Abstract
Objectives: Neoadjuvant chemoradiotherapy (CRT) is a viable treatment strategy for patients with pancreatic cancer. This study was conducted to evaluate the Virginia Mason Protocol (5-fluorouracil, cisplatin, interferon-α and radiation) given in the neoadjuvant setting for the treatment of locally advanced pancreatic cancer.
Methods: A Phase II pilot study evaluating interferon-based neoadjuvant CRT in patients with locally advanced pancreatic cancer was performed.
Results: A total of 23 patients were enrolled. The mean age of the patients was 58.6 years. Of the 23 patients, seven (30.4%) completed all treatments. In the remaining 16 (69.6%) patients, treatment was interrupted as a result of toxicity. The most commonly reported effects of toxicity were leucopoenia/cytopoenia (n = 19, 82.6%) and gastrointestinal effects (n = 19, 82.6%). Surgical resection was successful in seven (30.4%) patients. Margins were negative in six (85.7%) of these seven patients. Positive lymph nodes were identified in three (42.9%) of seven patients. Overall survival was 11.5 months. Surgery provided improved survival (22.6 months) compared with CRT alone (8.8 months). Disease-free survival in resected patients was 17.2 months.
Conclusions: Interferon-based neoadjuvant CRT may allow for resection of locally advanced pancreatic cancer, but with significant toxicity. In the absence of surgical resection, survival remains dismal.
Introduction
Pancreatic cancer is a highly lethal disease; an estimated 43 140 new cases are diagnosed and 36 800 deaths occur in the USA each year. Of newly diagnosed patients, approximately 80% will have inoperable disease at presentation as a result of locally advanced stage or metastases.1 As surgery offers the only possibility of cure, efforts have been made to increase the percentage of patients with locally advanced disease who are ultimately able to undergo resection with curative intent. Multiple investigators have reported rates of successful resection following neoadjuvant 5-fluorouracil (5-FU)-based chemoradiotherapy (CRT) that range from <3% to 30%.2–7 Snady and colleagues used cisplatin in combination neoadjuvant CRT in patients with locally advanced disease and found the combination to be associated with resectability rates of >80%.7 Subsequent to this promising report, several additional small trials have reported responses to neoadjuvant cisplatin-based CRT in the order of 45% to nearly 60%.8,9
Interferon-α (IFN-α), a cytokine with direct and indirect antitumor effects, has shown promising improvements in survival in multimodal adjuvant therapy. This was first reported by the Virginia Mason Medical Center (VMMC) group, who found a statistically significant improvement in survival over 5-FU-based adjuvant therapy at 26 months of follow-up, with 84% actuarial survival at 2 years.10 A subsequent Phase II study by Linehan and colleagues using combination CRT with post-radiation gemcitabine instead of 5-FU resulted in a 2-year actuarial survival rate of 56%,11 which is identical to that reported by Picozzi and colleagues in the multicentre Phase II ACOSOG Z05031 trial.12 The regimen initially described by the VMMC group includes IFN, cisplatin, 5-FU and radiation given concurrently (for 5.5–6.0 weeks), followed by continuous infusion of 5-FU (two courses of 6 weeks each).10
Given the remarkable outcomes reported by the VMMC, the present authors proposed to use this regimen in the preoperative setting in patients with locally advanced unresectable pancreas cancers in an effort to improve resectability rates. The present report describes a single-arm, Phase II trial of IFN-based chemoradiation given in the neoadjuvant setting. The study hypothesis was that neoadjuvant IFN-based CRT delivered as in the ACOSOG Z05031 trial would result in improved rates of resectability and provide superior overall survival (OS).
Materials and methods
The primary objective of this single-arm, Phase II pilot study was to determine the effect of neoadjuvant therapy with IFN, cisplatin, 5-FU and radiation in converting patients with locally advanced adenocarcinoma of the pancreas to resectable status. The secondary objectives were to determine the rate and severity of early and late toxicities, to improve the surgical morbidity profile and OS in resected patients, and to determine intent-to-treat overall and disease-free survival.
Initial enrolment to the study included patients with locally advanced pancreas cancer that was unresectable as a result of vascular involvement of tumour. Tumours were considered to be unresectable if there was evidence of arterial involvement, including encasement of the superior mesenteric artery (SMA) or major branches of the coeliac axis, or venous encasement not amenable to resection and reconstruction. As the trial progressed, enrolment was expanded to include patients with what would currently be considered ‘borderline resectable tumours’. These included cases of arterial abutment, but not encasement (<180 ° involvement) or portal venous involvement that was deemed to be not readily resectable by multidisciplinary review. The protocol was reviewed and approved by the Clinical Trials Office Cancer Protocol Review Committee of the University of Minnesota.
Patient enrolment
Patients aged ≥18 years with a minimum life expectancy of 12 weeks were eligible for inclusion if they had been diagnosed with locally advanced, non-metastatic (M0) adenocarcinoma of the pancreas with staging by computed tomography (CT) and endoscopic ultrasound (EUS). Metastatic disease was excluded by CT of the chest, EUS and CT with i.v. contrast [or magnetic resonance imaging (MRI)] of the abdomen or pelvis within 30 days prior to registration. Enrolled patients were required to have started treatment within 60 days of diagnosis. Patients were also required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1 documented within 14 days prior to registration, and to have adequate haematologic, renal and hepatic parameters, including a white blood cell (WBC) count of >3000 cells/mm3, absolute neutrophil count (ANC) of >1500/mm3, a platelet count of ≥100 000/mm3, haemoglobin of ≥9.5 g/dl, serum creatinine within 1.5 times the institutional upper limit of normal (ULN), total bilirubin of ≤3 mg/dl, aspartate aminotransferase (AST) within four times the institutional ULN, alanine aminotransferase (ALT) within four times the institutional ULN and alkaline phosphatase within four times the institutional ULN. Additionally, patients with reproductive potential were required to use effective contraception during treatment and for 3 months following chemotherapy. Female patients with reproductive potential were required to demonstrate a negative pregnancy test within 7 days of the initiation of treatment and were forbidden to breastfeed. Any patient with a prior diagnosis of cancer was required to satisfy all of the following criteria: receipt of potentially curative treatment; no evidence of prior malignancies for ≥5 years (excluding non-melanoma skin cancer, and breast or cervical carcinoma in situ), and no evidence of recurrence. Patients must also have been able to give informed consent.
Patients were excluded if they failed to meet any of the inclusion criteria and for pancreatic tumour histologies of adenosquamous carcinoma, ampullary carcinoma, carcinoid, cystadenoma or carcinoma, distal common bile duct carcinoma, duodenal carcinoma and islet cell carcinoma. A history of chronic immunotherapy for collagen vascular disease or another chronic immunologic abnormality was also an exclusion criterion. Finally, patients were excluded if they required concomitant use of any of granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage CSF (GM-CSF), oprevelkin, aminoglycoside antibiotics, loop diuretics, lithium, anticonvulsants, dexamethasone, theophylline, other myelosuppressive agents, and halogenated antiviral agents.
Study procedures and treatments
All patients underwent staging investigations with CT, EUS or MRI at diagnosis to rule out metastatic disease. All chemotherapy and radiotherapy were delivered at the University of Minnesota Medical Center. Figure 1 describes the treatment strategy. All patients were scheduled to receive one cycle of combination CRT followed by two cycles of chemotherapy alone. Resectability was comprehensively re-evaluated after each cycle. For the purposes of this study, patients required a central venous catheter (CVC) (e.g. PortaCath, Hickman, PICC), which was removed following the completion of therapy. Doses were modified before each subsequent cycle of treatment based on a recalculation of body surface area.
Figure 1.
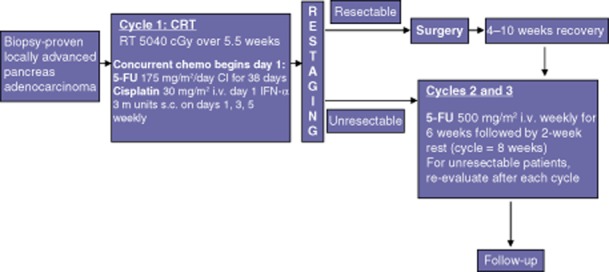
Treatment protocol in the current Phase II trial of neoadjuvant chemoradiotherapy (CRT) in patients with unresectable pancreatic cancers. RT, radiotherapy; 5-FU, 5-fluorouracil; CI, continuous infusion
Combination chemotherapy
All patients began chemotherapy within 60 days of enrolment. The regimen of cycle 1 included 5-FU administered by continuous infusion through a CVC using an ambulatory infusion pump at 175 mg/m2/day for 38 consecutive days (days 1–38), unless limited by toxicity. Cisplatin was infused on the first day only of each week for 6 weeks (i.e. days 1, 8, 15, 22, 29, 36). IFN-α-2b was given at 3 million units subcutaneously on days 1, 3 and 5 of each week until day 38. Figure 1 summarizes the treatment schedule.
Radiotherapy
Total radiation of 50.4 Gy was given concurrently with cycle 1 of combination chemotherapy. Radiation therapy was given in 28 fractions, at 180 cGy/fraction daily, on Monday–Friday, for 38 days.
Restaging
Patients were restaged by CT scanning at 2 weeks after the completion of cycle 1 and 2 weeks after each subsequent cycle as required. In the absence of metastatic disease, special attention was paid to the locally advanced tumour, taking into consideration growth and regression as they relate to resectability. If resectability was still undefined, a repeat EUS was performed by the original practitioner (if possible) and any pertinent tissues were sampled as required. If resection was deemed possible after multidisciplinary review, surgery was recommended. This protocol was repeated after each cycle to determine resectability in patients who did not respond to therapy, but had no demonstrable metastatic disease.
Surgery
Surgical exploration started with a diagnostic laparoscopy. If no evidence of carcinomatosis, liver metastases or other metastatic disease was encountered, a laparotomy was performed. In the absence of clear technical unresectability, a pancreaticoduodenectomy, distal or total pancreatectomy (and resection of any involved structures) was performed as mandated by the tumour anatomy. The operative findings, complications and histopathology were recorded.
Cycles 2 and 3 of 5-FU
In patients who underwent surgery, cycle 2 of 5-FU began at 4–10 weeks following recovery from surgery. If patients were found to be unresectable after cycle 1, post-CRT chemotherapy began in 4 weeks. Patients were given two cycles of chemotherapy with a 2-week rest between cycles. A bolus infusion of 5-FU at 500 mg/m2/week was administered once per week for 6 weeks (on days 1, 8, 15, 22, 29 and 35 of each cycle) and followed by rest and restaging (Fig. 1).
Dose modifications
Toxicity and adverse events were classified according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0. The intent was to give a full course of treatment that followed the treatment plan as closely as possible and to make up any missed doses. During cycle 1, if one modality (chemotherapy or radiotherapy) was withheld as a result of toxicity, the other modality was also withheld. Both were resumed following the resolution of toxicity. If three or more separate episodes of grade 3 or 4 non-haematologic toxicity occurred during cycle 1, or three or more separate episodes of grade 3 or 4 toxicity occurred during cycles 2 and 3, study treatment was terminated in the affected patient.
Statistical analysis
This was a Phase II clinical trial designed to determine if the therapy could convert patients with locally advanced pancreatic cancer to candidacy for surgery without excessive toxicity. The primary endpoint was tumour response (resectability). The secondary endpoints included toxicity and survival. Simon’s two-stage design is often used in Phase II clinical trials if only a primary endpoint is considered. However, the present study aimed not only to establish the tumour response rate, but also to gather additional information about the toxicity associated with the treatment. Therefore, Bryant and Day’s two-stage design, which extends Simon’s approach, was used to monitor both resectability and toxicity. The unacceptable response rate PR0 was determined to be 0.05 and the acceptable response rate PR1 to be 0.25, with a significance level αR = 0.10 and power 1-βR = 0.90; thus the study would be considered discouraging if no more than 5% of the study patients were successfully converted to candidacy for surgical resection (partial response), and would be considered promising if at least 25% of the cohort were successfully converted. In the VMMC study, only 30.2% (16 of 53) of patients did not experience toxicity of grade 3 or higher.10 However, in the present study, the unacceptable rate of non-toxicity (i.e. less than grade 3 toxicity) PT0 was set at 0.60 and the acceptable rate of non-toxicity PT1 at 0.80, with a significance level αT = 0.10 and power 1-βT = 0.90.
The present study group planned to accrue a total of 43 patients. Toxicity and survival were secondary endpoints. Toxicity data were analysed using a one-sample proportion test. Survival data include OS in all patients, disease-free survival in resected patients, locoregional disease control, and distant disease control. The corresponding survival functions were estimated according to the Kaplan–Meier method and the predictors for survival were evaluated by Cox’s proportional hazard model.
Results
A total of 23 patients were enrolled between January 2005 and October 2010. This trial was halted prematurely as a result of the incidence of grade 3 and 4 toxicity above the pre-trial predicted level of 80%. The initial target for enrolment had been 43 patients. The mean age at enrolment was 58.6 years. Men comprised 73.4% of the cohort. The majority of the patients were White (69.6%). Patient demographics are shown in Table 1. Clinical staging revealed T-stage 3 or 4 disease in 94.1% of patients. Disease stage based on imaging at trial enrolment is shown in Table 2. Indications for neoadjuvant treatment included involvement of one or more of the following: the SMA (≤180 ° in three patients; >180 ° in one patient); coeliac axis (≤180 ° in two patients; >180 ° in three patients); hepatic artery (HA) (>180 ° in six patients), and/or superior mesenteric vein/portal vein (≤180 ° in six patients; >180 ° in seven patients). Of 23 patients, seven (30.4%) completed all treatments. The remaining 16 (69.6%) patients did not complete all scheduled treatments as a result of severe side-effects (n = 7, 30.4%), progressive disease (n = 3, 13%), alternative treatment (n = 3, 13.0%), patient withdrawal (n = 1, 4.3%), other disease (n = 1, 4.3%), and death during the study (n = 1, 4.3%). These findings are summarized in Table 3. Overall, grade 3 or 4 toxicity occurred in 19 (82.6%) patients. Hospitalization for toxicity was required in 11 (47.8%) patients. The most commonly reported grade 3 or 4 toxicities were leucopoenia/cytopoenia (n = 19, 82.6%) and gastrointestinal toxicity (n = 19, 82.6%). Numbers of patients experiencing grade 3 and 4 toxicity are summarized in Table 4. Other toxicities were much less common (fatigue, weight loss, pain, skin rash; all n < 3). Complete details regarding specific toxicities, their severity and the point in the trial at which the toxicity occurred are summarized in Table 5. The single death that occurred during this study was caused by ascending cholangitis in an individual in whom a plastic biliary stent had been placed prior to neoadjuvant treatment. The stent became occluded and the patient developed ascending cholangitis. Despite immediate correction of the obstruction and aggressive antibiosis, the patient died. Subsequently, all patients receiving neoadjuvant therapy for pancreatic head cancers at this institution have been decompressed with metal stents because these stents have superior patency.
Table 1.
Demographic information
| Age, years, mean ± SD | 58.6 ± 9.01 |
| Gender, n (%) | |
| Male | 17 (73.9%) |
| Female | 6 (26.1%) |
| Ethnicity, n (%) | |
| American Indian/Alaska Native | 1 (4.3%) |
| African-American | 4 (17.4%) |
| White | 16 (69.6%) |
| Unknown | 2 (8.7%) |
SD, standard deviation.
Table 2.
Disease information at study onset
| T-stage | Patients, n (%) (n = 17) |
|---|---|
| 2 | 1 (5.9%) |
| 3 | 11 (64.7%) |
| 4 | 5 (29.4%) |
| N-stage | (n = 16) |
|---|---|
| 0 | 13 (81.3%) |
| 1 | 3 (18.7%) |
Table 3.
Summary of reasons for off-treatment and off-study shifts
| Reasons for stopping treatment | Patients, n (%) |
| Treatment completed | 7 (30.4%) |
| Progressive disease or relapse | 3 (13.0%) |
| AE, side-effects, complications | 7 (30.4%) |
| Death on study | 1 (4.3%) |
| Patient withdrawal | 1 (4.3%) |
| Alternative treatment | 3 (13.0%) |
| Other disease | 1 (4.3%) |
| Reasons for withdrawing from study | (n = 16) |
| Patient withdrawn | 15 (93.7%) |
| Death | 1 (6.3%) |
AE, adverse events.
Table 4.
Incidences of grade 3 or grade 4 toxicity in the 23 enrolled patients
| CRT and 4-week rest, n (%) | 5-FU, n (%) | End of trial, n (%) | |
|---|---|---|---|
| Blood/bone marrow | 19 (82.6%) | 4 (17.4%) | 2 (8.7%) |
| Leukocytes/total WBC | 11 (47.8%) | 3 (13.0%) | 1 (4.3%) |
| Neutrophils/granulocytes (ANC/AGC) | 6 (26.1%) | 1 (4.3%) | 1 (4.3%) |
| Platelets | 2 (8.7%) | 1 (4.3%) | |
| Gastrointestinal | 19 (82.6%) | 2 (8.7%) | 0% |
| Anorexia | 7 (30.4%) | ||
| Dehydration | 2 (8.7%) | ||
| Diarrhoea | 1 (4.3%) | ||
| Mucositis/stomatitis – oral cavity | 3 (13.0%) | ||
| Nausea | 4 (17.4%) | 2 (8.7%) | |
| Vomiting | 2 (8.7%) | ||
| Constitutional symptoms | 4 (17.4%) | 0% | 0% |
| Fatigue (asthenia, lethargy, malaise) | 3 (13.0%) | ||
| Weight loss | 1 (4.3%) | ||
| Dermatology/skin | 1 (4.3%) | 0% | 0% |
| Rash/hand foot skin reaction | 1 (4.3%) | ||
| Pain | 1 (4.3%) | 0% | 0% |
| Pain – abdomen NOS | 1 (4.3%) |
CRT, chemoradiotherapy; 5-FU, 5-fluorouracil; WBC, white blood cell; ANC/AGC, absolute neutrophil/granulocyte count; NOS, not otherwise specified.
Table 5.
Maximum severity of toxicity per patient (n = 23)
| Pre-study | CRT and 4-week rest | 5-FU | End of treatment | |
|---|---|---|---|---|
| Blood/bone marrow | ||||
| Grade 1 | 7 (30.4%) | 2 (8.7%) | 7 (58.3%) | |
| Grade 2 | 9 (39.1%) | 2 (16.7%) | ||
| Grade 3 | 10 (43.5%) | 3 (25.0%) | 1 (4.3%) | |
| Grade 4 | 1 (4.3%) | |||
| Cardiac general | ||||
| Grade 1 | 1 (4.3%) | |||
| Constitutional symptoms | ||||
| Grade 1 | 3 (13.0%) | 5 (21.7%) | 4 (33.3%) | 1 (4.3%) |
| Grade 2 | 13 (10.2%) | 3 (25.0%) | ||
| Grade 3 | 3 (13.0%) | |||
| Grade 4 | ||||
| Dermatology/skin | ||||
| Grade 1 | 6 (26.1%) | 2 (16.7%) | ||
| Grade 2 | 5 (21.7%) | 2 (16.7%) | ||
| Grade 3 | 1 (4.3%) | |||
| Grade 4 | ||||
| Endocrine | ||||
| Grade 1 | 1 (4.3%) | |||
| Gastrointestinal | ||||
| Grade 1 | 8 (34.8%) | 2 (8.7%) | 5 (41.7%) | |
| Grade 2 | 10 (43.5%) | 3 (25.0%) | 1 (4.3%) | |
| Grade 3 | 10 (43.5%) | 2 (16.7%) | ||
| Grade 4 | 1 (4.3%) | |||
| Infection | ||||
| Grade 1 | 1 (4.3%) | 1 (4.3%) | ||
| Grade 2 | 1 (8.3%) | |||
| Grade 3 | ||||
| Grade 4 | ||||
| Lymphatics | ||||
| Grade 1 | 1 (8.3%) | |||
| Metabolic/laboratory | ||||
| Grade 1 | 5 (21.7%) | 13 (56.5%) | 5 (41.7%) | |
| Grade 2 | 6 (26.1%) | 4 (33.3%) | 1 (4.3%) | |
| Grade 3 | ||||
| Grade 4 | ||||
| Neurology | ||||
| Grade 1 | 2 (8.7%) | 1 (8.3%) | ||
| Grade 2 | 3 (13.0%) | |||
| Grade 3 | ||||
| Grade 4 | ||||
| Ocular/visual | ||||
| Grade 1 | 1 (4.3%) | 1 (8.3%) | ||
| Pain | ||||
| Grade 1 | 10 (43.5%) | 9 (39.1%) | 6 (50.0%) | |
| Grade 2 | 1 (4.3%) | 1 (8.3%) | ||
| Grade 3 | 1 (13.0%) | |||
| Grade 4 | ||||
| Pulmonary/upper respiratory | ||||
| Grade 1 | 1 (4.3%) | 3 (13.0%) | ||
| Grade 2 | 1 (4.3%) | |||
| Grade 3 | ||||
| Grade 4 | ||||
| Renal/genitourinary | ||||
| Grade 1 | 1 (8.3%) |
CRT, chemoradiotherapy; 5-FU, 5-fluorouracil.
Following chemoradiation, all patients underwent restaging studies to evaluate for evidence of tumour regression and the possibility of surgical resection. Carbohydrate antigen 19-9 (CA 19-9) values were also obtained pre-and post-treatment. Median CA 19-9 values were 286 U/ml (range: 1–10 046 U/ml) pre-treatment and 155 U/ml (range: 19–2500 U/ml) post-treatment. This difference did not achieve statistical significance. No evidence of tumour regression was identified in any patient based on CT or EUS imaging. Conversely, progressive disease precluding surgical intervention was noted in three individuals.
Surgical resection was ultimately successful in seven (30.4%) patients. Two patients underwent distal pancreatectomy and splenectomy and five underwent pancreaticoduodenectomy. Venous resection and reconstruction were required during one pancreaticoduodenectomy, although post-treatment imaging suggested venous involvement in all five patients. No patient in whom arterial encasement had been observed pre-treatment was ultimately downstaged and resected. All of the patients brought to the operating room for exploration were ultimately resected. No operative palliative bypass procedures were performed. All surgical margins were negative in six of seven (85.7%) patients. One patient, who underwent a Whipple procedure, had microscopically positive margins at the retroperitoneal/uncinate margin and the proximal bile duct margin. No grossly positive (R2) margins were identified in this series. Positive lymph nodes were identified in three of seven (42.9%) patients. The mean lymph node count was 11.
Pathologic evaluation of all resected specimens revealed evidence of significant systemic treatment effect in three of seven (42.9%) patients. In these patients, extensive therapy-induced regressive changes were identified and only a few microscopic foci of tumour remained. Figure 2 shows a photomicrograph of a near-complete response, in which a single malignant gland remained within the muscularis of the duodenal wall. No complete pathologic responses were identified in this trial.
Figure 2.
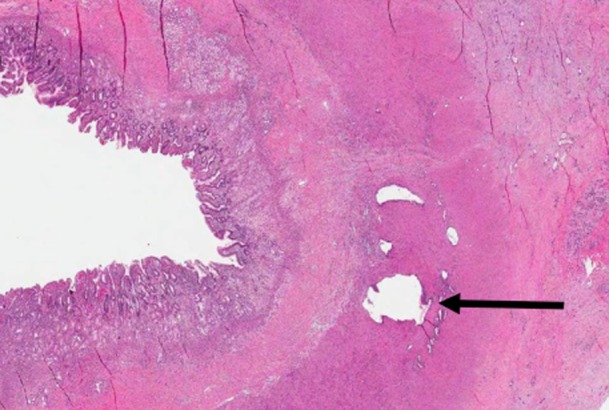
Microphotograph of infiltrative malignant glands within the muscularis propria. The duodenal lumen is on the left; benign pancreatic parenchyma with features of chronic pancreatitis is on the right. (Magnification ×1)
Mean OS in all patients enrolled in this trial was 11.5 months. Surgical resection provided significantly improved survival (22.6 months) compared with CRT alone (8.8 months). Disease-free survival in resected patients was 17.2 months (Fig. 3). Overall survival in individuals with pathologic evidence of tumour response to therapy did not differ significantly from that in patients who showed no evidence of response.
Figure 3.
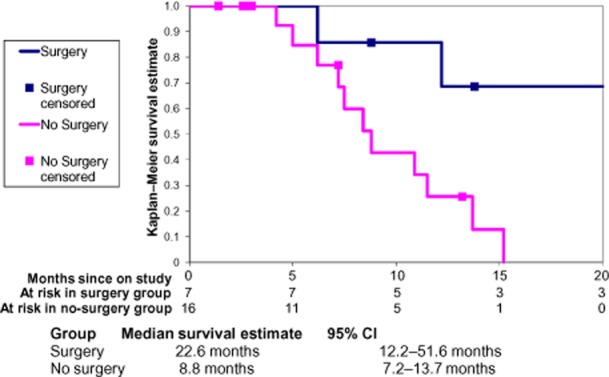
Overall survival across the study cohort (n = 23) in patients treated with chemoradiotherapy and submitted for resection (n = 7) or not submitted for resection (n = 16). 95% CI, 95% confidence interval
Discussion
This trial represents a novel effort to use IFN-based chemoradiation in the neoadjuvant setting in patients with locally advanced pancreatic cancer. Ultimately, seven of 23 tumours (30.4%) were brought to resection, all but one of which were removed with negative surgical margins. Although this is encouraging, it must be weighed against the high toxicity of this regimen, which resulted in one death during this trial.
The primary endpoint of the present study referred to ability to convert initially non-resectable tumours to resectable status, in which the study demonstrated promising results indicated by the removal of 30.4% of these tumours. When the protocol for this trial was written, the current definitions of ‘locally advanced’ and ‘borderline resectable’ cancer were not well established. Initially, only individuals with locally advanced unresectable disease were enrolled in this trial, the ultimate goal of which was to convert some of these individuals to resectable status. As the trial progressed, enrolment of patients who would now be considered as borderline resectable was allowed. All patients were reviewed by a multidisciplinary team and enrolled patients were considered to have disease that was not readily resectable as a result of vascular involvement. The present results indicate that all patients who were ultimately resected had what would now be considered borderline disease at presentation based on the extent of vascular involvement of the tumours. No patient in whom the SMA, coeliac axis or HA was encased by tumour was ultimately able to be resected. In addition, imaging evidence of tumour response to therapy was essentially non-existent, with no patient demonstrating a significant reduction in tumour volume during chemoradiation based on staging CT and EUS. Although these findings are disappointing, those on pathologic examination of the resected specimens were significantly more encouraging. Of the seven specimens removed, three (42.9%) showed significant pre-treatment effects with extensive therapy-induced regressive changes and only small islands of viable tumour cells remaining. In addition, in six of seven patients, tumours were resected with negative margins and only three of these seven patients were found to have lymph node involvement. Despite initial evidence of vascular invasion, only one individual in this group required portal vein resection in order to achieve negative margins. These factors indicate clearly that pre-treatment chemoradiation does provide a therapeutic effect.
Despite these seemingly positive findings, OS in individuals with evidence of tumour response did not improve compared with that in patients with no pathologic evidence of treatment response.
The fact that all patients demonstrated apparently significant vascular involvement but only one of five pancreaticoduodenectomies required venous resection raises concern that post-treatment imaging may not accurately reflect tumour resectability. Similar concerns are raised by the fact that the majority of these tumours were resected with negative margins, despite locally advanced disease. This trial did not explore patients for resectability if they had evidence of arterial encasement on post-treatment imaging. It is possible that additional patients may have been resectable, given current knowledge of the accuracy of CT scans following chemoradiation. Based on the results of this study, in the absence of tumour progression, the present group currently explores all patients for resectability following neoadjuvant therapy at this institution.
With reference to toxicity, the present findings support the observations of previous investigators. Over 80% of patients in the present trial suffered grade 3 or 4 toxicity as a result of treatment, which caused the trial to be halted prematurely. Although most instances of toxicity were non-life threatening, almost 50% of patients required hospitalization and one patient died as a result of treatment. It had been hoped initially that giving treatment prior to major surgical resection might enable patients to tolerate it better and to experience fewer severe side-effects. As the present findings show, this was not the case. Like the present observations, the results of ACOSOG Z0503112 also confirmed a significant toxicity associated with this regimen. In that study, 95% of patients experienced grade 3 or 4 toxicity (66% grade 3, 29% grade 4).12 In addition, only 17% of patients were able to complete all chemoradiation,12 whereas 30.4% did so in the present study. The issue of severe systemic toxicity continues to plague efforts to assess the full potential of IFN-based combination therapy.
The utility of neoadjuvant therapy in pancreatic cancer has now been widely published. Even in the absence of a survival advantage for individuals receiving neoadjuvant therapy, several other potential advantages must be considered. Firstly, neoadjuvant therapy allows immediate treatment for what is almost always systemic disease at the time of diagnosis. It ensures all patients will receive some form of systemic treatment, whereas in the adjuvant setting, a significant number of patients may not receive therapy following surgery. Secondly, neoadjuvant therapy allows the treatment of a tumour in situ, with an uncompromised blood supply and intact anatomy. Thirdly, neoadjuvant therapy allows for better patient selection as some individuals will have rapid tumour progression during systemic treatment. These cases are almost certain to gain no benefit from surgical resection.
In a recent meta-analysis of neoadjuvant therapy for pancreas adenocarcinoma, Assifi et al. found that of 134 patients, 31.6% of those with borderline and locally advanced tumours ultimately underwent resection following neoadjuvant treatment.13 Median survival was 22 months in individuals who underwent resection, whereas OS in all patients was 11 months. These findings are virtually identical to the observations in the present study. Also in Assifi et al.’s analysis, 46% of patients with locally advanced disease had grade 3 or 4 toxicity, according to the results reported from nine studies.13 This is significantly less than the proportion observed in any trial including an IFN-based chemoradiation regimen.13
The present study lends support to the principle of using neoadjuvant therapy in patients with locally advanced pancreatic adenocarcinomas with the ultimate aim of facilitating surgical resection. The benefit seen in the present trial was limited to those cancers that are truly borderline, based on < 180 ° abutment of major arterial structures. In patients with locally advanced disease made unresectable by arterial encasement, surgical resection was not possible. Encased vessels did not subsequently become non-encased and therefore these tumours remained unresectable. Given this, the present data support the proposals of others that aggressive neoadjuvant protocols should continue to be focused on those with borderline resectable disease, in whom the likelihood of achieving surgical therapy is most reasonable.
Since the initiation of this trial, several other neoadjuvant regimens have been investigated in Phase II trials with fairly similar outcomes in terms of OS. The present group felt that this IFN-based chemoradiation regimen held promise as the most effective therapy to date for pancreatic adenocarcinoma. Although the initial trial from the VMMC10 held significant promise, confirmatory trials have failed to reproduce its exceptional outcomes. Meanwhile, treatment-related toxicity has become a major problem and has caused many oncologists to hesitate to use such a regimen. Given the toxicity of this protocol, and its failure to show significantly improved outcomes over other less toxic regimens, efforts must continue to focus on identifying new drug combinations that provide tumour response while minimizing systemic toxicity.
Conflicts of interest
None declared.
References
- 1.Merchant NB, Parikh AA, Liu EH. Adjuvant chemoradiation therapy for pancreas cancer: who really benefits? Adv Surg. 2010;44:149–164. doi: 10.1016/j.yasu.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman JP, Lipsitz S, Pisansky T, Weese JL, Solin L, Benson AB., 3rd Phase II trial of preoperative radiation therapy and chemotherapy for patients with localized, resectable adenocarcinoma of the pancreas: an Eastern Cooperative Oncology Group Study. J Clin Oncol. 1998;16:317–323. doi: 10.1200/JCO.1998.16.1.317. [DOI] [PubMed] [Google Scholar]
- 3.Todd KE, Gloor B, Lane JS, Isacoff WH, Reber HA. Resection of locally advanced pancreatic cancer after downstaging with continuous-infusion 5-fluorouracil, mitomycin-C, leucovorin, and dipyridamole. J Gastrointest Surg. 1998;2:159–166. doi: 10.1016/s1091-255x(98)80008-5. [DOI] [PubMed] [Google Scholar]
- 4.Morganti AG, Trodella L, Valentini V, Doglietto GB, Ziccarelli P, Macchia G, et al. Preoperative radiochemotherapy in pancreatic cancer: preliminary results. Tumori. 1999;85(1 Suppl 1):27–32. [PubMed] [Google Scholar]
- 5.Bajetta E, Di Bartolomeo M, Stani SC, Artale S, Ricci SB, Bozzetti F, et al. Chemoradiotherapy as preoperative treatment in locally advanced unresectable pancreatic cancer patients: results of a feasibility study. Int J Radiat Oncol Biol Phys. 1999;45:285–289. doi: 10.1016/s0360-3016(99)00205-9. [DOI] [PubMed] [Google Scholar]
- 6.Kim HJ, Czischke K, Brennan MF, Conlon KC. Does neoadjuvant chemoradiation downstage locally advanced pancreatic cancer? J Gastrointest Surg. 2002;6:763–769. doi: 10.1016/s1091-255x(02)00017-3. [DOI] [PubMed] [Google Scholar]
- 7.Snady H, Bruckner H, Cooperman A, Paradiso J, Kiefer L. Survival advantage of combined chemoradiotherapy compared with resection as the initial treatment of patients with regional pancreatic carcinoma. An outcomes trial. Cancer. 2000;89:314–327. doi: 10.1002/1097-0142(20000715)89:2<314::aid-cncr16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 8.Al-Sukhun S, Zalupski MM, Ben-Josef E, Vaitkevicius VK, Philip PA, Soulen R, et al. Chemoradiotherapy in the treatment of regional pancreatic carcinoma: a Phase II study. Am J Clin Oncol. 2003;26:543–549. doi: 10.1097/01.coc.0000037143.60502.54. [DOI] [PubMed] [Google Scholar]
- 9.Magnin V, Moutardier V, Giovannini MH, Lelong B, Giovannini M, Viret F, et al. Neoadjuvant preoperative chemoradiation in patients with pancreatic cancer. Int J Radiat Oncol Biol Phys. 2003;55:1300–1304. doi: 10.1016/s0360-3016(02)04157-3. [DOI] [PubMed] [Google Scholar]
- 10.Nukui Y, Picozzi VJ, Traverso LW. Interferon-based adjuvant chemoradiation therapy improves survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2000;179:367–371. doi: 10.1016/s0002-9610(00)00369-x. [DOI] [PubMed] [Google Scholar]
- 11.Linehan DC, Tan MC, Strasberg SM, Drebin JA, Hawkins WG, Picus J, et al. Adjuvant interferon-based chemoradiation followed by gemcitabine for resected pancreatic adenocarcinoma: a single-institution Phase II study. Ann Surg. 2008;248:145–151. doi: 10.1097/SLA.0b013e318181e4e9. [DOI] [PubMed] [Google Scholar]
- 12.Picozzi VJ, Abrams RA, Decker PA, Traverso W, O’Reilly EM, Greeno E, et al. Multicentre Phase II trial of adjuvant therapy for resected pancreatic cancer using cisplatin, 5-fluorouracil, and interferon-alpha-2b-based chemoradiation: ACOSOG Trial Z05031. Ann Oncol. 2011;22:348–354. doi: 10.1093/annonc/mdq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Assifi MM, Lu X, Eibl G, Reber HA, Li G, Hines OJ. Neoadjuvant therapy in pancreatic adenocarcinoma: a meta-analysis of Phase II trials. Surgery. 2011;150:466–473. doi: 10.1016/j.surg.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


