i) over the past decade, peri-operative chemotherapy followed by a hepatic resection is increasingly performed in patients with resectable CLM. In a randomized controlled study, 5-FU-based peri-operative chemotherapy followed by a hepatic resection was associated with increased disease-free survival and a trend towards increased overall survival when compared with a hepatic resection alone.11 Furthermore, modern chemotherapy regimens using a combination of 5-FU plus oxaliplatin and/or irinotecan has produced even better response rates with a partial response rate up to 50% and a median survival approaching 2 years in patients with unresectable metastatic colorectal cancer.29,30 The addition of biological agents such as bevacizumab and cetuximab has been shown to further improve the response rate;31,32 (ii) in patients with intact colorectal cancer and synchronous CLM, it has been shown that pre-operative chemotherapy with oxaliplatin-and/or irinotecan-based regimens induced a major histological response of 70% in colorectal cancer. Such a response was also significantly correlated with a response of the corresponding CLM; (iii) recent studies demonstrated that colorectal cancer resection in patients with metastatic colorectal cancer was associated with a significantly higher 30-day mortality of 10% when compared with a colorectal cancer resection in the non-metastatic setting; 33,34 (iv) the potential colonic complications of leaving the colorectal cancer intact may have been overstated. The rate of surgical intervention for colonic complications was only 20% in one review of 255 patients with metastatic colorectal cancer treated primarily with 5-FU.27 Poultsides et al. 27 published the Memorial Sloan-Kettering Cancer Centre experience of 233 patients with metastatic colorectal cancer with an intact primary tumour. The incidence of colonic complications was 7% with the use of modern oxaliplatin-or irinotecan-based chemotherapy. A recent multi-institutional NSABP Trial C-10 prospectively followed 86 patients with asymptomatic colon cancer and unresectable metastases. All patients were treated with infusional 5-FU, leucovorin and oxaliplatin (mFOLFOX6) combined with bevacizumab. The incidence of a significant colonic complication was 12/86 (14%) with 10 patients requiring surgery (8 for an obstruction, 1 for perforation and 1 for abdominal pain) and 2 patients died with symptoms of colon cancer;35 (v) even if patients develop symptoms related to advanced colorectal cancer, endoscopic treatment has been increasingly used. One systematic review demonstrated that self-expanding metallic stents were safe and effective for relieving malignant colorectal obstruction with a median clinical success rate of 92%.36 Endoscopic ablative therapies might also be useful in controlling haemorrhagic symptoms;37 (vi) an anastomotic leak after colorectal resection is not uncommon. The rate of an anastomotic leak after a rectal resection in particular was up to 12%, with a morbidity rate of up to 50%. Such complications could lead to a delay or even cancellation of both hepatic surgery and chemotherapy. One study also demonstrated that up to 50% of patients did not undergo further optimal post-operative chemotherapy treatment because of post-operative complications after rectal surgery; (vii) metastatic disease rather than primary colorectal cancer has been proposed to be the main determinant of patient survival and thus treatment of the CLM should be the first priority.7,17 One systematic review also demonstrated that downstaging chemotherapy could convert 22.5% of patients with initially unresectable CLM into ‘resectable’.38 On this basis many argue that modern chemotherapy should be administered first and not delayed by the colorectal cancer resection, especially if surgical complications occur.25 All these findings lend support to the LFA with upfront modern chemotherapy followed by hepatic resection in patients with synchronous asymptomatic colorectal cancer and CLM. This conclusion is further supported by a decision analysis demonstrating that the optimal management in patients with colorectal cancer and synchronous resectable CLM should be upfront systemic chemotherapy.39
The majority of patients in this review of LFA received up-front chemotherapy. Although peri-operative chemotherapy (both before and after liver resection) is associated with improved oncological outcomes, it appears to come at the cost of increased post-operative morbidity.11 However, given that no high-quality study compares pre-and post-operative chemotherapy to post-operative chemotherapy alone, the optimal sequence of liver surgery, colonic resection and systemic chemotherapy in synchronous CLM is as yet unresolved. Consideration should be give in particular to liver damage owing to chemotherapy in the form of steohepatitis (associated with irinotecan exposure) and sinusoidal injury (linked to oxaliplatin administration). This may increase the risk of liver failure after a resection, particularly if extended in nature. A liver first approach, prior to systemic treatment, circumvents these issues and may reduce the risks of post-operative surgical complications.
In the past decade, a simultaneous colorectal cancer resection and hepatic resection of CLM has been increasingly performed. Brouquet et al.18 compared the ‘Bowel First’, ‘Simultaneous’ and ‘Liver First’ approaches in the management of 156 consecutive patients with colorectal cancer and synchronous CLM and found an equivalent peri-operative morbidity and mortality as well as survival outcome among all three strategies. One systematic review comparing simultaneous and staged resection for synchronous CLM also demonstrated the safety and efficacy of the simultaneous approach in selected patients with comparable oncological outcomes and fewer overall complications.10 The major limitation of this review was that all the included studies were retrospective. In addition, the patients who underwent simultaneous colorectal and hepatic resection were more likely to have a right-sided primary tumour, fewer and smaller synchronous CLM and were more likely to require a minor hepatic resection. Nevertheless, if a simultaneous resection can be performed without added morbidity and mortality, the potential benefits of one surgical procedure over two are clear with a shorter total hospital stay and thus less financial costs.40
In this study, a pooled analysis was conducted where the data from observational studies were combined as if it were derived from a single sample. The application of any formal meta-analytic methods, particularly simple pooling, to observational studies has been controversial.41 Although combining data by meta-analytic methods is preferable, it was not feasible in this study.42 It must be acknowledged that the studies in this review each include a small number of patients and that there is considerable heterogeneity evident in their design. Nevertheless, pooling of data provides an indication of real-world outcomes, but its results must be interpreted with caution.
In conclusion, this systematic review demonstrated that LFA is safe and feasible in selected patients with colorectal cancer and synchronous CLM and is associated with acceptable peri-operative and survival outcomes The experience of LFA is in its infancy and future prospective controlled studies are required to further define the patient selection criteria for LFA and the exact role of LFA in the management of synchronous CLM.
Conflicts of interest
None declared.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.McMillan DC, McArdle CS. Epidemiology of colorectal liver metastases. Surg Oncol. 2007;16:3–5. doi: 10.1016/j.suronc.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Köhne C-H, Hitre E, Zaluski J, Chang Chien C-R, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 4.Chua TC, Saxena A, Chu F, Zhao J, Morris DL. Predictors of cure after hepatic resection of colorectal liver metastases: an analysis of actual 5-and 10-year survivors. J Surg Oncol. 2011;103:796–800. doi: 10.1002/jso.21864. [DOI] [PubMed] [Google Scholar]
- 5.Pulitano C, Castillo F, Aldrighetti L, Bodingbauer M, Parks RW, Ferla G, et al. What defines ‘cure’ after liver resection for colorectal metastases? Results after 10 years of follow-up. HPB. 2010;12:244–249. doi: 10.1111/j.1477-2574.2010.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomlinson JS, Jarnagin WR, Dematteo RP, Fong Y, Kornprat P, Gönen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 7.Mentha G, Majno PE, Andres A, Rubbia-Brandt L, Morel P, Roth AD. Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br J Surg. 2006;93:872–878. doi: 10.1002/bjs.5346. [DOI] [PubMed] [Google Scholar]
- 8.Law WL, Choi HK, Lee YM, Ho JW. The impact of postoperative complications on long-term outcomes following curative resection for colorectal cancer. Ann Surg Oncol. 2007;14:2559–2566. doi: 10.1245/s10434-007-9434-4. [DOI] [PubMed] [Google Scholar]
- 9.Reddy SK, Pawlik TM, Zorzi D, Gleisner AL, Ribero D, Assumpcao L, et al. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol. 2007;14:3481–3491. doi: 10.1245/s10434-007-9522-5. [DOI] [PubMed] [Google Scholar]
- 10.Hillingsø JG, Wille-Jørgensen P. Staged or simultaneous resection of synchronous liver metastases from colorectal cancer – a systematic review. Colorectal Dis. 2009;11:3–10. doi: 10.1111/j.1463-1318.2008.01625.x. [DOI] [PubMed] [Google Scholar]
- 11.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehmann K, Rickenbacher A, Weber A, Pestalozzi BC, Clavien P-A. Chemotherapy before liver resection of colorectal metastases. Ann Surg. 2012;255:237–247. doi: 10.1097/SLA.0b013e3182356236. [DOI] [PubMed] [Google Scholar]
- 13.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. Third Symposium on Systematic Reviews: beyond the Basics. Oxford: 2000. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. July. [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 15.National Health and Medical Research Council. A Guide to the Development, Implementation and Evaluation of Clinical Practice Guidelines. Canberra, ACT: AusInfo; 1999. [Google Scholar]
- 16.Mentha G, Roth AD, Terraz S, Giostra E, Gervaz P, Andres A, et al. The ‘Liver first’ approach in the treatment of colorectal cancer with synchronous liver metastases. Dig Surg. 2008;25:430–435. doi: 10.1159/000184734. [DOI] [PubMed] [Google Scholar]
- 17.Verhoef C, van der Pool AEM, Nuyttens JJ, Planting AST, Eggermont AMM, de Wilt JHW. The liver-first approach for patients with locally advanced rectal cancer and synchronous liver metastases. Dis Colon Rectum. 2009;52:23–30. doi: 10.1007/DCR.0b013e318197939a. [DOI] [PubMed] [Google Scholar]
- 18.Brouquet A, Mortenson MM, Vauthey JN, Rodriguez-Bigas MA, Overman MJ, Chang GJ, et al. Surgical strategies for synchronous colorectal liver metastases in 156 consecutive patients: classic, combined or reverse strategy? J Am Coll Surg. 2010;210:934–941. doi: 10.1016/j.jamcollsurg.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 19.De Jong MC, van Dam RM, Maas M, Bemelmans MHA, Olde Damink SWM, Beets GL, et al. The liver-first approach for synchronous colorectal liver metastasis: a 5-year single-centre experience. HPB. 2011;13:745–752. doi: 10.1111/j.1477-2574.2011.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Pool AEM, de Wilt JHW, Lalmahomed ZS, Eggermont AMM, Ijzermans JNM, Verhoef C. Optimizing the outcome of surgery in patients with rectal cancer and synchronous liver metastases. Br J Surg. 2010;97:383–390. doi: 10.1002/bjs.6947. [DOI] [PubMed] [Google Scholar]
- 21.Radunz S, Heuer M, Trarbach T, Mathe Z, Baba HA, Paul A, et al. Long-term survival after ‘liver first’ approach for locally advanced rectal cancer and synchronous liver metastases. Int J Colorectal Dis. 2011;26:1219–1220. doi: 10.1007/s00384-010-1101-8. [DOI] [PubMed] [Google Scholar]
- 22.Charnsangavej C, Clary B, Fong Y, Grothey A, Pawlik TM, Choti MA. Selection of patients for resection of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1261–1268. doi: 10.1245/s10434-006-9023-y. [DOI] [PubMed] [Google Scholar]
- 23.Ayez N, Van Der Pool A, Burger JWA, Eggermont AMM, Ijzermans JNM, Verhoef C. The long term results of the liver first approach in patients with locally advanced rectal cancer and synchronous liver metastases. Eur J Cancer. 2011;47:S409. doi: 10.1097/DCR.0b013e318279b743. [DOI] [PubMed] [Google Scholar]
- 24.Van Der Pool AE, De Wilt JH, Nuytens JJ, Eggermont AM, Verhoef C. The liver-first approach for patients with locally advanced rectal cancer and synchronous liver metastases: long-term results. J Clin Oncol. 2010;28(15 Suppl):e14027. [Google Scholar]
- 25.Zitt M. Bowel first? Simultaneous resection? Liver first? Treatment options in patients with colorectal cancer and resectable synchronous liver metastases. Mag Eur Med Oncol. 2011;4:79–81. [Google Scholar]
- 26.Damjanov N, Weiss J, Haller DG. Resection of the primary colorectal cancer is not necessary in nonobstructed patients with metastatic disease. Oncologist. 2009;14:963–969. doi: 10.1634/theoncologist.2009-0022. [DOI] [PubMed] [Google Scholar]
- 27.Poultsides GA, Servais EL, Saltz LB, Patil S, Kemeny NE, Guillem JG, et al. Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol. 2009;27:3379–3384. doi: 10.1200/JCO.2008.20.9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambert LA, Colacchio TA, Barth RJ. Interval hepatic resection of colorectal metastases improves patient selection. Arch Surg. 2000;135:473–479. doi: 10.1001/archsurg.135.4.473. [DOI] [PubMed] [Google Scholar]
- 29.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 30.Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 31.Folprecht G, Gruenberger T, Bechstein WO, Raab R, Lordick F, Hartmann JT, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjavant chemotherapy with cetuximan: the CELIM randomized phase 2 trial. Lancet Oncol. 2010;11:38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 32.Masi G, Loupakis F, Salvatore L, Fornaro L, Cremolini C, Cupini S, et al. Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first-line treatment for metastatic colorectal cancer: a phase 2 trial. Lancet Oncol. 2010;11:845–852. doi: 10.1016/S1470-2045(10)70175-3. [DOI] [PubMed] [Google Scholar]
- 33.Temple LKF, Hsieh L, Wong WD, Saltz L, Schrag D. Use of surgery among elderly patients with stage IV colorectal cancer. J Clin Oncol. 2004;22:3475–3484. doi: 10.1200/JCO.2004.10.218. [DOI] [PubMed] [Google Scholar]
- 34.Stillwell AP, Buettner PG, Siu SK, Stitz RW, Stevenson AR, Ho YH. Predictors of postoperative mortality, morbidity, and long-term survival after palliative resection in patients with colorectal cancer. Dis Colon Rectum. 2011;54:535–544. doi: 10.1007/DCR.0b013e3182083d9d. [DOI] [PubMed] [Google Scholar]
- 35.McCahill LE, Yothers G, Sharif S, Petrelli NJ, Lai LL, Bechar N, et al. Primary mFOLFOX6 plus bevacizumab without resection of the primary tumor for patients presenting with surgically unresectable metastatic colon cancer and an intact asymptomatic colon cancer: definitive analysis of NSABP trial C-10. J Clin Oncol. 2012;30:3223–3228. doi: 10.1200/JCO.2012.42.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watt AM, Faragher IG, Griffin TT, Rieger NA, Maddern GJ. Self-expanding metallic stents for relieving malignant colorectal obstruction. Ann Surg. 2007;246:24–30. doi: 10.1097/01.sla.0000261124.72687.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tebbutt NC, Norman AR, Cunningham D, Hill ME, Tait D, Oates J, et al. Intestinal complications after chemotherapy for patients with unresected primary colorectal cancer and synchronous metastases. Gut. 2003;52:568–573. doi: 10.1136/gut.52.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lam VWT, Spiro C, Laurence JM, Johnston E, Hollands MJ, Pleass HCC, et al. A systematic review of clinical response and survival outcomes of downsizing systemic chemotherapy and rescue liver surgery in patients with initially unresectable colorectal liver metastases. Ann Surg Oncol. 2012;19:1292–1301. doi: 10.1245/s10434-011-2061-0. [DOI] [PubMed] [Google Scholar]
- 39.Aloia TA, Fahy BN. A decision analysis model predicts the optimal treatment pathway for patients with colorectal cancer and resectable synchronous liver metastases. Clin Colorectal Cancer. 2008;7:197–201. doi: 10.3816/CCC.2008.n.026. [DOI] [PubMed] [Google Scholar]
- 40.Abbott DE, Cantor SB, Hu C-Y, Aloia TA, You YN, Nguyen S, et al. Optimizing clinical and economic outcomes of surgical therapy for patients with colorectal cancer and synchronous liver metastases. J Am Coll Surg. 2012;215:262–270. doi: 10.1016/j.jamcollsurg.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blettner M, Sauerbrei W, Schlehofer B, Scheuchenpflug T, Friedenreich C. Traditional reviews, meta-analyses and pooled analyses in epidemiology. Int J Epidemiol. 1999;28:1–9. doi: 10.1093/ije/28.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Bravata DM, Olkin I. Simple pooling versus combining in meta-analysis. Eval Health Prof. 2001;24:218–230. doi: 10.1177/01632780122034885. [DOI] [PubMed] [Google Scholar]


